Electrochemical Sensor for Simple and Sensitive Determination of Hydroquinone in Water Samples Using Modified Glassy Carbon Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Materials
2.2. Synthesis of MnO2 NRs/GO Nanocomposite
2.3. Modified Electrode Preparation
2.4. Preparation of Water Specimens
3. Results and Discussions
3.1. Characterization of MnO2 NRs/GO Nanocomposite by Field Emission Scanning Electron Microscopy (FE-SEM)
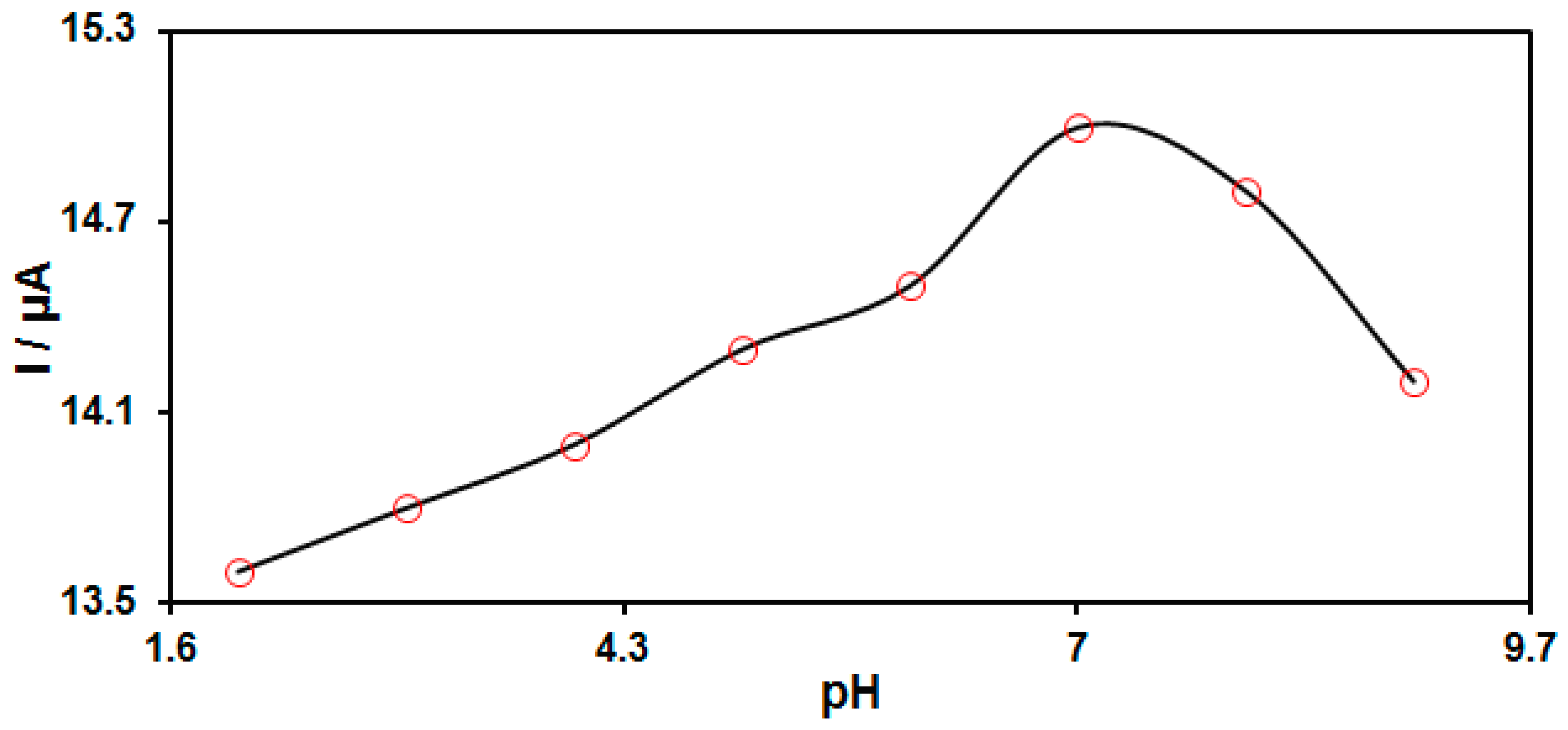
3.2. Electrochemical Behaviors of HQ on the MnO2 NRs/GO/GCE
3.3. Scan Rate Exploration
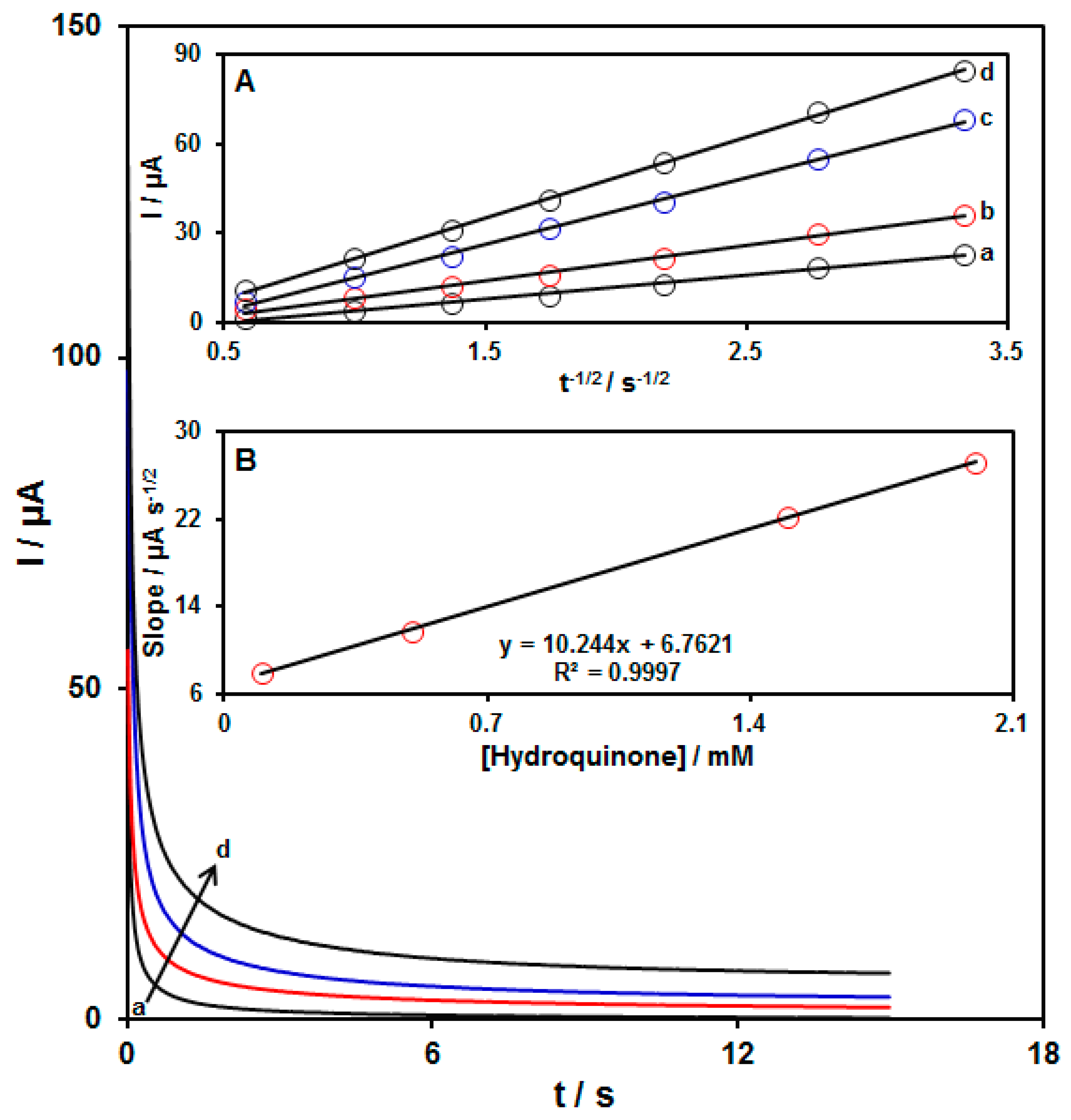
3.4. Chronoamperometric Determinations
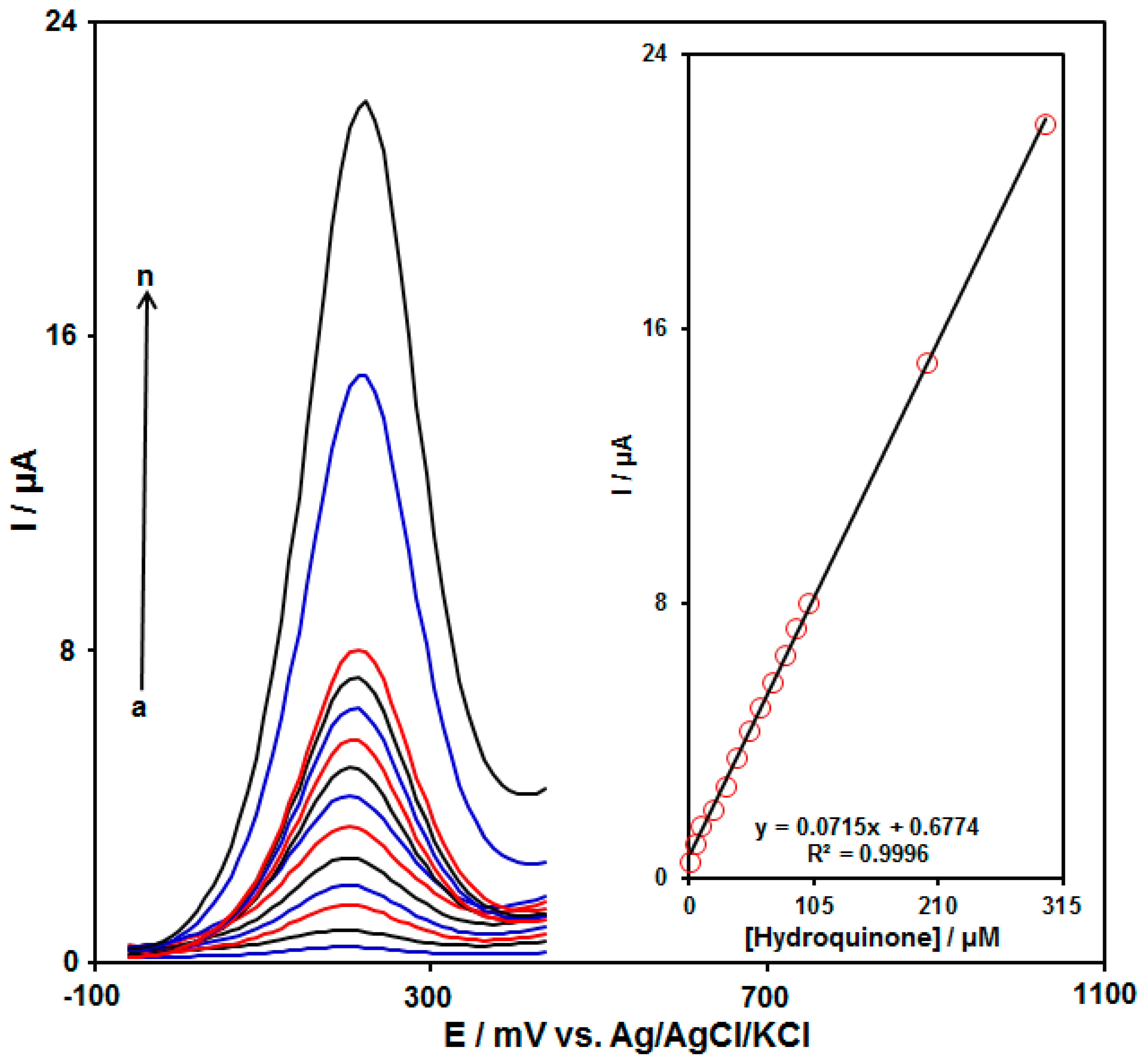
3.5. Calibration Plot
3.6. Interference Studies
3.7. Stability, Repeatability, and Reproducibility Studies
3.8. Application of the MnO2 NRs/GO/GCE Sensor for the Determination of HQ in Real Samples (River Water and Tap Water)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apetrei, C.; Iticescu, C.; Georgescu, L.P. Multisensory system used for the analysis of the water in the lower area of River Danube. Nanomaterials 2019, 9, 891. [Google Scholar] [CrossRef]
- Ghrissi, H.; Veloso, A.C.; Marx, Í.M.; Dias, T.; Peres, A.M. A potentiometric electronic tongue as a discrimination tool of water-food indicator/contamination bacteria. Chemosensors 2021, 9, 143. [Google Scholar] [CrossRef]
- Martoni, L.V.; Gomes, N.O.; Prado, T.M.; Calegaro, M.L.; Oliveira, O.N., Jr.; Machado, S.A.; Raymundo-Pereira, P.A. Carbon spherical shells in a flexible photoelectrochemical sensor to determine hydroquinone in tap water. J. Environ. Chem. Eng. 2022, 10, 107556. [Google Scholar] [CrossRef]
- Nordlund, J.J.; Grimes, P.E.; Ortonne, J.P. The safety of hydroquinone. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, W.; Zhang, L.; Jia, Y.; Liu, Y. Simultaneous determination of catechol and hydroquinone using gold nanoparticles/poly (p-aminobenzenesulfonic acid)/multi-walled carbon nanotubes modified glassy carbon electrode. J. Electrochem. Soc. 2022, 169, 107504. [Google Scholar] [CrossRef]
- Cabrera-Alonso, R.; Guevara, E.; Ramírez-Elías, M.G.; Moncada, B.; González, F.J. Surface-enhanced Raman scattering of hydroquinone assisted by gold nanorods. J. Nanophotonics 2019, 13, 036006. [Google Scholar] [CrossRef]
- Bhanger, M.I.; Niaz, A.; Shah, A.; Rauf, A. Ultra-trace level determination of hydroquinone in waste photographic solutions by UV–vis spectrophotometry. Talanta 2007, 72, 546–553. [Google Scholar]
