Unveiling the Silent Danger of Childhood Obesity: Non-Invasive Biomarkers Such as Carotid Intima-Media Thickness, Arterial Stiffness Surrogate Markers, and Blood Pressure Are Useful in Detecting Early Vascular Alterations in Obese Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Physical Examination
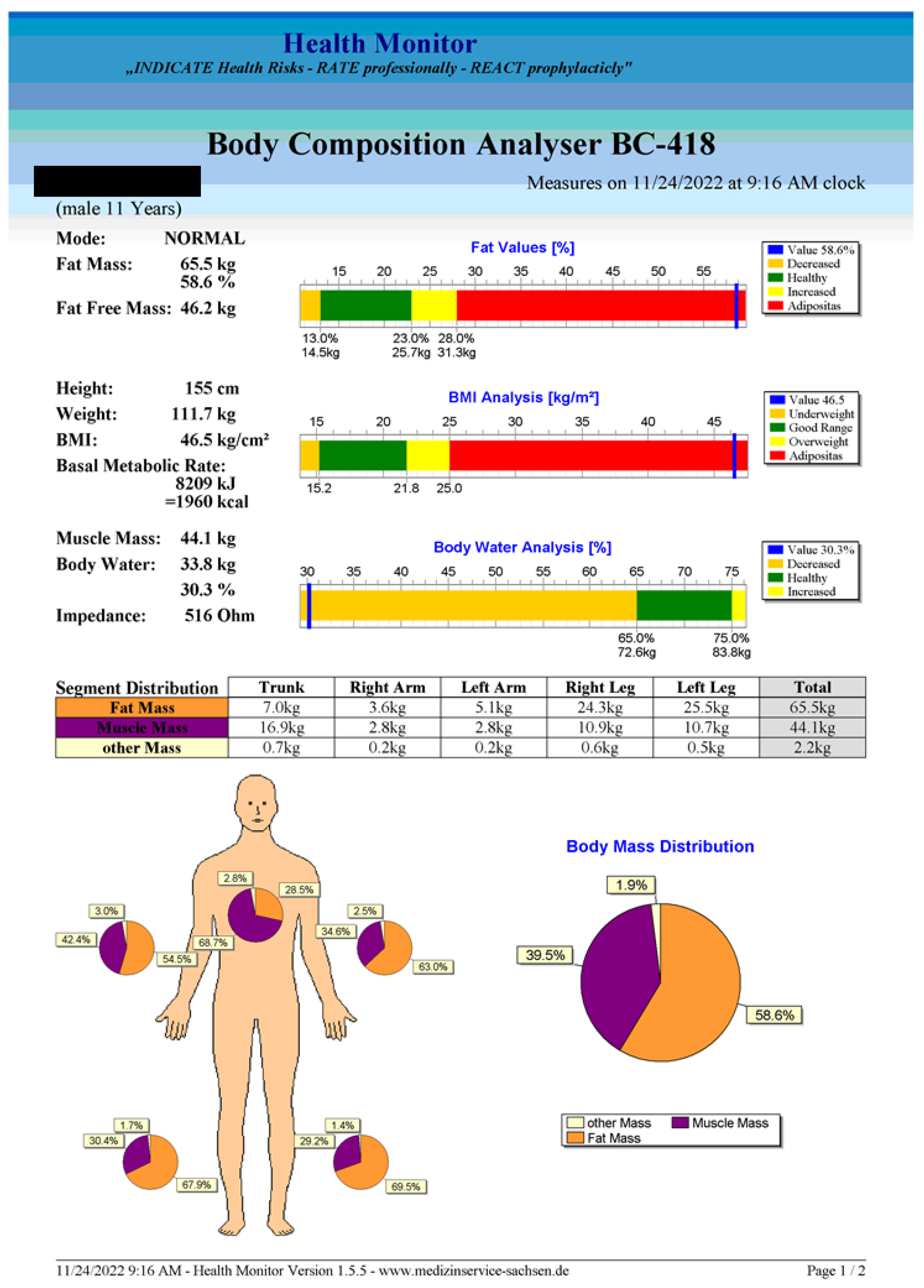
2.2. Body Composition Analysis
- ❖
- According to the manufacturer, the use of this device is advised only for children over 6 years old;
- ❖
- The software requires the examiner to enter the following data for each patient: identification data, birth date, sex, weight, height, and clothes’ weight;
- ❖
- The subject steps with bare feet on the device’s foot electrodes, making sure that their feet are positioned exactly in the pre-determined shapes, and then grabs both handles, which contain the hand electrodes;
- ❖
- The subject needs to maintain a stable and straight position throughout the measurement;
- ❖
- After the measurement is accomplished, the software provides an explicit report (an example is given in Figure A1, Appendix A).
2.3. Arterial Stiffness Measurements
- ❖
- There had been no ongoing acute illness, and no exposure to smoke for at least four hours prior to the measurements, with no consumption of caffeinated drinks allowed for at least a day prior, and a full eight-hour night sleep was had the night before the measurement;
- ❖
- Ten-minutes rest in a supine position was taken prior to the measurement;
- ❖
- The appropriate cuff size had to be chosen for each patient according to the measurement of their upper arm: extra small: 14–20 cm, small: 20–24 cm, medium: 24–32 cm, and large: 32–38 cm;
- ❖
- The measurement was made in the supine position and the subjects needed to be still, silent, and relaxed;
- ❖
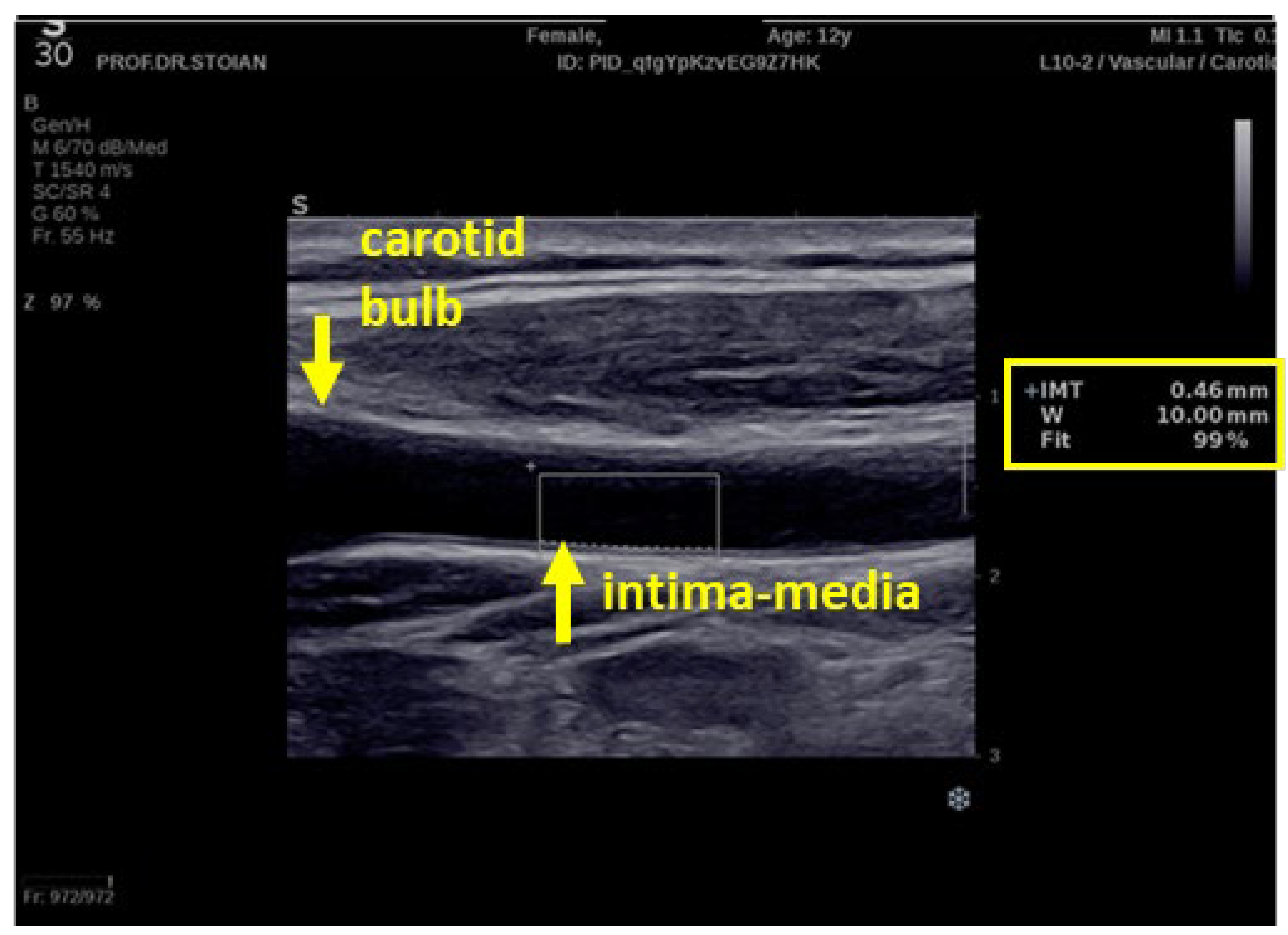
2.4. The Carotid Intima-Media Thickness Measurement
- ❖
- The ultrasonography technique
2.5. Blood Tests
- ❖
- HOMA-IR = [Glucose (mg/dl) × Insulin (µU/mL)]/405 [60].
- ❖
- For pre-pubertal children (Tanner stage 1) the HOMA-IR cut-off was considered 2.3, and for pubertal children (Tanner stages 2, 3, and 4), 3.4, as defined in a large study on Italian Caucasian obese children aged 8–15 [61];
- ❖
- For post-pubertal children (Tanner stage 5), the HOMA-IR cut-off remains 3.4, as a study on normal-weight and obese children aged 2–17.8 years old placed the cut-off value for obese children at 3.42, at the 75% percentile, for individuals that may develop cardiovascular risk factors very early in life [62].
2.6. Statistical Analysis
3. Results
3.1. The Vascular Biomarkers in Relation to the Weight Excess
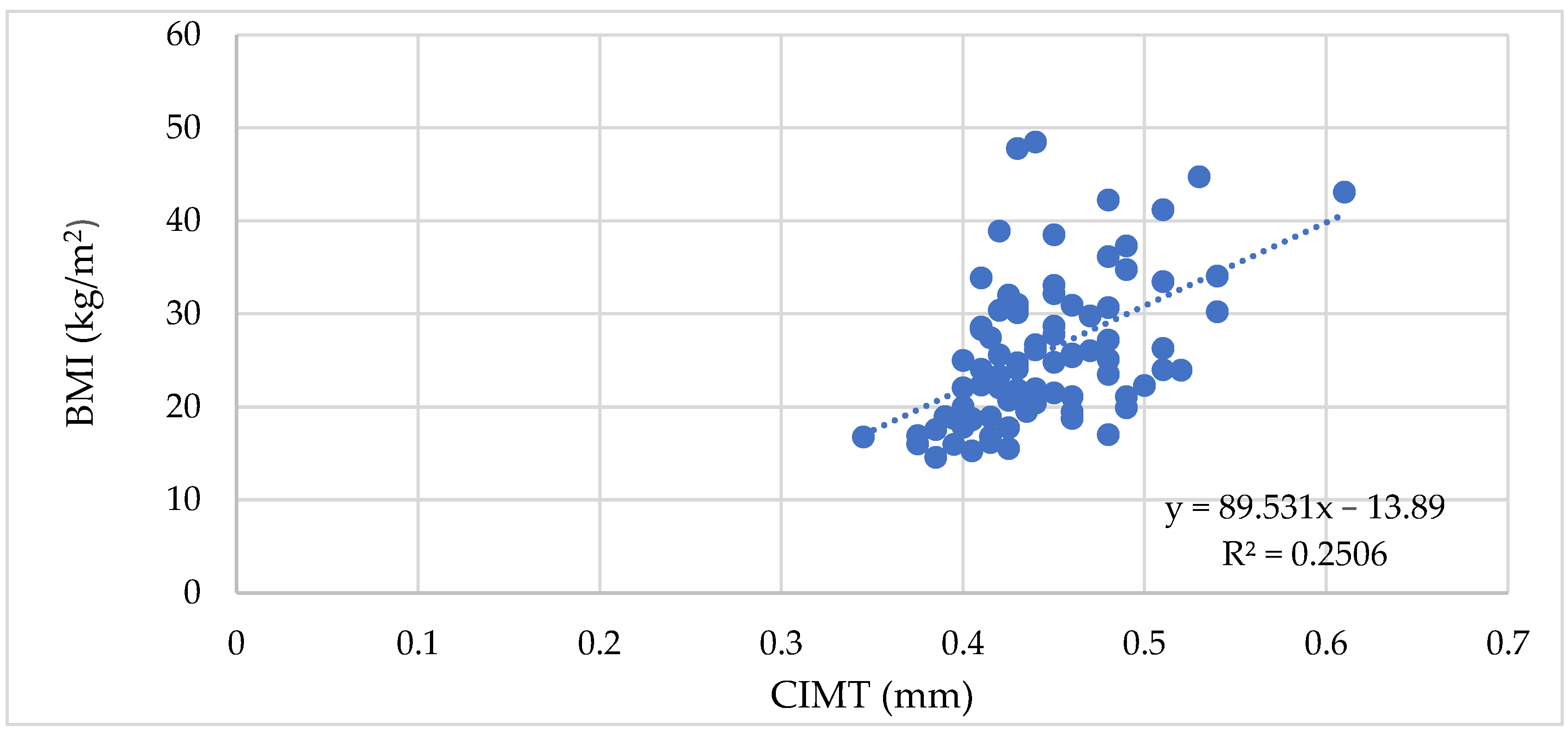
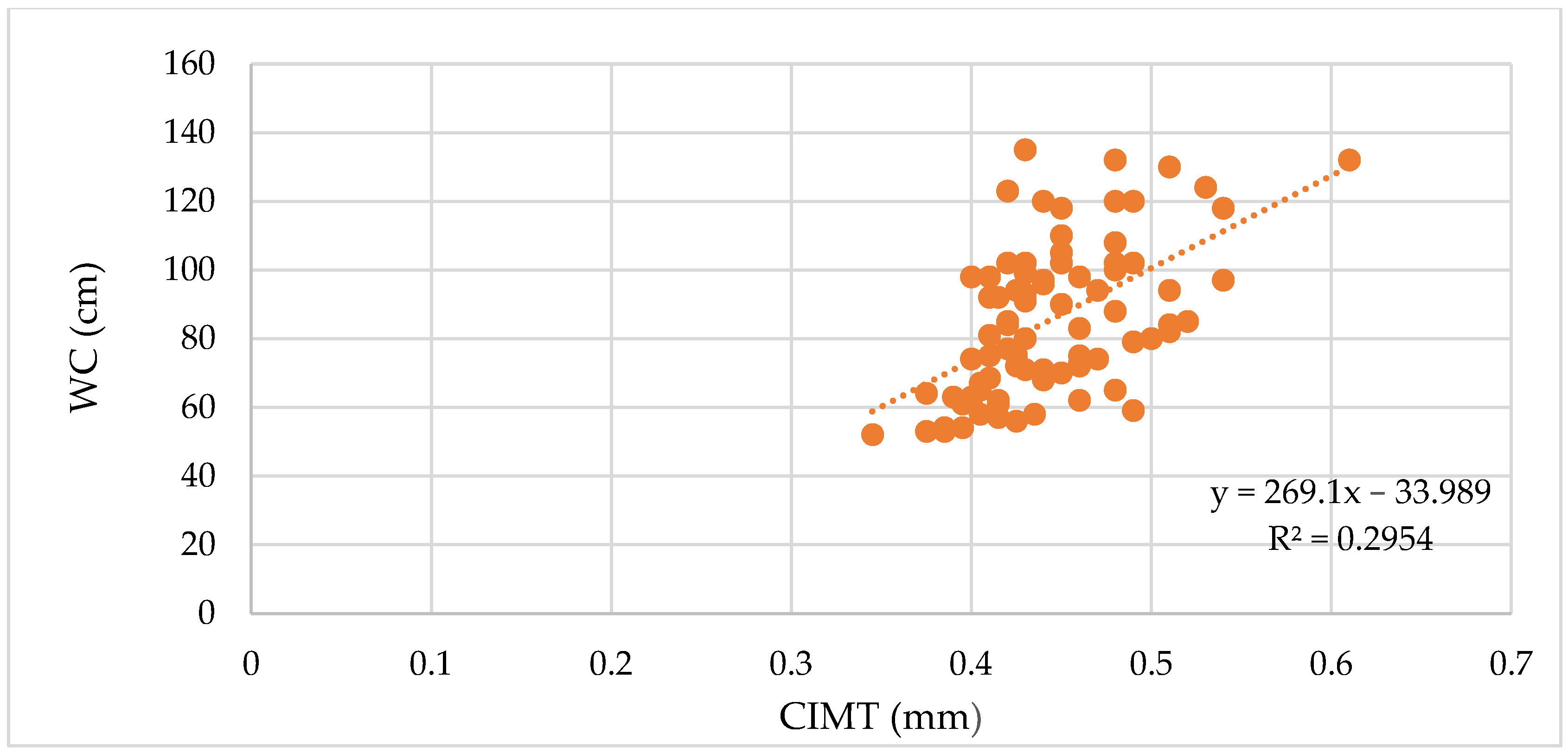
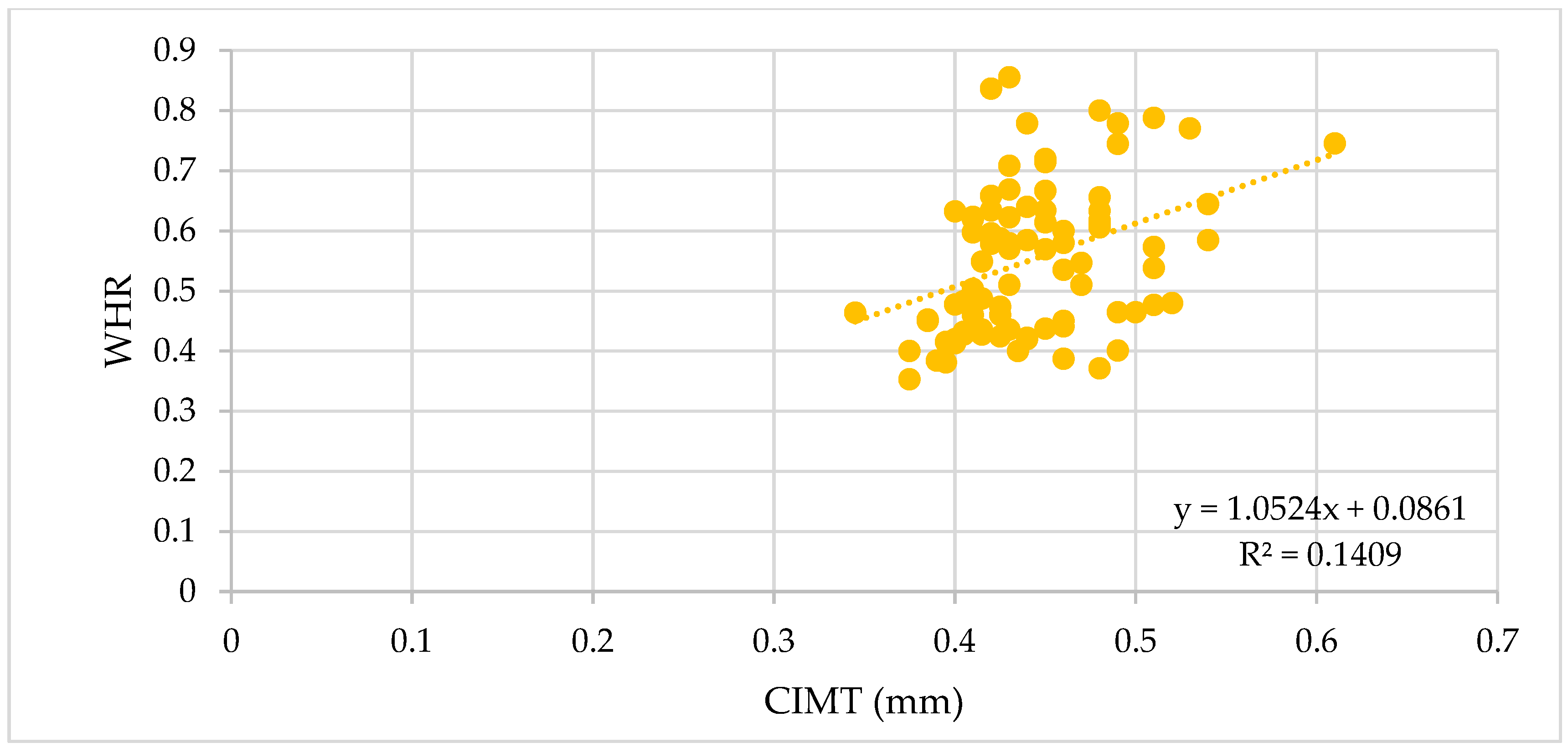
3.1.1. The Vascular Biomarkers and the BMI, WC, and WHR
3.1.2. The Vascular Biomarkers and the Body Composition Analysis
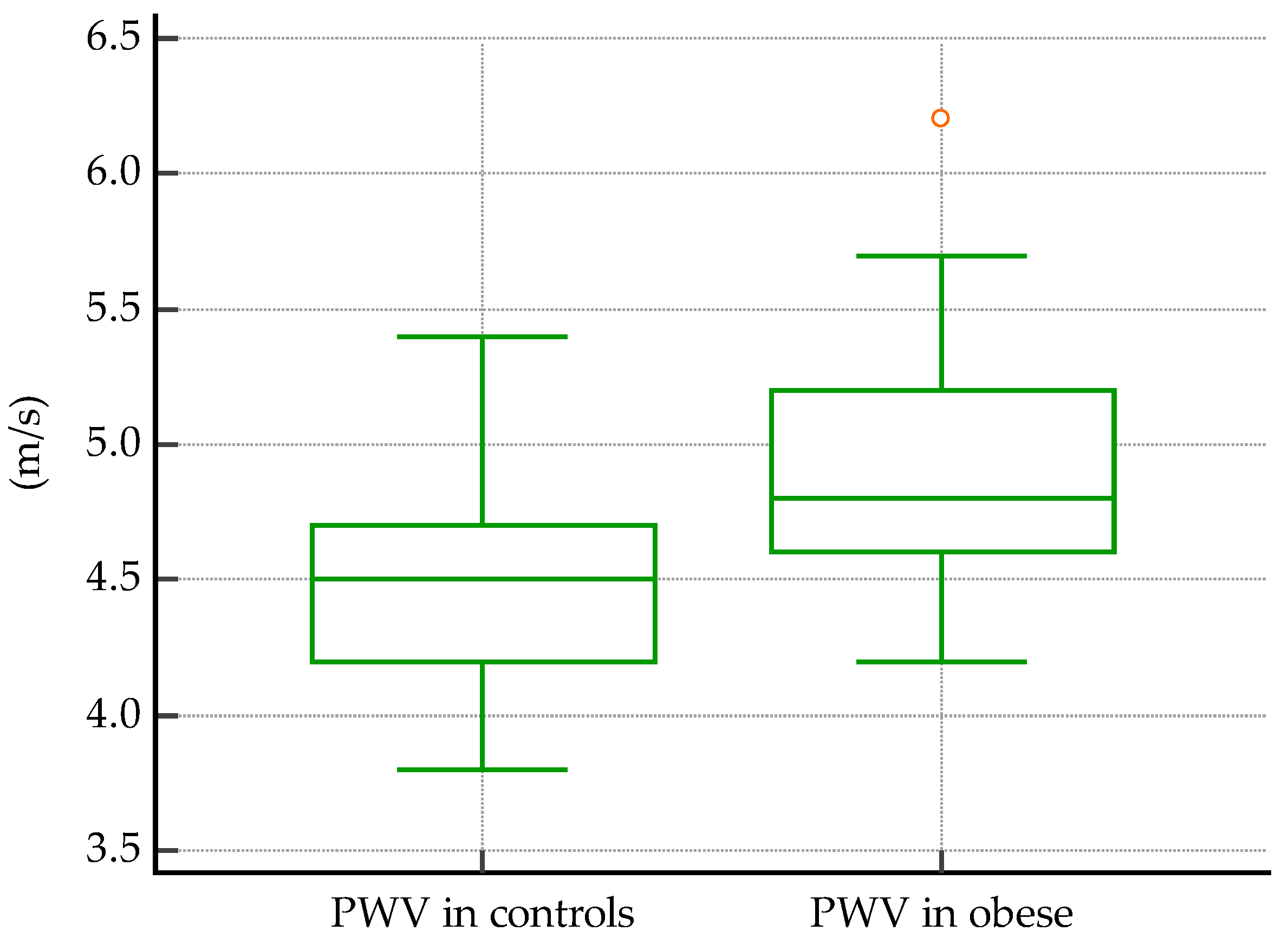
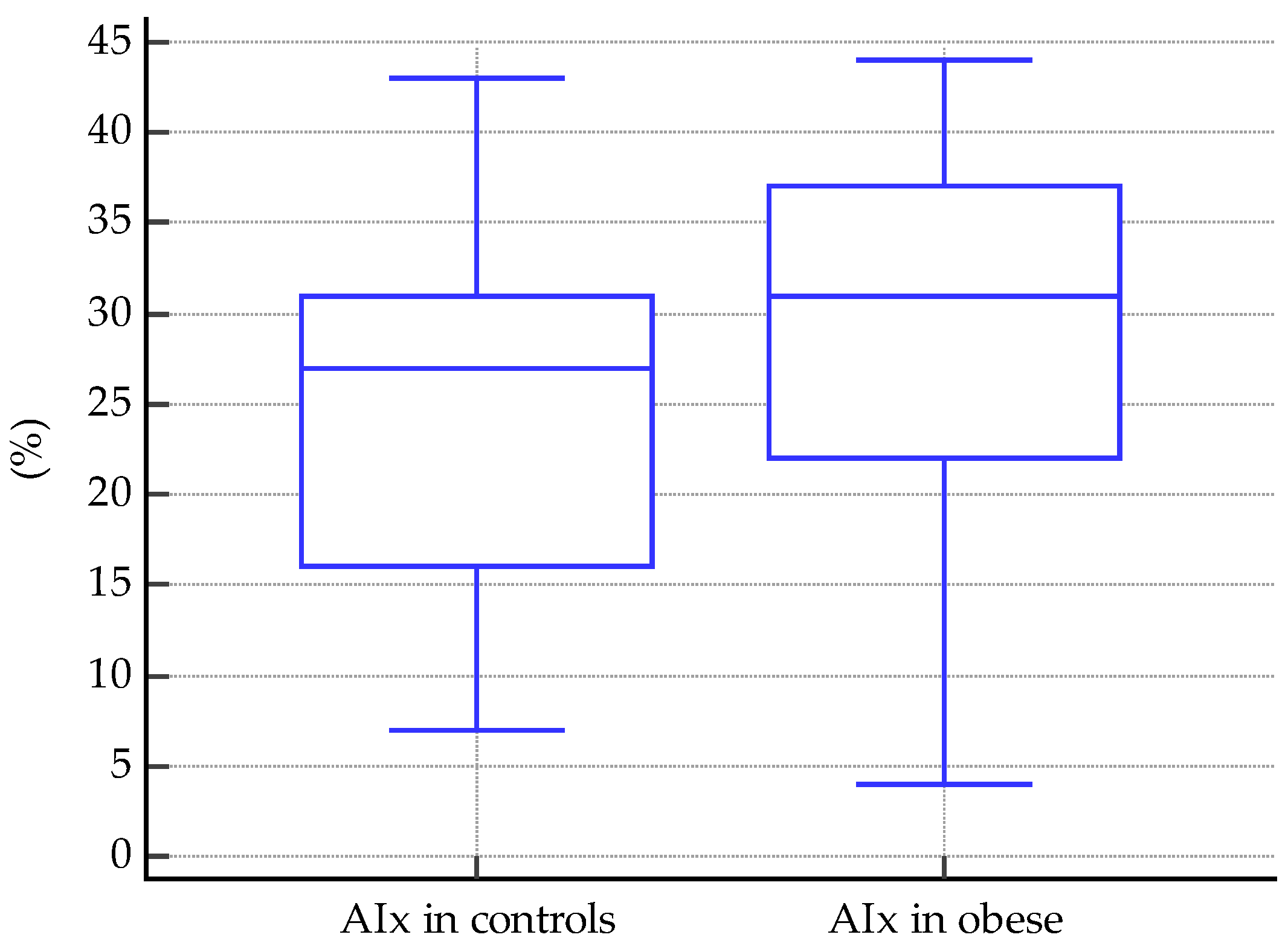
3.1.3. Comparisons of Vascular Markers between Obese and Normal-Weight Subjects
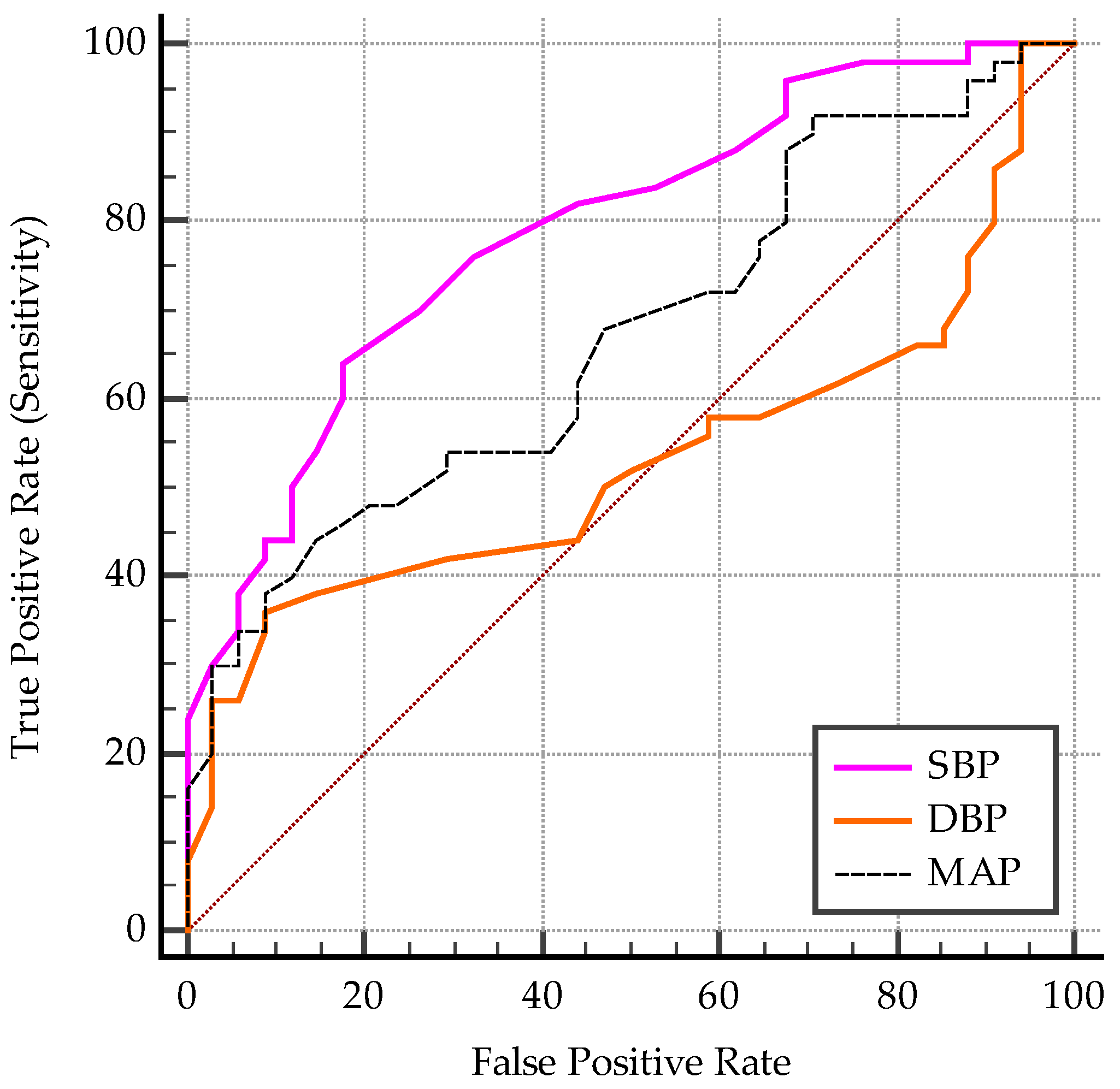
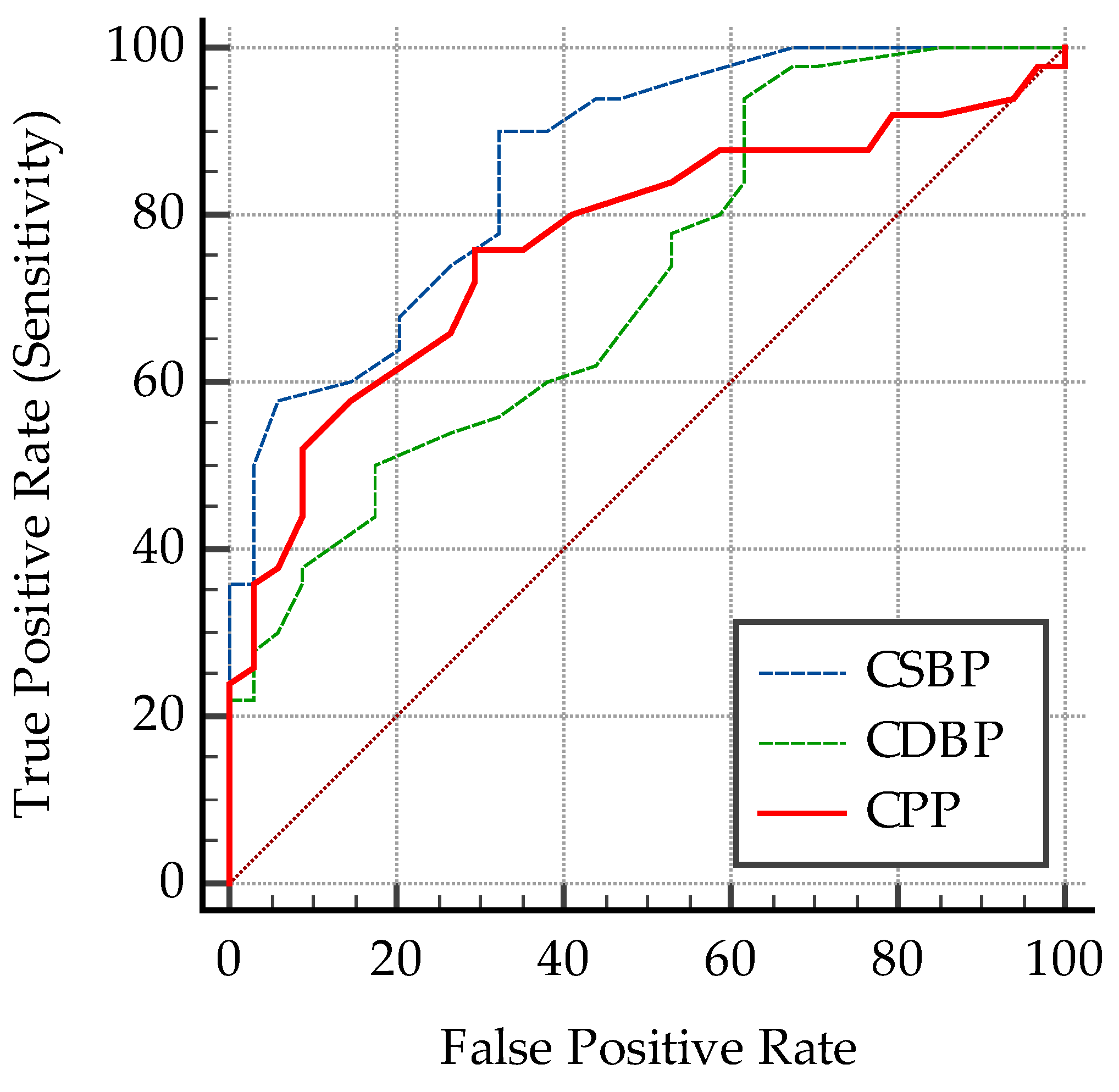
3.1.4. Predictive Cut-Off Values for the Vascular Biomarkers
- ❖
- Predictive cut-off values for the vascular biomarkers in all subjects
- ❖
- Predictive cut-off values for the vascular biomarkers according to age groups