- Lin, Y.H.; Yang, Y.H.; Wu, S.M. Experimental design and capillary electrophoresis for simultaneous analysis of arbutin, kojic acid and hydroquinone in cosmetics. J. Pharm. Biomed. Anal. 2007, 44, 279–282. [Google Scholar] [CrossRef]
- Zhao, L.; Lv, B.; Yuan, H.; Zhou, Z.; Xiao, D. A sensitive chemiluminescence method for determination of hydroquinone and catechol. Sensors 2007, 7, 578–588. [Google Scholar] [CrossRef]
- Wolf, A.; Hüttl, R.; Wolf, G. Enzymatic reactions for the calorimetric detection of phenolic compounds. J. Therm. Anal. Calorim. 2000, 61, 37–44. [Google Scholar] [CrossRef]
- Jurica, K.; Karaconji, I.B.; Šegan, S.; Milojković-Opsenica, D.; Kremer, D. Quantitative analysis of arbutin and hydroquinone in strawberry tree (Arbutus unedo L., Ericaceae) leaves by gas chromatography-mass spectrometry. Arh. Hig. Rada Toksikol. 2015, 66, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Legido-Quigley, C. Fast and sensitive high performance liquid chromatography analysis of cosmetic creams for hydroquinone, phenol and six preservatives. J. Chromatogr. A 2011, 1218, 4307–4311. [Google Scholar] [CrossRef] [PubMed]
- Cotchim, S.; Promsuwan, K.; Dueramae, M.; Duerama, S.; Dueraning, A.; Thavarungkul, P.; Limbut, W. Development and application of an electrochemical sensor for hydroquinone in pharmaceutical products. J. Electrochem. Soc. 2020, 167, 155528. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Min, A.; Choi, M.Y. Fabrication of nonenzymatic electrochemical sensor based on Zn@ZnO core-shell structures obtained via pulsed laser ablation for selective determination of hydroquinone. Environ. Res. 2022, 204, 112340. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, C. Simultaneous determination of hydroquinone and catechol at a glassy carbon electrode modified with multiwall carbon nanotubes. Electroanalysis 2005, 17, 832–838. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, K.; Shi, Y.; Gui, X.; Lv, C.; Tao, H. Reduced graphene oxide cross-linked L-cysteine modified glassy carbon electrode for detection of environmental pollutant of hydroquinone. FlatChem 2021, 25, 100214. [Google Scholar] [CrossRef]
- Chen, T.; Xu, J.; Arsalan, M.; Sheng, Q.; Zheng, J.; Cao, W.; Yue, T. Controlled synthesis of Au@ Pd core-shell nanocomposites and their application for electrochemical sensing of hydroquinone. Talanta 2019, 198, 78–85. [Google Scholar] [CrossRef]