3.2. The Vascular Biomarkers and the Glucose Metabolism
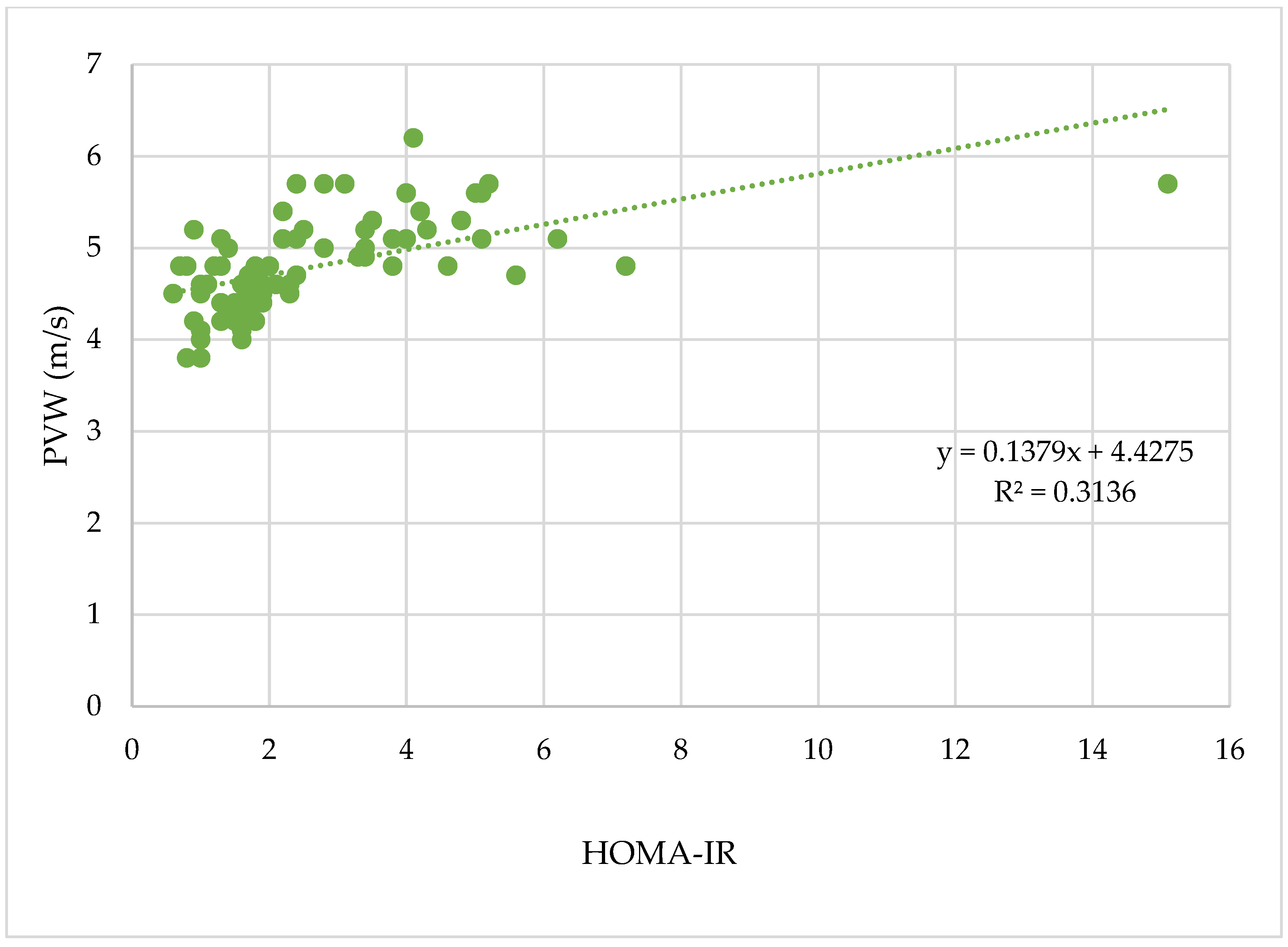
3.2.1. HOMA-IR–A Biomarker of Insulin Resistance
3.2.2. Acanthosis Nigricans—A Clinical Sign of Insulin Resistance
3.3. The Vascular Biomarkers in Relation to the Menstrual Cycle Regularity
3.4. The Vascular Biomarkers in Relation to the Blood Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIx | Index of augmentation |
| AUC | Area under the receiver operating characteristic curve |
| BMI | Body mass index |
| BP | Blood pressure |
| cDBP | Central diastolic blood pressure |
| CIMT | Carotid intima-media thickness |
| cPP | Central pulse pressure |
| cSBP | Central systolic blood pressure |
| DBP | Diastolic blood pressure |
| GOT | Aspartate aminotransferase |
| GPT | Alanine aminotransferase |
| H | Height |
| HDL-c | High-density lipoprotein cholesterol |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| HR | Heart rate |
| LDL-c | Low-density lipoprotein cholesterol |
| MAP | Mean arterial pressure |
| n | Number of subjects |
| PCOS | Polycystic ovary syndrome |
| PWV | Pulse wave velocity |
| ROC | Receiver operating characteristic |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| Se | Sensitivity |
| Sp | Specificity |
| Std. | Standard |
| TC | Total cholesterol |
| TG | Triglycerides |
| vs. | Versus |
| W | Weight |
| WC | Waist circumference |
| WHR | Waist-to-height ratio |
| y | Years |
Appendix A

References
- WHO European Regional Obesity Report 2022. Copenhagen: WHO Regional Office for Europe; 2022. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://apps.who.int/iris/bitstream/handle/10665/353747/9789289057738-eng.pdf (accessed on 6 March 2023).