- Zhao, D.M.; Zhang, X.H.; Feng, L.J.; Jia, L.; Wang, S.F. Simultaneous determination of hydroquinone and catechol at PASA/MWNTs composite film modified glassy carbon electrode. Colloids Surf. B Biointerfaces 2009, 74, 317–321. [Google Scholar] [CrossRef]
- Chen, T.W.; Palanisamy, S.; Chen, S.M.; Velusamy, V.; Liu, Y.H.; Tseng, T.W.; Liu, X. Sensitive and low-potential electrochemical detection of hydroquinone using a nanodiamond modified glassy carbon electrode. Int. J. Electrochem. Sci. 2017, 12, 8021–8032. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Zhang, Y.; Huang, L.; Wan, Q. A hydroquinone sensor based on a new nanocrystals modified electrode. J. Chem. Technol. Biotechnol. 2014, 89, 259–264. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Huang, K.; Song, L.; Li, Y. A graphene oxide–mesoporous MnO2 nanocomposite modified glassy carbon electrode as a novel and efficient voltammetric sensor for simultaneous determination of hydroquinone and catechol. Sens. Actuators B Chem. 2013, 177, 412–418. [Google Scholar] [CrossRef]
- Roshanfekr, H. A simple specific dopamine aptasensor based on partially reduced graphene oxide–Au NPs composite. Prog. Chem. Biochem. Res. 2023, 6, 79–88. [Google Scholar]
- Beitollahi, H.; Tajik, S.; Karimi-Maleh, H.; Hosseinzadeh, R. Application of a 1-benzyl-4-ferrocenyl-1H-[1,2,3]-triazole/carbon nanotube modified glassy carbon electrode for voltammetric determination of hydrazine in water samples. Appl. Organomet. Chem. 2013, 27, 444–450. [Google Scholar] [CrossRef]
- Ariavand, S.; Ebrahimi, M.; Foladi, E. Design and construction of a novel and an efficient potentiometric sensor for determination of sodium ion in urban water samples. Chem. Methodol. 2022, 6, 886–904. [Google Scholar]
- Mohanraj, J.; Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S.; Rajendran, S.; Karimi-Maleh, H. Facile synthesis of paper based graphene electrodes for point of care devices: A double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 2020, 566, 463–472. [Google Scholar] [CrossRef]
- Hasanpour, F.; Taei, M.; Fouladgar, M.; Salehi, M. Au nano dendrites/composition optimized nd-dopped cobalt oxide as an efficient electrocatalyst for ethanol oxidation. J. Appl. Organomet. Chem. 2022, 2, 188–196. [Google Scholar]