- Report on the Fifth Round of Data Collection, 2018–2020: WHO European Childhood Obesity Surveillance Initiative (COSI). Copenhagen: WHO Regional Office for Europe; 2022. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://apps.who.int/iris/rest/bitstreams/1476879/retrieve (accessed on 6 March 2023).
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.-C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. Natl. Health Stat. Rep. 2021. NHSR No. 158. Available online: https://stacks.cdc.gov/view/cdc/106273 (accessed on 6 March 2023).
- Lange, S.J.; Kompaniyets, L.; Freedman, D.S.; Kraus, E.M.; Porter, R.; Blanck, H.M. Longitudinal Trends in Body Mass Index Before and During the COVID-19 Pandemic Among Persons Aged 2–19 Years—United States, 2018–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Woolford, S.J.; Sidell, M.; Li, X.; Else, V.; Young, D.; Resnicow, K.; Koebnick, C. Changes in Body Mass Index Among Children and Adolescents During the COVID-19 Pandemic. JAMA 2021, 326, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, P.; Krishna, S. Obesity Effects on Child Health. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570613/ (accessed on 6 March 2023).
- Zieske, A.W.; Malcom, G.T.; Strong, J.P. Natural history and risk factors of atherosclerosis in children and youth: The PDAY study. Pediatr. Pathol. Mol. Med. 2002, 21, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.M.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation 2015, 132, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Mihuta, M.-S.; Paul, C.; Ciulpan, A.; Dacca, F.; Velea, I.P.; Mozos, I.; Stoian, D. Subclinical Atherosclerosis Progression in Obese Children with Relevant Cardiometabolic Risk Factors Can Be Assessed through Carotid Intima Media Thickness. Appl. Sci. 2021, 11, 10721. [Google Scholar] [CrossRef]
- Mihuta, M.S.; Stoian, D.; Borlea, A.; Roi, C.M.; Velea-Barta, O.-A.; Mozos, I.; Paul, C. Evaluating the Arterial Stiffness as a Useful Tool in the Management of Obese Children. Children 2023, 10, 183. [Google Scholar] [CrossRef]
- Nikiforov, N.G.; Zlenko, D.V.; Orekhova, V.A.; Melnichenko, A.A.; Orekhov, A.N. Local Accumulation of Lymphocytes in the Intima of Human Aorta Is Associated with Giant Multinucleated Endothelial Cells: Possible Explanation for Mosaicism of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1059. [Google Scholar] [CrossRef]
- Yasin, J.; Sharma, C.; Hashim, M.J.; Al Hamed, S.; AlKaabi, J.; Aburawi, E.H. Cross-Sectional Association Between Body Fat Composition and Biomarkers of Inflammation and Endothelial Dysfunction in Children with Overweight/Obesity. Diabetes. Metab. Syndr. Obes. 2023, 16, 483–493. [Google Scholar] [CrossRef]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef]
- Tounian, P.; Aggoun, Y.; Dubern, B.; Varille, V.; Guy-Grand, B.; Sidi, D.; Girardet, J.P.; Bonnet, D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: A prospective study. Lancet 2001, 358, 1400–1404. [Google Scholar] [CrossRef]
- Bots, M.L.; Hofman, A.; De Jong, P.T.; Grobbee, D.E. Common carotid intima-media thickness as an indicator of atherosclerosis at other sites of the carotid artery. The Rotterdam Study. Ann. Epidemiol. 1996, 6, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Beauloye, V.; Zech, F.; Tran, H.T.; Clapuyt, P.; Maes, M.; Brichard, S.M. Determinants of early atherosclerosis in obese children and adolescents. J. Clin. Endocrinol. Metab. 2007, 92, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Farello, G.; Antenucci, A.; Stagi, S.; Mazzocchetti, C.; Ciocca, F.; Verrotti, A. Metabolically healthy and metabolically unhealthyobese children both have increased carotid intima-media thickness: A case control study. BMC Cardiovasc. Disord. 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic reviewand meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef]
- Reusz, G.S.; Cseprekal, O.; Temmar, M.; Kis, E.; Cherif, A.B.; Thaleb, A.; Fekete, A.; Szabó, A.J.; Benetos, A.; Salvi, P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension 2010, 56, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P., 3rd; Tracy, R.E.; Wattigney, W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef]
- Sakuragi, S.; Abhayaratna, K.; Gravenmaker, K.J.; O’Reilly, C.; Srikusalanukul, W.; Budge, M.M.; Telford, R.D.; Abhayaratna, W.P. Influence of adiposity and physical activity on arterial stiffness in healthy children: The lifestyle of our kids study. Hypertens 2009, 53, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Kulsum-Mecci, N.; Goss, C.; Kozel, B.A.; Garbutt, J.M.; Schechtman, K.B.; Dharnidharka, V.R. Effects of Obesity and Hypertension on Pulse Wave Velocity in Children. J. Clin. Hypertens. 2017, 19, 221–226. [Google Scholar] [CrossRef]
- Herouvi, D.; Karanasios, E.; Karayianni, C.; Karavanaki, K. Cardiovascular disease in childhood: The role of obesity. Eur. J. Pediatr. 2013, 172, 721–732. [Google Scholar] [CrossRef]
- Wilenius, M.; Tikkakoski, A.J.; Tahvanainen, A.M.; Haring, A.; Koskela, J.; Huhtala, H.; Kähönen, M.; Kööbi, T.; Mustonen, J.T.; Pörsti, I.H. Central wave reflection is associated with peripheral arterial resistance in addition to arterial stiffness in subjects without antihypertensive medication. BMC Cardiovasc. Disord. 2016, 16, 131. [Google Scholar] [CrossRef]
- Močnik, M.; Nikolić, S.; Varda, N.M. Arterial Compliance Measurement in Overweight and Hypertensive Children. Indian J. Pediatr. 2016, 83, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Kario, K. Review: Role of the augmentation index in hypertension. TACA 2008, 2, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Park, C.; Davies, J.; Francis, D.; Thom, S.A.M.G.; Mayet, J.; Parker, K.H. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS ONE 2013, 8, e59371. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Ding, S. Obesity-associated sympathetic overactivity in children and adolescents: The role of catecholamine resistance in lipid metabolism. J. Pediatr. Endocrinol. Metab. 2016, 29, 113–125. [Google Scholar] [CrossRef]
- Mihuta, M.S.; Paul, C.; Borlea, A.; Cepeha, C.M.; Velea, I.P.; Mozos, I.; Stoian, D. The Oscillometric Pulse Wave Analysis Is Useful in Evaluating the Arterial Stiffness of Obese Children with Relevant Cardiometabolic Risks. J. Clin. Med. 2022, 11, 5078. [Google Scholar] [CrossRef]
- Reilly, J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef]
- Neuhauser, H.K.; Büschges, J.; Schaffrath Rosario, A.; Anja Schienkiewitz, A.; Sarganas, G.; Königstein, K.; Schweizer, D.; Schmidt-Trucksäss, A. Carotid Intima-Media Thickness Percentiles in Adolescence and Young Adulthood and Their Association with Obesity and Hypertensive Blood Pressure in a Population Cohort. Hypertens 2022, 79, 1167–1176. [Google Scholar] [CrossRef]
- Urbina, E.M.; Khoury, P.R.; McCoy, C.; Daniels, S.R.; Kimball, T.R.; Dolan, L.M. Cardiac and vascular consequences of prehypertension in youth. J. Clin. Hypertens. 2011, 13, 332–342. [Google Scholar] [CrossRef]
- Urbina, E.M.; Kimball, T.R.; Khoury, P.R.; Daniels, S.R.; Dolan, L.M. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J. Hypertens. 2010, 28, 1692–1698. [Google Scholar] [CrossRef]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central blood pressure: Current evidence and clinical importance. Eur. Heart J. 2014, 35, 1719–1725. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kizer, J.R.; Lee, E.T.; Galloway, J.M.; Ali, T.; Umans, J.G.; Howard, B.V. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: The strong heart study. Hypertens 2007, 50, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Pichler, G.; Martinez, F.; Vicente, A.; Solaz, E.; Calaforra, O.; Lurbe, E.; Redon, J. Influence of obesity in central blood pressure. J. Hypertens. 2015, 33, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; He, J.; Cutler, J.A.; Wildman, R.P.; Whelton, P.K. Trends in blood pressure among children and adolescents. JAMA 2004, 291, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.A.; Ness, A.R.; Davey Smith, G.; Leary, S.D. Association between body composition and blood pressure in a contemporary cohort of 9-year-old children. J. Hum. Hypertens. 2007, 21, 283–290. [Google Scholar] [CrossRef]
- Bittencourt, J.C.; Scheinbein, G.H.A.; de Oliveira Junior, W.C.; Bassi, R.L.; Moura, L.B.; Correa, A.L.D.; de Lima Bernardes, R.G.; Freitas, L.S.; Lemos, J.C.; Gonçalves, G.K.N.; et al. Arterial stiffness indices, pulse wave velocity and central systolic blood pressure, are able to discriminate between obese and non-obese children. Eur. J. Pediatr. 2023, 21, 1–13. [Google Scholar] [CrossRef]
- Wójtowicz, J.; Łempicka, A.; Łuczyński, W.; Szczepański, W.; Zomerfeld, A.; Semeran, K.; Bossowski, A. Central aortic pressure, arterial stiffness and echocardiographic parameters of children with overweight/obesity and arterial hypertension. Adv. Clin. Exp. Med. Off Organ. Wroclaw. Med. Univ. 2017, 26, 1399–1404. [Google Scholar] [CrossRef]
- Niiranen, T.J.; Kalesan, B.; Mitchell, G.F.; Ramachandran, S.V. Relative Contributions of Pulse Pressure and Arterial Stiffness to Cardiovascular Disease—The Framingham Heart Study. Hypertension 2019, 73, 712–717. [Google Scholar] [CrossRef]
- Hale, P.M.; Cushman, T.T.; Kimball, E.S.; Nair, A.; Shaffer, R.G. Secondary Causes of Obesity in Childhood. In Management of Pediatric Obesity and Diabetes. Nutrition and Health; Ferry, R., Jr., Ed.; Humana Press: Totowa, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Velea, I.P.; Albulescu, R.; Arghirescu, S.T. Obezitatea la copil. In Pediatrie-Curs pentru Studentii Facultătii de Medicină: Obezitatea la Copil; Velea, I., Ed.; Editura Victor Babes: Timisoara, Romania, 2016; pp. 289–297. [Google Scholar]
- Cheung, Y.F. Arterial stiffness in the young: Assessment, determinants, and implications. Korean Circ. J. 2010, 40, 153–162. [Google Scholar] [CrossRef]
- NHLBI Obesity Education Initiative Expert Panel on the Identification Evaluation and Treatment Obesity in Adults. In Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults; National Heart, Lung and Blood Institute, Ed.; NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US): Bethesda, MD, USA, 1998. [Google Scholar]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Edwards, K.A.; Eneli, I.; Hamre, R.; Joseph, M.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef]
- Xi, B.; Zong, X.; Kelishadi, R.; Litwin, M.; Hong, Y.M.; Poh, B.K.; Steffen, L.M.; Galcheva, S.V.; Herter-Aeberli, I.; Nawarycz, T.; et al. International Waist Circumference Percentile Cutoffs for Central Obesity in Children and Adolescents Aged 6 to 18 Years. J. Clin. Endocrinol. Metab. 2020, 1, e1569–e1583. [Google Scholar] [CrossRef]
- Santoro, N.; Amato, A.; Grandone, A.; Brienza, C.; Savarese, P.; Tartaglione, N.; Marzuillo, P.; Perrone, L.; Miraglia Del Giudice, E. Predicting metabolic syndrome in obese children and adolescents: Look, measure and ask. Obes. Facts 2013, 6, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, M.; Bokor, B.R. Tanner Stages. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470280/ (accessed on 5 June 2022).