- Guo, Q.; Zhang, M.; Zhou, G.; Zhu, L.; Feng, Y.; Wang, H.; Hou, H. Highly sensitive simultaneous electrochemical detection of hydroquinone and catechol with three-dimensional N-doping carbon nanotube film electrode. J. Electroanal. Chem. 2016, 760, 15–23. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.Y.; Cheng, C. Electrochemical detection of hydroquinone by graphene and Pt-graphene hybrid material synthesized through a microwave-assisted chemical reduction process. Electrochim. Acta 2011, 56, 2712–2716. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Li, Z. Nanohybrid of Co3O4 and histidine-functionalized graphene quantum dots for electrochemical detection of hydroquinone. Electrochim. Acta 2017, 255, 323–334. [Google Scholar] [CrossRef]
- Li, S.J.; Xing, Y.; Wang, G.F. A graphene-based electrochemical sensor for sensitive and selective determination of hydroquinone. Microchim. Acta 2012, 176, 163–168. [Google Scholar] [CrossRef]
- Nasr, B.; Abdellatif, G.; Canizares, P.; Sáez, C.; Lobato, J.; Rodrigo, M.A. Electrochemical oxidation of hydroquinone, resorcinol, and catechol on boron-doped diamond anodes. Environ. Sci. Technol. 2005, 39, 7234–7239. [Google Scholar]
- Hu, S.; Wang, Y.; Wang, X.; Xu, L.; Xiang, J.; Sun, W. Electrochemical detection of hydroquinone with a gold nanoparticle and graphene modified carbon ionic liquid electrode. Sens. Actuators B Chem. 2012, 168, 27–33. [Google Scholar] [CrossRef]
- Bijad, M.; Karimi-Maleh, H.; Farsi, M.; Shahidi, S.A. An electrochemical-amplified-platform based on the nanostructure voltammetric sensor for the determination of carmoisine in the presence of tartrazine in dried fruit and soft drink samples. J. Food Meas. Charact. 2018, 12, 634–640. [Google Scholar] [CrossRef]
- Hosseini Fakhrabad, A.; Sanavi Khoshnood, R.; Abedi, M.R.; Ebrahimi, M. Fabrication a composite carbon paste electrodes (CPEs) modified with Multi-Wall Carbon Nano-Tubes (MWCNTs/N, N-Bis (salicyliden)-1,3-propandiamine) for determination of lanthanum (III). Eurasian Chem. Commun. 2021, 3, 627–634. [Google Scholar]
- Zhang, Z.; Karimi-Maleh, H. Label-free electrochemical aptasensor based on gold nanoparticles/titanium carbide MXene for lead detection with its reduction peak as index signal. Adv. Compos. Hybrid Mater. 2023, 6, 68. [Google Scholar] [CrossRef]
- Peyman, H. Design and fabrication of modified DNA-Gp nano-biocomposite electrode for industrial dye measurement and optical confirmation. Prog. Chem. Biochem. Res. 2022, 5, 391–405. [Google Scholar]