- Itriyeva, K. The effects of obesity on the menstrual cycle. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101241. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.M.; Carsote, M.; Dumitrascu, M.C.; Sandru, F. Acanthosis Nigricans: Pointer of Endocrine Entities. Diagnostics 2022, 12, 2519. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.; Gohlisch, C.; Harsch-Gladisch, C.; Tölle, M.; Zidek, W.; van der Giet, M. Oscillometric estimation of central bloodpressure: Validation of the Mobil-O-Graph in comparison with the SphygmoCor device. Blood Press. Monit. 2012, 17, 128–131. [Google Scholar] [CrossRef]
- Mynard, J.P.; Goldsmith, G.; Springall, G.; Eastaugh, L.; Lane, G.K.; Zannino, D.; Smolich, J.J.; Avolio, A.; Cheung, M.M.H. Central aortic blood pressure estimation in children and adolescents: Results of the KidCoreBP study. J. Hypertens. 2020, 38, 821–828. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, S.H. Blood pressure measurements and hypertension in infants, children, and adolescents: From the postmercury to mobile devices. Clin. Exp. Pediatr. 2022, 65, 73–80. [Google Scholar] [CrossRef]
- Mozos, I.; Gug, C.; Mozos, C.; Stoian, D.; Pricop, M.; Jianu, D. Associations between Intrinsic Heart Rate, P Wave and QT Interval Durations and Pulse Wave Analysis in Patients with Hypertension and High Normal Blood Pressure. Int. J. Environ. Res. Public Health 2020, 17, 4350. [Google Scholar] [CrossRef]
- Gómez-Marcos, M.A.; Recio-Rodríguez, J.I.; Patino-Alonso, M.C.; Agudo-Conde, C.; Gómez-Sanchez, L.; Gómez-Sanchez, M.; Rodríguez-Sánchez, E.; García-Ortiz, L. Protocol for measuring carotid intima-media thickness that best correlates with cardio-vascular risk and target organ damage. Am. J. Hypertens. 2012, 25, 955–961. [Google Scholar] [CrossRef]
- El Jalbout, R. Intima-Media Thickness Measurement in Children; Techniques and Reference Values. Am. J. Biomed. Sci. Res. 2020, 7, 101–103. [Google Scholar] [CrossRef]
- Polak, J.F.; Meisner, A.; Pencina, M.J.; Wolf, P.A.; D’Agostino, R.B. Variations in common carotid artery intima-media thickness during the cardiac cycle: Implications for cardiovascular risk assessment. J. Am. Soc. Echocardiogr. 2012, 25, 1023–1028. [Google Scholar] [CrossRef]
- Vermeersch, S.; Rietzschel, E.; De Buyzere, M.; Van Bortel, L.M.; D’Asseler, Y.; Gillebert, T.C.; Verdonck, P.R.; Segers, P. Validation of a new automated IMT measurement algorithm. J. Hum. Hypertens. 2007, 21, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Vizzuso, S.; Del Torto, A.; Dilillo, D.; Calcaterra, V.; Di Profio, E.; Leone, A.; Gilardini, L.; Bertoli, S.; Battezzati, A.; Zuccotti, G.V.; et al. Visceral Adiposity Index (VAI) in Children and Adolescents with Obesity: No Association with Daily Energy Intake but Promising Tool to Identify Metabolic Syndrome (MetS). Nutrients 2021, 13, 413. [Google Scholar] [CrossRef]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta. Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Ultrasound assessment of flow-mediated dilation. Hypertens 2010, 55, 1075–1085. [Google Scholar] [CrossRef]
- Khanna, D.; Peltzer, C.; Kahar, P.; Parmar, M.S. Body Mass Index (BMI): A Screening Tool Analysis. Cureus 2022, 14, e22119. [Google Scholar] [CrossRef]
- Savant, J.D.; Furth, S.L.; Meyers, K.E.C. Arterial stiffness in children: Pediatric measurement and considerations. Pulse 2015, 2, 69–80. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Lentferink, Y.E.; Kromwijk, L.A.J.; van der Aa, M.P.; Knibbe, C.A.J.; van der Vorst, M.M.J. Increased Arterial Stiffness in Adolescents with Obesity. Glob. Pediatr. Health 2019, 6, 2333794X19831297. [Google Scholar] [CrossRef]
- Jakab, A.E.; Hidvégi, E.V.; Illyés, M.; Cziráki, A.; Kalmár, T.; Maróti1, Z.; Bereczki, C. Childhood Obesity: Does it Have Any Effect on Young Arteries? Front. Pediatr. 2020, 8, 398. [Google Scholar] [CrossRef]
- Dangardt, F.; Chen, Y.; Berggren, K.; Osika, W.; Friberg, P. Increased rate of arterial stiffening with obesity in adolescents: A five-year follow-up study. PLoS ONE 2013, 8, e57454. [Google Scholar] [CrossRef]
- Zhao, M.; López-Bermejo, A.; Caserta, C.A.; Medeiros, C.C.M.; Kollias, A.; Bassols, J.; Romeo, E.; Ramos, T.D.A.; Stergiou, G.; Yang, L.; et al. International Childhood Vascular Structure Evaluation Consortium. Metabolically Healthy Obesity and High Carotid Intima-Media Thickness in Children and Adolescents: International Childhood Vascular Structure Evaluation Consortium. Diabetes Care. 2019, 42, 119–125. [Google Scholar] [CrossRef]
- Brady, T.M. Obesity-Related Hypertension in Children. Front. Pediatr. 2017, 25, 197. [Google Scholar] [CrossRef]
- Kalil, G.Z.; Haynes, W.G. Sympathetic nervous system in obesity-related hypertension: Mechanisms and clinical implications. Hypertens. Res. 2012, 35, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Vecchiola, A.; Lagos, C.F.; Carvajal, C.A.; Baudrand, R.; Fardella, C.E. Aldosterone production and signaling dysregulation in obesity. Curr. Hypertens. Rep. 2016, 18, 20. [Google Scholar] [CrossRef]
- Parker, E.D.; Sinaiko, A.R.; Kharbanda, E.O.; Margolis, K.L.; Daley, M.F.; Trower, N.K.; Sherwood, N.; Greenspan, L.; Lo, J.; Magid, D.; et al. Change in weight status and development of hypertension. Pediatrics 2016, 137, e20151662. [Google Scholar] [CrossRef]
- Reinehr, T.; de Sousa, G.; Toschke, A.M.; Andler, W. Long-term follow-up of cardiovascular disease risk factors in children after an obesity intervention. Am. J. Clin. Nutr. 2006, 84, 490–496. [Google Scholar] [CrossRef]
- Rocchini, A.P.; Katch, V.; Anderson, J.; Hinderliter, J.; Becque, D.; Martin, M.; Marks, C. Blood pressure in obese adolescents: Effect of weight loss. Pediatrics 1988, 82, 16–23. [Google Scholar] [PubMed]
- Henning, R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: A review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc. Dis. 2021, 11, 50–529. [Google Scholar]
- Harbin, M.M.; Hultgren, N.E.; Kelly, A.S.; Dengel, D.R.; Evanoff, N.G.; Ryder, J.R. Measurement of central aortic blood pressure in youth: Role of obesity and sex. Am. J. Hypertens. 2018, 31, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.Y.; Qasem, A.; Ayer, J.G.; Butlin, M.; O’Meagher, S.; Melki, C.; Marks, G.B.; Avolio, A.; Celermajer, D.S.; Skilton, M.R. Central blood pressure in children and adolescents: Non-invasive development and testing of novel transfer functions. J. Hum. Hypertens. 2017, 31, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.M. Obesity and Central Blood Pressure in Children and Adolescents. Am. J. Hypertens 2018, 31, 1266–1267. [Google Scholar] [CrossRef] [PubMed]
- Köchli, S.; Deiseroth, A.; Hauser, C.; Streese, L.; Schmidt-Trucksäs, A.; Faude, O.; Hanssen, H. Body Composition and Physical Fitness Affect Central Hemodynamics in Young Children. Front. Pediatr. 2021, 9, 750398. [Google Scholar] [CrossRef] [PubMed]
- Bassali, R.; Waller, J.L.; Gower, B.; Allison, J.; Davis, C.L. Utility of waist circumference percentile for risk evaluation in obese children. Int. J. Pediatric Obes. 2010, 5, 97–101. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Yoo, E.G. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J. Pediatr. 2016, 59, 425–431. [Google Scholar] [CrossRef]
- Brambilla, P.; Bedogni, G.; Heo, M.; Pietrobelli, A. Waist circumference-to-height ratio predicts adiposity better than body mass index in children and adolescents. Int. J. Obes. 2013, 37, 943–946. [Google Scholar] [CrossRef]
- Khoury, M.; Manlhiot, C.; McCrindle, B.W. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol. 2013, 62, 742–751. [Google Scholar] [CrossRef]
- Baroncini, L.A.; de Sylvestre, L.C.; Pecoits Filho, R. Assessment of Intima-Media Thickness in Healthy Children Aged 1 to 15 Years. Arq. Bras. Cardiol. 2016, 106, 327–332. [Google Scholar] [CrossRef]
- Sinning, C.; Wild, P.S.; Echevarria, F.M.; Wilde, S.; Schnabel, R.; Lubos, E.; Herkenhoff, S.; Bickel, C.; Klimpe, S.; Gori, T.; et al. Gutenberg-Heart Study. Sex dif-ferences in early carotid atherosclerosis (from the community-based Gutenberg Heart Study). Am. J. Cardiol. 2011, 107, 1841–1847. [Google Scholar] [CrossRef]
- Ahimastos, A.A.; Formosa, M.; Dart, A.M.; Kingwell, B.A. Gender differences in large artery stiffness pre- and post-puberty. J. Clin. Endocrinol. Metab. 2003, 88, 5375–5380. [Google Scholar] [CrossRef] [PubMed]
- Du pond, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
- Robb, A.O.; Mills, N.L.; Din, J.N.; Smith, I.B.; Paterson, F.; Newby, D.E.; Denison, F.C. Influence of the menstrual cycle, pregnancy, and preeclampsia on arterial stiffness. Hypertension 2009, 53, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Stamatelopoulos, K.S.; Georgiopoulos, G.; Papaioannou, T.; Lambrinoudaki, I.; Kouzoupis, A.; Vlachopoulos, C.; Sfikakis, P.P. Can premenstrual syndrome affect arterial stiffness or blood pressure? Atherosclerosis 2012, 224, 170–176. [Google Scholar] [CrossRef]