- Buledi, J.A.; Mahar, N.; Mallah, A.; Solangi, A.R.; Palabiyik, I.M.; Qambrani, N.; Karimi, F.; Vasseghian, Y.; Karimi-Maleh, H. Electrochemical quantification of mancozeb through tungsten oxide/reduced graphene oxide nanocomposite: A potential method for environmental remediation. Food Chem. Toxicol. 2022, 161, 112843. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, Z.; Shahidi, S.A.; Ghorbani-HasanSaraei, A.; Limooei, M.B.; Bijad, M. Monitoring of amaranth in drinking samples using voltammetric amplified electroanalytical sensor. Chem. Methodol. 2022, 6, 246–252. [Google Scholar]
- Husain, Z.; Raheman, A.S.; Ansari, K.B.; Pandit, A.B.; Khan, M.S.; Qyyum, M.A.; Lam, S.S. Nano-sized mesoporous biochar derived from biomass pyrolysis as electrochemical energy storage supercapacitor. Mater. Sci. Technol. 2022, 5, 99–109. [Google Scholar] [CrossRef]
- Janitabar Darzi, S.; Bastami, H. Au decorated mesoporous TiO2 as a high performance photocatalyst towards crystal violet dye. Adv. J. Chem. A 2022, 5, 22–30. [Google Scholar]
- Kavade, R.; Khanapure, R.; Gawali, U.; Patil, A.; Patil, S. Degradation of methyl orange under visible light by ZnO-Polyaniline nanocomposites. J. Appl. Organomet. Chem. 2022, 2, 101–112. [Google Scholar]
- Sheikhshoaie, I.; Rezazadeh, A.; Ramezanpour, S. Removal of Pb (II) from aqueous solution by gel combustion of a new nano sized Co3O4/ZnO composite. Asian J. Nanosci. Mater. 2022, 5, 336–345. [Google Scholar]
- Dormanesh, R.; Mohseni, S. Green synthesis of zinc oxide nanoparticles and their application in ring opening of epoxides. Prog. Chem. Biochem. Res. 2021, 4, 403–413. [Google Scholar]
- Muhi-Alden, Y.Y.; Saleh, K.A. Removing methylene blue dye from industrial wastewater using polyacrylonitrile/iron oxide nanocomposite. Eurasian Chem. Commun. 2021, 3, 755–762. [Google Scholar]
- Tallapaneni, V.; Mude, L.; Pamu, D.; Karri, V.V.S.R. Formulation, characterization and in vitro evaluation of dual-drug loaded biomimetic chitosan-collagen hybrid nanocomposite scaffolds. J. Med. Chem. Sci. 2022, 5, 1059–1074. [Google Scholar]
- Abadi, M.A.A.; Masrournia, M.; Abedi, M.R. Simultaneous extraction and preconcentration of benzene, toluene, ethylbenzene and xylenes from aqueous solutions using magnetite-graphene oxide composites. Chem. Methodol. 2021, 5, 11–20. [Google Scholar]
- Khazaeli, P.; Alaei, M.; Khaksarihadad, M.; Ranjbar, M. Preparation of PLA/chitosan nanoscaffolds containing cod liver oil and experimental diabetic wound healing in male rats study. J. Nanobiotechnol. 2020, 18, 176. [Google Scholar] [CrossRef]