- Solorzano, C.M.B.; Helm, K.D.; Patrie, J.T.; Shayya, R.F.; Cook-Andersen, H.L.; Chang, R.J.; McCartney, C.R.; Marshall, J.C. In-creased adrenal androgens in overweight peripubertal girls. J. Endocr. Soc. 2017, 1, 538–552. [Google Scholar] [CrossRef]
- Dockery, F.; Bulpitt, C.J.; Donaldson, M.; Fernandez, S.; Rajkumar, C. The relationship between androgens and arterial stiffness in older men. JAGS 2003, 51, 1627–1632. [Google Scholar] [CrossRef]
- Webb, C.M.; Elkington, A.G.; Kraidly, M.M.; Keenan, N.; Pennell, D.J.; Collins, P. Effects of oral testosterone treatment on myocardial perfusion and vascular function in men with low plasma testosterone and coronary heart disease. Am. J. Card. 2008, 101, 618–624. [Google Scholar] [CrossRef]
- Wilson, D.P. Is Atherosclerosis a Pediatric Disease? In Endotext. South Dartmouth (MA); Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK395576/ (accessed on 12 February 2023).
- Qiu, H.; Zhu, Y.; Sun, Z.; Trzeciakowski, J.P.; Gansner, M.; Depre, C.; Vatner, S.F. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 2010, 107, 615–619. [Google Scholar] [CrossRef]
- van den Munckhof, I.C.L.; Jones, H.; Hopman, M.T.E.; de Graaf, J.; Nyakayiru, J.; van Dijk, B.; Eijsvogels, T.M.H.; Thijssen, D.H.J. Relation between age and carotid artery intima-medial thickness: A systematic review. Clin. Cardiol. 2018, 41, 698–704. [Google Scholar] [CrossRef]
- Hidvégi, E.V.; Illyés, M.; Benczúr, B.; Böcskei, R.M.; Rátgéber, L.; Lenkey, Z.; Molnár, F.T.; Cziráki, A. Reference values of aortic pulse wave velocity in a large healthy population aged between 3 and 18 years. J. Hypertens. 2012, 30, 2314–2321. [Google Scholar] [CrossRef]
- Jo, C.O.; Lande, M.B.; Meagher, C.C.; Wang, H.; Vermilion, R.P. A simple method of measuring thoracic aortic pulse wave velocity in children: Methods and normal values. J. Am. Soc. Echocardiogr. 2010, 23, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Voges, I.; Jerosch-Herold, M.; Hedderich, J.; Pardun, E.; Hart, C.; Gabbert, D.D.; Hansen, J.H.; Petko, C.; Kramer, H.H.; Rickers, C. Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: A cross-sectional study. J. Cardiovasc. Magn. Reson. 2012, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, M.M.; Zeitler, P.S. Insulin Resistance of Puberty. Curr. Diab. Rep. 2016, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Tagi, V.M.; Chiarelli, F. Obesity and insulin resistance in children. Curr. Opin. Pediatr. 2020, 32, 582–588. [Google Scholar] [CrossRef]
- Chiarelli, F.; Marcovecchio, M.L. Insulin resistance and obesity in childhood. Eur. J. Endocrinol. 2008, 159, S67–S74. [Google Scholar] [CrossRef]
- Kurtoğlu, S.; Hatipoğlu, N.; Mazıcıoğlu, M.; Kendirici, M.; Keskin, M.; Kondolot, M. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 100–106. [Google Scholar] [CrossRef]
- Sabin, M.A.; Hunt, L.P.; Ford, A.L.; Werther, G.A.; Crowne, E.C.; Shield, J.P.H. Elevated glucose concentrations during an oral glucose tolerance test are associated with the presence of metabolic syndrome in childhood obesity. Diab. Med. 2008, 25, 3. [Google Scholar] [CrossRef]
- Roman, R.; Zeitler, P.S. Oral Glucose Tolerance Testing in Asymptomatic Obese Children: More Questions than Answers. J. Clin. Endocrinol. MetabVolume. 2008, 93, 4228–4230. [Google Scholar] [CrossRef] [PubMed]
- Kluczynik, C.E.; Mariz, L.S.; Souza, L.C.; Solano, G.B.; Albuquerque, F.C.; Medeiros, C.C. Acanthosis nigricans and insulin resistance in overweight children and adolescents. An. Bras. Dermatol. 2012, 87, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Chueh, H.W.; Cho, G.R.; Yoo, J.H. Clinical significance of acanthosis nigricans in children and adolescents with obesity induced metabolic complications. Korean J. Pediatr. 2007, 50, 987–994. [Google Scholar] [CrossRef]
- Slyper, A.H.; Kashmer, L.; Huang, W.M.; Re’em, Y. Acanthosis nigricans, vitamin D, and insulin resistance in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2014, 27, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Patidar, P.P.; Ramachandra, P.; Philip, R.; Saran, S.; Agarwal, P.; Gutch, M.; Gupta, K. Correlation of acanthosis nigricans with insulin resistance, anthropometric, and other metabolic parameters in diabetic Indians. Indian J. Endocrinol. Metab. 2012, 16, S436–S437. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Gutiérrez, E.; Tlacuilo-Parra, A.; Gutiérrez-Fajardo, P.; Sánchez-Tenorio, T.; Barba-Gómez, F.; Miranda-Díaz, A. A study of the association of acanthosis nigricans with subclinical atherosclerosis. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.; Kinra, S.; Wong, I.; Viner, R. Arterial stiffening, insulin resistance and acanthosis nigricans in a community sample of adolescents with obesity. Int. J. Obes. 2017, 41, 1454–1456. [Google Scholar] [CrossRef]
- Jabbour, R.; Ott, J.; Eppel, W.; Frigo, P. Carotid intima-media thickness in polycystic ovary syndrome and its association with hormone and lipid profiles. PLoS ONE 2020, 15, e0232299. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Truong, U.; King, M.; Ferland, A.; Moreau, K.L.; Dorosz, J.; Hokanson, J.E.; Wang, H.; Kinney, G.L.; Maahs, D.M.; et al. Obese adolescents with polycystic ovarian syndrome have elevated cardiovascular disease risk markers. Vasc. Med. 2017, 22, 85–95. [Google Scholar] [CrossRef]
- Cree-Green, M.; Truong, U.; Dorosz, J.L.; Moreau, K.; Coe, G.; Baumgartner, A.; Newnes, L.; Nadeau, K.J. Abstract 12078: Obese Girls With PCOS Have Evidence of Vascular Stiffening and Left Ventricular Hypertrophy Which Relate to Insulin Resistance. Circulation 2014, 130, A12078. [Google Scholar] [CrossRef]
- Ketel, I.J.; Stehouwer, C.D.; Henry, R.M.; Serné, E.H.; Hompes, P.; Homburg, R.; Smulders, Y.M.; Lambalk, C.B. Greater arterial stiffness in polycystic ovary syndrome (PCOS) is an obesity--but not a PCOS-associated phenomenon. J. Clin. Endocrinol. Metab. 2010, 95, 4566–4575. [Google Scholar] [CrossRef]
- Jørgensen, R.M.; Bøttger, B.; Vestergaard, E.T.; Kremke, B.; Bahnsen, R.F.; Nielsen, B.W.; Bruun, J.M. Uric Acid Is Elevated in Children with Obesity and Decreases After Weight Loss. Front. Pediatr. 2022, 9, 814166. [Google Scholar] [CrossRef]
- Tsouli, S.G.; Liberopoulos, E.N.; Mikhailidis, D.P.; Athyros, V.G.; Elisaf, M.S. Elevated serum uric acid levels in metabolic syndrome: An active component or an innocent bystander? Metabolism 2006, 55, 1293–1301. [Google Scholar] [CrossRef]
- Mortada, I. Hyperuricemia, type 2 diabetes mellitus, and hypertension: An emerging association. Curr. Hypertens. Rep. 2017, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Agabiti-Rosei, E.; Johnson, R.J.; Kielstein, J.T.; Lurbe, E.; Mancia, G.; Redon, J.; Stack, A.; Tsioufis, K. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Int. Med. 2020, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, S.; Montelisciani, L.; Viazzi, F.; Giussani, M.; Lieti, G.; Patti, I.; Orlando, A.; Antolini, L.; Salvi, P.; Parati, G. Uric acid and arterial stiffness in children and adolescents: Role of insulin resistance and blood pressure. Front. Cardiovasc. Med. 2022, 11, 978366. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Cantisani, V.; Anania, C.; Bonaiuto, E.; Martino, F.; Pascone, R.; Chiesa, C. Serum uric acid and its association with metabolic syndrome and carotid atherosclerosis in obese children. Eur. J. Endocrinol. 2009, 160, 45–52. [Google Scholar] [CrossRef]
- Hatami, M.; Tohidi, M.; Mohebi, R.; Khalili, D.; Azizi, F.; Hadaegh, F. Adolescent lipoprotein classifications according to National Health and Nutrition Examination Survey (NHANES) vs. National Cholesterol Education Program (NCEP) for predicting abnormal lipid levels in adulthood in a Middle East population. Lipids Health Dis. 2012, 11, 107. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs. Context. 2018, 7, 212525. [Google Scholar] [CrossRef]
- Frontini, M.G.; Srinivasan, S.R.; Xu, J.; Tang, R.; Bond, M.G.; Berenson, G.S. Usefulness of childhood non-high density lipoprotein cholesterol levels versus other lipoprotein measures in predicting adult subclinical atherosclerosis: The Bogalusa HeartStudy. Pediatrics 2008, 121, 924–929. [Google Scholar] [CrossRef]
- Boullart, A.C.; de Graaf, J.; Stalenhoef, A.F. Serum triglycerides and risk of cardiovascular disease. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 867–875. [Google Scholar] [CrossRef]
- Krawczyk, M.; Rumińska, M.; Witkowska-Sędek, E.; Majcher, A.; Pyrżak, B. Usefulness of the triglycerides to high-density lipoprotein cholesterol ratio (TG/HDL-C) in prediction of metabolic syndrome in polish obese children and adolescents. Acta-. Biochim. Pol. 2018, 65, 605–611. [Google Scholar] [CrossRef]
- Iwani, N.A.K.Z.; Jalaludin, M.Y.; Zin, R.M.W.M.; Fuziah, M.Z.; Hong, J.Y.H.; Abqariyah, Y.; Mokhtar, A.H.; Nazaimoon, W.M.W. TG: HDL-C Ratio Is a Good Marker to Identify Children Affected by Obesity with Increased Cardiometabolic Risk and Insulin Resistance. Int. J. Endocrinol. 2019, 2019, 8586167. [Google Scholar] [CrossRef]
- Lemieux, I.; Lamarche, B.; Couillard, C.; Pascot, A.; Cantin, B.; Bergeron, J.; Dagenais, G.R.; Després, J.P. Total Cholesterol/HDL Cholesterol Ratio vs LDL Cholesterol/HDL Cholesterol Ratio as Indices of Ischemic Heart Disease Risk in Men: The Quebec Cardiovascular Study. Arch. Intern. Med. 2001, 161, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Artha, I.M.J.R.; Bhargah, A.; Dharmawan, N.K.; Pande, U.W.; Triyana, K.A.; Mahariski, P.A.; Yuwono, J.; Bhargah, V.; Prabawa, I.P.Y.; Manuaba, I.B.A.P.; et al. High level of individual lipid profile and lipid ratio as a predictive marker of poor glycemic control in type-2 diabetes mellitus. Vasc. Health Risk Manag. 2019, 15, 149–157. [Google Scholar] [CrossRef]
- Magnussen, C.G.; Venn, A.; Thomson, R.; Juonala, M.; Srinivasan, S.R.; Viikari, J.S.; Berenson, G.S.; Dwyer, T.; Raitakari, O.T. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J. Am. Coll. Cardiol. 2009, 53, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Man, E.; Cheung, P.T.; Cheung, Y.F. Associations between arterial structure and function and serum levels of liver enzymes in obese adolescents. J. Paediatr. Child. Health 2017, 53, 691–697. [Google Scholar] [CrossRef]
- Al Mheid, I.; Patel, R.; Murrow, J.; Morris, A.; Rahman, A.; Fike, L.; Kavtaradze, N.; Uphoff, I.; Hooper, C.; Tangpricha, V.; et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J. Am. Coll. Cardiol. 2011, 58, 186–192. [Google Scholar] [CrossRef]
- Peterson, C.A.; Belenchia, A.M. Vitamin D deficiency & childhood obesity: A tale of two epidemics. Mo Med. 2014, 111, 49–53. [Google Scholar]
- Rajakumar, K.; Moore, C.G.; Khalid, A.T.; Vallejo, A.N.; Virji, M.A.; Holick, M.F.; Greenspan, S.L.; Arslanian, S.; Reis, S.E. Effect of vitamin D3 supplementation on vascular and metabolic health of vitamin D-deficient overweight and obese children: A randomized clinical trial. Am. J. Clin. Nutr. 2020, 111, 757–768. [Google Scholar] [CrossRef] [PubMed]



| Obese (n = 50) | % | Controls (n = 34) | % | ||
|---|---|---|---|---|---|
| Sex | Female | 23 | 46 | 18 | 53 |
| Male | 27 | 54 | 16 | 47 | |
| Age | <12 y | 22 | 44 | 13 | 38 |
| 12–15 y | 17 | 34 | 13 | 38 | |
| >15 y | 11 | 22 | 8 | 24 | |
| Mean Values | p-Values | ||||||
|---|---|---|---|---|---|---|---|
| ANOVA p-value | <12 y | 12–15 y | >15 y | <12 y vs. 12–15 y | <12 y vs. >15 y | 12–15 y vs. >15 y | |
| CIMT | 0.02 | 0.44 | 0.47 | 0.45 | 0.02 | 0.68 | 0.66 |
| PWV | 0.01 | 4.8 | 5 | 5.2 | 0.24 | 0.01 | 0.65 |
| cSBP | 0.04 | 115.4 | 118.94 | 125.45 | 0.84 | 0.02 | 0.5 |
| Mean Values | p-Values | ||||||
|---|---|---|---|---|---|---|---|
| ANOVA p-value | Tanner 1 | Tanner 2–4 | Tanner 5 | Tanner 1 vs. Tanner 2–4 | Tanner 1 vs. Tanner 5 | Tanner 2–4 vs. Tanner 5 | |
| CIMT | 0.0009 | 0.42 | 0.44 | 0.46 | 0.39 | 0.002 | 0.04 |
| PWV | 0.002 | 4.5 | 4.6 | 5 | 0.9 | 0.003 | 0.001 |
| AIx | 0.008 | 23.31 | 26.39 | 31.58 | 0.25 | 0.004 | 0.02 |
| SBP | 0.003 | 117.81 | 119.6 | 127.82 | 0.95 | 0.02 | 0.003 |
| DBP | 0.01 | 77.86 | 75 | 81.9 | 0.75 | 0.36 | 0.003 |
| MAP | 0.002 | 97.84 | 97.3 | 104.86 | 0.96 | 0.057 | 0.0006 |
| cSBP | 0.004 | 106.22 | 111.33 | 118.24 | 0.42 | 0.01 | 0.054 |
| cDBP | 0.09 | 73.4 | 71.78 | 77.03 | 0.97 | 0.69 | 0.04 |
| cPP | 0.005 | 32.81 | 39.54 | 41.2 | 0.02 | 0.003 | 0.98 |
| HR | 0.21 | 88.6 | 84.2 | 83 | 0.12 | 0.09 | 0.45 |
| BMI (kg/m2) | WC (cm) | WHR | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Obese | 29.23 | 6.78 | 99.41 | 16.68 | 0.62 | 0.09 |
| Controls | 19.01 | 2.48 | 65.41 | 8.96 | 0.43 | 0.03 |
| p-value | <0.0001 | <0.0001 | <0.0001 | |||
| Correlations | CIMT | PWV | AIx | SPB | DBP | MAP | cSBP | cDBP | cPP | HR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | ρ | 0.52 | 0.7 | 0.36 | 0.7 | 0.32 | 0.56 | 0.75 | 0.52 | 0.49 | 0.37 |
| p-value | <0.0001 | <0.0001 | 0.0007 | <0.0001 | 0.003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.04 | |
| WC | ρ | 0.53 | 0.73 | 0.39 | 0.72 | 0.29 | 0.55 | 0.76 | 0.51 | 0.5 | 0.24 |
| p-value | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.007 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.21 | |
| WHR | ρ | 0.38 | 0.58 | 0.33 | 0.58 | 0.2 | 0.42 | 0.64 | 0.38 | 0.43 | 0.13 |
| p-value | 0.0004 | <0.0001 | 0.001 | <0.0001 | 0.06 | 0.0001 | <0.0001 | 0.0004 | <0.0001 | 0.27 |
| Dependent Variable | F | p-Value | R2 | Variance % |
|---|---|---|---|---|
| CIMT | 6.39 | <0.001 | 0.44 | 43.74 |
| PWV | 14.68 | <0.001 | 0.64 | 64.09 |
| AIx | 2.7 | 0.008 | 0.25 | 24.74 |
| SBP | 13.89 | <0.001 | 0.63 | 62.81 |
| DBP | 3.28 | 0.002 | 0.29 | 28.55 |
| MAP | 9.43 | <0.001 | 0.53 | 53.42 |
| cSBP | 16.86 | <0.001 | 0.67 | 67.21 |
| cDBP | 6.46 | <0.001 | 0.44 | 43.98 |
| cPP | 3.63 | <0.001 | 0.31 | 30.62 |
| HR | 0.61 | 0.78 | 0.07 | 6.92 |
| Dependent Variable | Independent Variable | B Coefficient | Beta Coefficient | Std. Error | t | p-Value |
|---|---|---|---|---|---|---|
| SBP | WC | 1.52 | 2.77 | 0.6 | 2.51 | 0.01 |
| WHR | −204.26 | −2.11 | 89.23 | −2.29 | 0.02 | |
| DBP | BMI | 2.17 | 1.88 | 0.99 | 2.19 | 0.03 |
| WC | 1.32 | 3.17 | 0.64 | 2.08 | 0.04 | |
| WHR | −216.47 | −2.94 | 93.89 | −2.31 | 0.02 | |
| MAP | BMI | 1.9 | 1.58 | 0.83 | 2.28 | 0.02 |
| WC | 1.42 | 3.27 | 0.53 | 2.65 | 0.01 | |
| WHR | −210.36 | −2.75 | 78.95 | −2.66 | 0.009 | |
| cSBP | WC | 1.7 | 2.8 | 0.63 | 2.71 | 0.008 |
| WHR | −225.68 | −2.11 | 92.71 | −2.43 | 0.01 | |
| Tanner | 7.13 | 0.24 | 3.52 | 2.02 | 0.04 | |
| cDBP | WHR | −179.4 | −2.31 | 87.96 | −2.04 | 0.04 |
| Tanner | 9.88 | 0.45 | 3.34 | 2.95 | 0.004 |
| Vascular Biomarkers | Correlations | Fat Mass (kg) | Trunk Fat Mass (kg) | Muscle Mass (kg) | Body Water (kg) |
|---|---|---|---|---|---|
| CIMT | ρ/r | 0.39 | 0.41 | 0.41 | 0.4 |
| p-value | 0.005 | 0.002 | 0.003 | 0.004 | |
| PWV | ρ/r | 0.65 | 0.6 | 0.48 | 0.47 |
| p-value | <0.0001 | <0.0001 | 0.0004 | 0.0005 | |
| AIx | ρ/r | 0.34 | 0.32 | 0.17 | 0.19 |
| p-value | 0.01 | 0.02 | 0.22 | 0.18 | |
| SBP | ρ/r | 0.62 | 0.53 | 0.41 | 0.4 |
| p-value | <0.0001 | 0.0001 | 0.003 | 0.004 | |
| DBP | ρ/r | 0.42 | 0.29 | 0.24 | 0.24 |
| p-value | 0.002 | 0.04 | 0.08 | 0.08 | |
| MAP | ρ/r | 0.55 | 0.44 | 0.38 | 0.37 |
| p-value | <0.0001 | 0.001 | 0.006 | 0.007 | |
| cSBP | ρ/r | 0.63 | 0.55 | 0.46 | 0.45 |
| p-value | <0.0001 | <0.0001 | 0.0006 | 0.0009 | |
| cDBP | ρ/r | 0.48 | 0.38 | 0.33 | 0.33 |
| p-value | 0.0004 | 0.006 | 0.01 | 0.01 | |
| cPP | ρ/r | 0.18 | 0.23 | 0.18 | 0.18 |
| p-value | 0.19 | 0.1 | 0.19 | 0.2 | |
| HR | ρ/r | 0.38 | 0.47 | 0.26 | 0.24 |
| p-value | 0.003 | 0.02 | 0.12 | 0.18 |
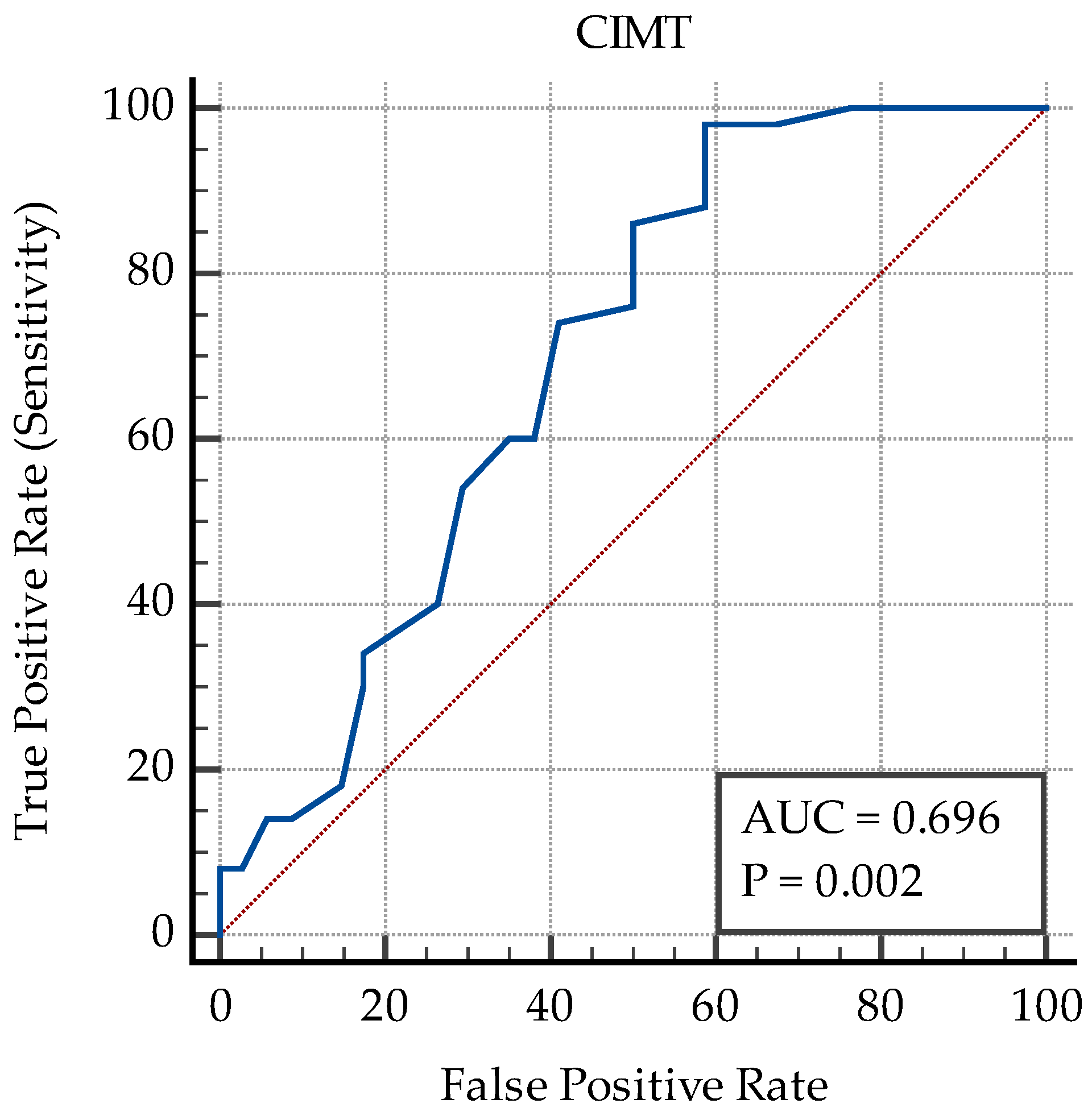
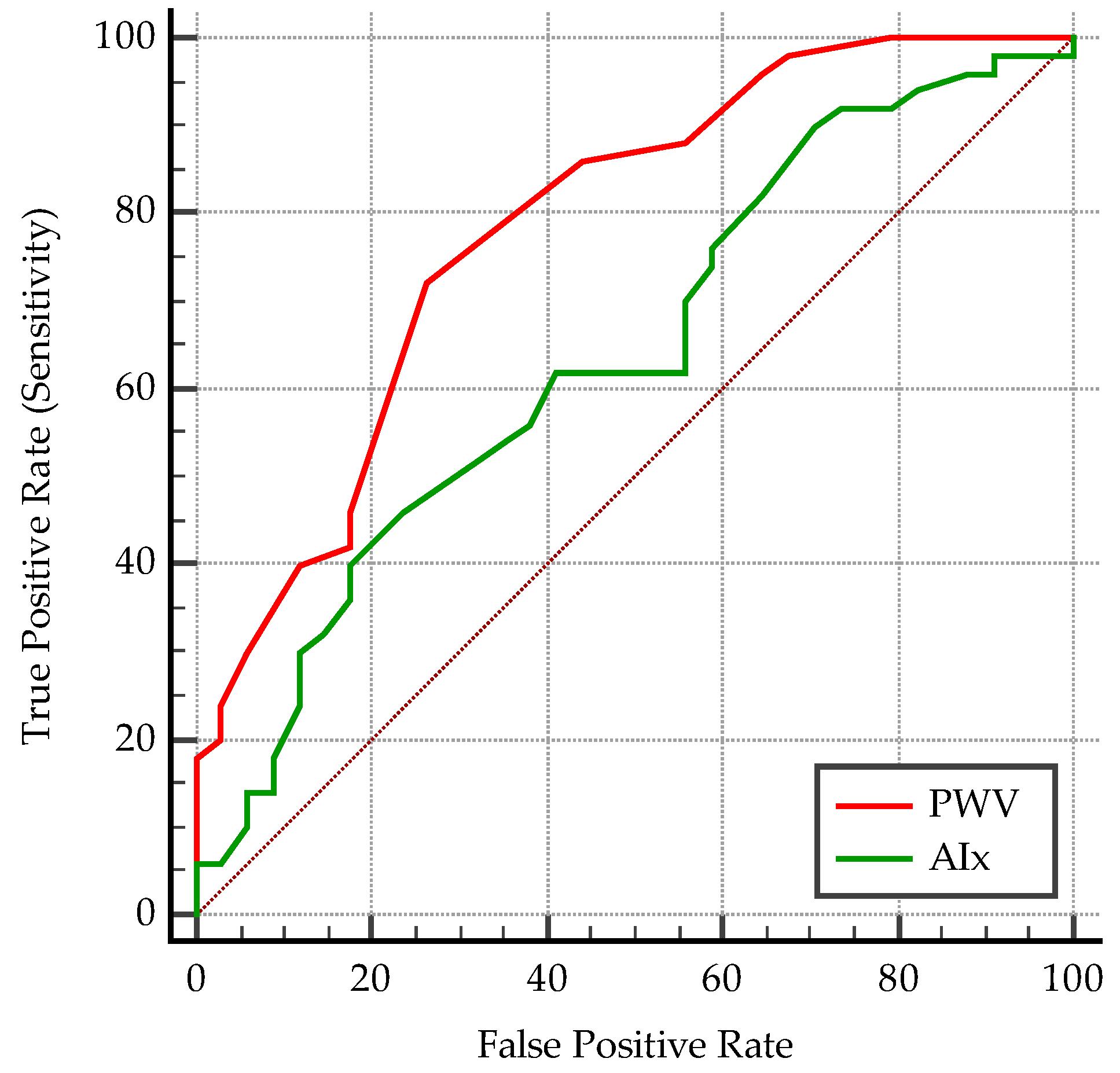
| Criterion (Cut-Off) | AUC | p | Se % | 95% CI | Sp % | 95% CI | PPV % | NPV % | |
|---|---|---|---|---|---|---|---|---|---|
| CIMT | >0.4 | 0.69 | 0.002 | 98 | 89.4–99.9 | 41.2 | 24.6–59.3 | 71 | 93.3 |
| PWV | >4.6 | 0.78 | <0.0001 | 72 | 57.5–83.8 | 73.5 | 55.6–87.1 | 79.61 | 64.1 |
| AIx | >31 | 0.64 | 0.02 | 46 | 31.8–60.7 | 76.5 | 58.8–89.3 | 74.22 | 49.13 |
| SBP | >119 | 0.79 | <0.0001 | 64 | 49.2–77.1 | 82.3 | 65.5–93.2 | 84.13 | 60.81 |
| DBP | >84 | 0.53 | 0.62 | 36 | 22.9–50.8 | 91.2 | 76.3–98.1 | 85.73 | 49.23 |
| MAP | >102.5 | 0.67 | 0.003 | 44 | 30–58.7 | 85.3 | 68.9–95 | 91.47 | 50.9 |
| cSBP | >106 | 0.86 | <0.0001 | 90 | 78.2–96.7 | 67.6 | 49.5–82.6 | 80.31 | 82.14 |
| cDBP | >76 | 0.71 | 0.0001 | 50 | 35.5–64.5 | 82.3 | 65.5–93.2 | 80.58 | 52.83 |
| cPP | >36 | 0.77 | <0.0001 | 76 | 61.8–86.9 | 70.6 | 52.5–84.9 | 79.15 | 66.68 |
| Age Group | Vascular Biomarker | Criterion (Cut-Off) | AUC | p | Se % | Sp % | PPV % | NPV % |
|---|---|---|---|---|---|---|---|---|
| <12 y | CIMT | >0.4 | 0.83 | 0.0001 | 100 | 61.5 | 81.42 | 100 |
| PWV | >4.6 | 0.8 | 0.001 | 72.75 | 84.6 | 88.85 | 64.73 | |
| SBP | >114 | 0.86 | <0.0001 | 90.9 | 69.2 | 83.28 | 81.83 | |
| MAP | >93.5 | 0.74 | 0.01 | 90.9 | 61.5 | 81.21 | 80.76 | |
| cSBP | >106 | 0.91 | <0.0001 | 95.45 | 84.6 | 91.27 | 91.67 | |
| cPP | >34 | 0.88 | <0.0001 | 81.8 | 94.6 | 89.96 | 73.35 | |
| 12–15 y | CIMT | >0.46 | 0.84 | <0.0001 | 52.9 | 100 | 100 | 61.89 |
| PWV | >4.5 | 0.86 | <0.0001 | 94.1 | 69.2 | 80 | 90 | |
| AIx | >31 | 0.68 | 0.06 | 52.9 | 92.3 | 90 | 60 | |
| SBP | >124 | 0.71 | 0.03 | 52.9 | 100 | 100 | 61.9 | |
| cSBP | >115 | 0.86 | <0.0001 | 64.7 | 100 | 100 | 68.4 | |
| cDBP | >75 | 0.77 | 0.001 | 64.7 | 84.6 | 84.6 | 64.7 | |
| cPP | >36 | 0.71 | 0.03 | 76.5 | 69.2 | 76.5 | 69.2 | |
| >15 y | PWV | >5 | 0.74 | 0.04 | 81.8 | 62.5 | 75 | 71.4 |
| SBP | >126 | 0.82 | 0.0008 | 81.8 | 75 | 81.8 | 75 | |
| cSBP | >123 | 0.88 | <0.0001 | 72.7 | 100 | 100 | 72.7 | |
| cDBP | >79 | 0.76 | 0.02 | 63.6 | 87.5 | 87.5 | 63.6 | |
| cPP | >44 | 0.76 | 0.03 | 63.6 | 100 | 100 | 66.7 |
| CIMT | PWV | AIx | SPB | DBP | MAP | cSBP | cDBP | cPP | HR | |
|---|---|---|---|---|---|---|---|---|---|---|