- Tajik, S.; Lohrasbi-Nejad, A.; Mohammadzadeh Jahani, P.; Askari, M.B.; Salarizadeh, P.; Beitollahi, H. Co-detection of carmoisine and tartrazine by carbon paste electrode modified with ionic liquid and MoO3/WO3 nanocomposite. J. Food Meas. Charact. 2022, 16, 722–730. [Google Scholar] [CrossRef]
- Mohammadi, S.; Beitollahi, H.; Mohadesi, A. Electrochemical behaviour of a modified carbon nanotube paste electrode and its application for simultaneous determination of epinephrine, uric acid and folic acid. Sens. Lett. 2013, 11, 388–394. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Fakude, C.T.; Mabuba, N.; Peleyeju, G.M.; Arotiba, O.A. The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 2019, 554, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Sheikhshoaie, I. Novel nanostructure-based electrochemical sensor for simultaneous determination of dopamine and acetaminophen. Mater. Sci. Eng. C 2012, 32, 375–380. [Google Scholar] [CrossRef]
- Roy, S.; Soin, N.; Bajpai, R.; Misra, D.S.; McLaughlin, J.A.; Roy, S.S. Graphene oxide for electrochemical sensing applications. J. Mater. Chem. 2011, 21, 14725–14731. [Google Scholar] [CrossRef]
- Ardakani, M.M.; Taleat, Z.; Beitollahi, H.; Salavati-Niasari, M.; Mirjalili, B.B.F.; Taghavinia, N. Electrocatalytic oxidation and nanomolar determination of guanine at the surface of a molybdenum (VI) complex–TiO2 nanoparticle modified carbon paste electrode. J. Electroanal. Chem. 2008, 624, 73–78. [Google Scholar] [CrossRef]
- Ratinac, K.R.; Yang, W.; Gooding, J.J.; Thordarson, P.; Braet, F. Graphene and related materials in electrochemical sensing. Electroanalysis 2011, 23, 803–826. [Google Scholar] [CrossRef]
- Almarbd, Z.Z.; Abbass, N.M. Synthesis and characterization of TiO2, Ag2O, and graphene oxide nanoparticles with polystyrene as a nonocomposites and some of their applications. Eurasian Chem. Commun. 2022, 4, 1033–1043. [Google Scholar]
- Garkani-Nejad, F.; Beitollahi, H.; Alizadeh, R. Sensitive determination of hydroxylamine on ZnO nanorods/graphene oxide nanosheets modified graphite screen printed electrode. Anal. Bioanal. Electrochem. 2017, 9, 134–144. [Google Scholar]
- Li, J.; Liu, J.; Tan, G.; Jiang, J.; Peng, S.; Deng, M.; Liu, Y. High-sensitivity paracetamol sensor based on Pd/graphene oxide nanocomposite as an enhanced electrochemical sensing platform. Biosens. Bioelectron. 2014, 54, 468–475. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, J.; Zhang, H.; Liu, C.C. Highly sensitive electrochemical analysis of tunnel structured MnO2 nanoparticle-based sensors on the oxidation of nitrite. Sens. Actuators B Chem. 2019, 281, 746–750. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Di Bartolomeo, A. Application of MnO2 nanorod–ionic liquid modified carbon paste electrode for the voltammetric determination of sulfanilamide. Micromachines 2022, 13, 598. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Zhang, X.; Liu, N.; Li, H.; Yu, Z.; Yan, X. Electrochemical sensor for mercuric chloride based on graphene-MnO2 composite as recognition element. Electrochim. Acta 2015, 174, 221–229. [Google Scholar] [CrossRef]
- Obodo, R.M.; Onah, E.O.; Nsude, H.E.; Agbogu, A.; Nwanya, A.C.; Ahmad, I.; Ezema, F.I. Performance evaluation of graphene oxide based Co3O4@GO, MnO2@GO and Co3O4/MnO2@GO electrodes for supercapacitors. Electroanalysis 2020, 32, 2786–2794. [Google Scholar] [CrossRef]
- Li, L.; Du, Z.; Liu, S.; Hao, Q.; Wang, Y.; Li, Q.; Wang, T. A novel nonenzymatic hydrogen peroxide sensor based on MnO2/graphene oxide nanocomposite. Talanta 2010, 82, 1637–1641. [Google Scholar] [CrossRef]
- Kumar, P.; Khan, M.Q.; Khan, R.A.; Ahmad, K.; Kim, H. Hydrothermal synthesis of MnO2/reduced graphene oxide composite for 4-Nitrophenol sensing applications. Inorganics 2022, 10, 219. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, P.; Wu, Y.; Li, J.; Liu, J.; Li, G.; He, Q. MnO2 nanowires-decorated reduced graphene oxide modified glassy carbon electrode for sensitive determination of bisphenol A. J. Electrochem. Soc. 2020, 167, 046514. [Google Scholar] [CrossRef]
- Lei, J.; Lu, X.; Wang, W.; Bian, X.; Xue, Y.; Wang, C.; Li, L. Fabrication of MnO2/graphene oxide composite nanosheets and their application in hydrazine detection. RSC Adv. 2012, 2, 2541–2544. [Google Scholar] [CrossRef]



| Electrode | Anodic Peak Current (µA) | Anodic Peak Potential (mV) | Cathodic Peak Current (µA) | Cathodic Peak Potential (mV) |
|---|---|---|---|---|
| Bare GCE | 5.4 | 290 | −4.0 | −140 |
| MnO2 NRs/GCE | 8.5 | 240 | −6.5 | −130 |
| GO/GCE | 11.7 | 215 | −9.9 | −115 |
| MnO2 NRs/GO/GCE | 15.0 | 200 | −13.4 | −100 |
| Electrochemical Sensor | Analytical Methods | Dynamic Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| Zinc @ zinc oxide core-shell/glassy carbon electrode | Cyclic voltammetry | 10.0 to 90.0 µM | 0.10443 µM | [14] |
| Glassy carbon electrode modified with multiwall carbon nanotubes | Differential pulse voltammetry | 1.0 × 10−6 M to 1.0 × 10−4 M | 7.5 × 10−7 M | [15] |
| Reduced graphene oxide cross-linked L-cysteine/glassy carbon electrode | Differential pulse voltammetry | 2.0 to 160.0 µM | 1.5 µM | [16] |
| Au@Pd nanocomposites/glassy carbon electrode | Differential pulse voltammetry | 4.0 to 5000.0 µM | 0.63 µM | [17] |
| Poly-amidosulfonic acid and multi-wall carbon nanotubes composite electropolymerization on glassy carbon electrode | Differential pulse voltammetry | 6.0 × 10−6 to 4.0 × 10−4 M | 1.0 × 10−6 M | [18] |
| Nanodiamond/glassy carbon electrode | Differential pulse voltammetry | 1.0 to 78.0 μM | 0.19 μM | [19] |
| CuS nanocrystals/chitosan/glassy carbon electrode | Cyclic voltammetry | 4.5 µM to 4.5 mM | 1.5 µM | [20] |
| Electrodeposition of reduced graphene oxide on glassy carbon electrode | Differential pulse voltammetry | 6.0 to 200.0 μM | 0.2 μM | [21] |
| GO–mesoporous MnO2 nanocomposite/glassy carbon electrode | Differential pulse voltammetry | 0.01 to 0.7 µM | 7.0 nM | [22] |
| MnO2 NRs/GO/GCE | Differential pulse voltammetry | 0.5 to 300.0 μM | 0.012 µM | This Work |
| Sample | Spiked | Found | Recovery (%) | R.S.D. (%) |
|---|---|---|---|---|
| River water | 0 | - | - | - |
| 5.0 | 4.9 | 98.0 | 3.5 | |
| 7.0 | 7.1 | 101.4 | 1.9 | |
| Tap Water | 0 | - | - | - |
| 5.5 | 5.7 | 103.6 | 2.8 | |
| 7.5 | 7.3 | 97.3 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karami-Kolmoti, P.; Beitollahi, H.; Modiri, S. Electrochemical Sensor for Simple and Sensitive Determination of Hydroquinone in Water Samples Using Modified Glassy Carbon Electrode. Biomedicines 2023, 11, 1869. https://doi.org/10.3390/biomedicines11071869
Karami-Kolmoti P, Beitollahi H, Modiri S. Electrochemical Sensor for Simple and Sensitive Determination of Hydroquinone in Water Samples Using Modified Glassy Carbon Electrode. Biomedicines. 2023; 11(7):1869. https://doi.org/10.3390/biomedicines11071869
Chicago/Turabian StyleKarami-Kolmoti, Parisa, Hadi Beitollahi, and Sina Modiri. 2023. "Electrochemical Sensor for Simple and Sensitive Determination of Hydroquinone in Water Samples Using Modified Glassy Carbon Electrode" Biomedicines 11, no. 7: 1869. https://doi.org/10.3390/biomedicines11071869
APA StyleKarami-Kolmoti, P., Beitollahi, H., & Modiri, S. (2023). Electrochemical Sensor for Simple and Sensitive Determination of Hydroquinone in Water Samples Using Modified Glassy Carbon Electrode. Biomedicines, 11(7), 1869. https://doi.org/10.3390/biomedicines11071869