| ρ | 0.44 | 0.64 | 0.4 | 0.63 | 0.43 | 0.6 | 0.59 | 0.47 | 0.34 | 0.22 |
| p-value | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.001 | 0.17 |
| Tanner Stage | Group | n | Boys | Girls | Mean Age | SD | Age Limits | HOMA-IR Median Values | SD | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Obese | 12 | 9 | 3 | 8.6 | 1.6 | 6–11 y | 1.85 | 3.9 | 0.01 |
| Controls | 10 | 6 | 4 | 7.6 | 1.42 | 6–10 y | 1 | 0.25 | ||
| 2 3 4 | Obese | 20 | 12 | 8 | 11.6 | 1.6 | 9–15.5 y | 1.8 | 1.49 | 0.34 |
| Controls | 13 | 6 | 7 | 12 | 1.52 | 9–14 y | 1.63 | 0.2 | ||
| 5 | Obese | 18 | 6 | 12 | 16 | 1.26 | 14–18 y | 3.91 | 1.39 | 0.002 |
| Controls | 11 | 4 | 7 | 16 | 1.26 | 14–18 y | 2.2 | 0.35 |
| CIMT | PWV | AIx | SPB | DBP | MAP | cSBP | cDBP | cPP | HR | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tanner 1 (n = 22) | ρ | 0.39 | 0.61 | 0.56 | 0.67 | 0.45 | 0.64 | 0.66 | 0.43 | 0.52 | 0.16 |
| p-value | 0.07 | 0.002 | 0.006 | 0.0007 | 0.03 | 0.01 | 0.0007 | 0.04 | 0.01 | 0.34 | |
| Tanner 2, 3, 4 (n = 33) | ρ | 0.13 | 0.22 | −0.08 | 0.15 | 0.02 | 0.17 | 0.15 | 0.16 | 0.11 | 0.13 |
| p-value | 0.46 | 0.2 | 0.62 | 0.4 | 0.9 | 0.32 | 0.37 | 0.37 | 0.52 | 0.27 | |
| Tanner 5 (n=29) | ρ | 0.31 | 0.66 | 0.43 | 0.68 | 0.57 | 0.7 | 0.68 | 0.66 | 0.23 | 0.09 |
| p-value | 0.09 | 0.0001 | 0.02 | <0.0001 | 0.001 | <0.0001 | <0.0001 | 0.0001 | 0.22 | 0.45 | |
| HOMA-IR Cut-Off Values in Obese Subjects | Puberty Development | n | n of Patients with HOMA-IR >Cut-Off | Acanthosis Nigricans Present in: | n Of Patients With HOMA-IR <Cut-Off | Acanthosis Nigricans Present in: |
|---|---|---|---|---|---|---|
| 2.3 | Pre-pubertal | 12 | 5 | 3 | 7 | 1 |
| 3.4 | Pubertal and post-pubertal | 38 | 15 | 13 | 23 | 7 |
| Criterion (Cut-Off) | AUC | p | Se % | 95% CI | Sp % | 95% CI | PPV % | NPV % | |
|---|---|---|---|---|---|---|---|---|---|
| CIMT | >0.47 | 0.67 | 0.02 | 50 | 29.1–70.9 | 88.4 | 69.8–97.6 | 86.36 | 54.61 |
| PWV | >4.8 | 0.77 | 0.0001 | 75 | 53.3–90.2 | 80.8 | 60.6–93.4 | 85.14 | 68.74 |
| AIx | >28 | 0.58 | 0.31 | 66.7 | 44.7–84.4 | 53.8 | 33.4–73.4 | 67.95 | 52.37 |
| SBP | >125 | 0.79 | <0.0001 | 75 | 53.3–90.2 | 84.6 | 65.1–95.6 | 87.73 | 69.72 |
| DBP | >72 | 0.73 | 0.001 | 87.5 | 67.6–97.3 | 53.8 | 33.4–73.4 | 73.56 | 74.55 |
| MAP | >97 | 0.82 | <0.0001 | 91.7 | 73–99 | 65.4 | 44.3–82.8 | 79.56 | 84.28 |
| cSBP | >117 | 0.8 | >0.0001 | 70.8 | 48.9–87.4 | 80.8 | 60.6–93.4 | 84.41 | 65.32 |
| cDBP | >74 | 0.7 | 0.007 | 79.2 | 57.8–92.9 | 65.4 | 44.3–82.8 | 77.07 | 68.15 |
| cPP | >43 | 0.6 | 0.22 | 54.2 | 32.8–74.4 | 76.9 | 56.4–91 | 77.51 | 53.33 |
| Menstrual Cycles | n | Mean HOMA-IR | Acanthosis Nigricans Present In: | BMI kg/m2 | WC cm | WHR | ||
|---|---|---|---|---|---|---|---|---|
| Obese | Regular | 6 | 3.43 | 3 girls | 33.45 | 105 | 0.65 | |
| Irregular | 6 | 4.8 | 5 girls | 40.56 | 121 | 0.76 | ||
| Controls | Regular | 7 | 2.01 | No girl | 20.29 | 70.14 | 0.42 | |
| CIMT | PWV | AIx | SPB | DBP | MAP | cSBP | cDBP | cPP | |
|---|---|---|---|---|---|---|---|---|---|
| Regular menses | 0.43 | 4.76 | 28.16 | 122.83 | 77 | 99.91 | 114.33 | 77.33 | 37 |
| Irregular menses | 0.48 | 5.38 | 37.16 | 135.33 | 89.33 | 112.33 | 126.83 | 82.66 | 44.16 |
| p-value | 0.06 | 0.002 | 0.09 | 0.006 | 0.01 | 0.003 | 0.009 | 0.27 | 0.26 |
| Variables | Obese Subjects | Normal-Weight Subjects | |||
|---|---|---|---|---|---|
| Mean/Median | SD | Mean/Median | SD | p-Value | |
| Fasting glucose | 81 | 12.71 | 80.6 | 10.1 | 0.6 |
| HDL-c | 42.7 | 7.95 | 41 | 10.25 | 0.8 |
| LDL-c | 109.8 | 33.82 | 85.55 | 24.86 | 0.04 |
| TC | 174.28 | 33.83 | 160.76 | 27.62 | 0.06 |
| TG | 116.27 | 45.15 | 81 | 47.67 | 0.02 |
| Non-HDL-c | 131.56 | 36.62 | 118.26 | 27.92 | 0.07 |
| LDL-c/HDL-c | 2.37 | 1.27 | 2.5 | 0.77 | 0.67 |
| TG/HDL-c | 2.91 | 1.47 | 2 | 1.31 | 0.1 |
| TC/HDL-c | 3.9 | 1.4 | 3.7 | 0.88 | 0.42 |
| GPT | 30.5 | 15.07 | 24 | 10.85 | 0.003 |
| GOT | 27 | 10.98 | 21 | 8.74 | 0.005 |
| Creatinine | 0.52 | 0.13 | 0.4 | 0.1 | 0.01 |
| Uric acid | 4.9 | 1.44 | 3.88 | 0.74 | 0.0001 |
| TSH | 3.5 | 1.87 | 3.47 | 0.88 | 0.77 |
| FreeT4 | 14.99 | 1.69 | 15.06 | 1.67 | 0.86 |
| 8 am cortisol | 17.22 | 5.09 | 17 | 2.14 | 0.88 |
| Ionized calcium | 3.93 | 0.43 | 4.1 | 0.2 | 0.009 |
| 25-OH-vitamin D | 19.84 | 9.93 | 24 | 8.4 | 0.22 |
| Correlations | CIMT | PWV | AIx | SBP | DBP | MAP | cSBP | cDBP | cPP | HR |
|---|---|---|---|---|---|---|---|---|---|---|
| Fasting glucose | −0.19 | −0.11 | 0.05 | 0.04 | 0.21 | 0.16 | −0.001 | 0.27 | −0.25 | 0.03 |
| p-value | 0.17 | 0.53 | 0.69 | 0.77 | 0.13 | 0.26 | 0.99 | 0.06 | 0.07 | 0.89 |
| Uric acid | 0.27 | 0.41 | 0.09 | 0.35 | 0.32 | 0.4 | 0.46 | 0.33 | 0.12 | 0.16 |
| p-value | 0.05 | 0.02 | 0.53 | 0.01 | 0.02 | 0.04 | 0.0008 | 0.01 | 0.4 | 0.78 |
| Creatinine | 0.14 | 0.24 | 0.09 | 0.14 | 0.01 | 0.08 | 0.16 | 0.02 | 0.15 | 0.04 |
| p-value | 0.33 | 0.08 | 0.49 | 0.31 | 0.93 | 0.55 | 0.24 | 0.85 | 0.29 | 0.91 |
| HDL-c | −0.02 | −0.2 | 0.07 | −0.21 | −0.19 | −0.21 | −0.36 | −0.34 | 0.1 | 0.16 |
| p-value | 0.86 | 0.15 | 0.62 | 0.14 | 0.16 | 0.13 | 0.02 | 0.01 | 0.42 | 0.64 |
| LDL-c | 0.31 | 0.39 | 0.06 | 0.42 | 0.21 | 0.24 | 0.36 | 0.2 | 0.19 | 0.07 |
| p-value | 0.03 | 0.02 | 0.65 | 0.02 | 0.09 | 0.07 | 0.03 | 0.16 | 0.16 | 0.94 |
| Total cholesterol | 0.21 | 0.24 | 0.1 | 0.22 | 0.06 | 0.15 | 0.17 | 0.03 | 0.25 | 0.08 |
| p-value | 0.27 | 0.15 | 0.52 | 0.08 | 0.9 | 0.27 | 0.24 | 0.87 | 0.14 | 0.69 |
| TG | 0.27 | 0.47 | 0.16 | 0.32 | 0.15 | 0.22 | 0.38 | 0.09 | 0.06 | 0.04 |
| p-value | 0.07 | 0.01 | 0.34 | 0.04 | 0.22 | 0.17 | 0.02 | 0.66 | 0.79 | 0.78 |
| Non-HDL-c | 0.24 | 0.33 | 0.15 | 0.22 | 0.14 | 0.16 | 0.17 | 0.18 | 0.21 | 0.004 |
| p-value | 0.09 | 0.03 | 0.32 | 0.12 | 0.33 | 0.35 | 0.26 | 0.22 | 0.13 | 0.96 |
| LDL-c/HDL-c ratio | 0.14 | 0.17 | 0.05 | 0.12 | 0.22 | 0.16 | 0.09 | 0.23 | 0.08 | 0.18 |
| p-value | 0.2 | 0.12 | 0.64 | 0.27 | 0.04 | 0.13 | 0.4 | 0.03 | 0.43 | 0.09 |
| TG/HDL-c ratio | 0.19 | 0.23 | 0.11 | 0.18 | 0.09 | 0.12 | 0.15 | 0.19 | 0.08 | 0.01 |
| p-value | 0.22 | 0.1 | 0.43 | 0.34 | 0.53 | 0.46 | 0.39 | 0.17 | 0.54 | 0.57 |
| TC/HDL-c ratio | 0.1 | 0.04 | 0.1 | 0.04 | 0.17 | 0.1 | 0.05 | 0.24 | 0.23 | −0.07 |
| p-value | 0.9 | 0.76 | 0.49 | 0.78 | 0.21 | 0.45 | 0.73 | 0.09 | 0.11 | 0.66 |
| GPT | 0.31 | 0.48 | 0.16 | 0.35 | 0.55 | 0.44 | 0.19 | 0.2 | 0.21 | 0.21 |
| p-value | 0.03 | 0.001 | 0.19 | 0.03 | 0.001 | 0.01 | 0.12 | 0.16 | 0.16 | 0.29 |
| GOT | 0.09 | 0.25 | 0.02 | 0.31 | 0.42 | 0.37 | 0.17 | 0.16 | 0.28 | −0.13 |
| p-value | 0.54 | 0.17 | 0.54 | 0.004 | 0.003 | 0.02 | 0.92 | 0.25 | 0.04 | 0.48 |
| 8 a.m. cortisol | 0.28 | 0.16 | 0.23 | 0.11 | 0.21 | 0.19 | 0.07 | 0.2 | −0.13 | −0.06 |
| p-value | 0.04 | 0.25 | 0.1 | 0.41 | 0.12 | 0.17 | 0.61 | 0.15 | 0.36 | 0.72 |
| TSH | 0.12 | −0.7 | −0.5 | −0.004 | −0.2 | −0.11 | 0.05 | −0.15 | 0.34 | −0.03 |
| p-value | 0.4 | 0.63 | 0.7 | 0.97 | 0.14 | 0.44 | 0.71 | 0.3 | 0.04 | 0.88 |
| FreeT4 | 0.17 | 0.16 | 0.08 | 0.16 | 0.06 | 0.13 | 0.09 | 0.1 | 0.02 | 0.09 |
| p-value | 0.21 | 0.26 | 0.55 | 0.26 | 0.66 | 0.36 | 0.53 | 0.47 | 0.87 | 0.59 |
| 25-OH- vitamin D | −0.19 | −0.41 | −0.09 | −0.37 | −0.44 | −0.43 | −0.35 | −0.42 | 0.1 | 0.12 |
| p-value | 0.17 | 0.003 | 0.51 | 0.007 | 0.001 | 0.001 | 0.01 | 0.002 | 0.47 | 0.65 |
| Ionized calcium | −0.2 | −0.35 | 0.01 | −0.38 | −0.35 | −0.39 | −0.39 | −0.32 | −0.03 | 0.11 |
| p-value | 0.14 | 0.01 | 0.9 | 0.006 | 0.01 | 0.005 | 0.004 | 0.02 | 0.8 | 0.28 |
| Dependent Variable | Independent Variable | B Coefficient | Beta Coefficient | Std. Error | t | p-Value |
|---|---|---|---|---|---|---|
| CIMT | uric acid | 0.009 | 0.09 | 0.003 | 2.68 | 0.008 |
| PWV | HOMA-IR | 0.1 | 0.41 | 0.02 | 4.4 | <0.0001 |
| GPT | 0.008 | 0.28 | 0.003 | 2.74 | 0.007 | |
| uric acid | 0.07 | 0.19 | 0.03 | 2.21 | 0.02 | |
| AIx | HOMA-IR | 1.37 | 0.27 | 0.52 | 2.95 | 0.004 |
| SBP | HOMA-IR | 3.36 | 0.51 | 0.53 | 6.3 | <0.0001 |
| GPT | 0.18 | 0.31 | 0.07 | 2.44 | 0.01 | |
| DBP | HOMA-IR | 1.92 | 0.37 | 0.46 | 4.15 | 0.0001 |
| MAP | HOMA-IR | 2.83 | 0.5 | 0.42 | 6.63 | <0.0001 |
| cSBP | HOMA-IR | 2.71 | 0.41 | 0.65 | 4.17 | 0.0001 |
| GPT | 0.18 | 0.27 | 0.08 | 2.11 | 0.03 | |
| uric acid | 2.19 | 0.29 | 0.95 | 2.29 | 0.02 | |
| cDBP | HOMA-IR | 2.15 | 0.37 | 0.46 | 4.63 | <0.0001 |
| TC/HDL-c ratio | 1.87 | 1.72 | 0.74 | 2.51 | 0.01 | |
| cPP | uric acid | 1.91 | 0.24 | 0.78 | 2.44 | 0.01 |
| TG | 0.45 | 2.16 | 0.18 | 2.53 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihuta, M.S.; Paul, C.; Borlea, A.; Roi, C.M.; Velea-Barta, O.-A.; Mozos, I.; Stoian, D. Unveiling the Silent Danger of Childhood Obesity: Non-Invasive Biomarkers Such as Carotid Intima-Media Thickness, Arterial Stiffness Surrogate Markers, and Blood Pressure Are Useful in Detecting Early Vascular Alterations in Obese Children. Biomedicines 2023, 11, 1841. https://doi.org/10.3390/biomedicines11071841
Mihuta MS, Paul C, Borlea A, Roi CM, Velea-Barta O-A, Mozos I, Stoian D. Unveiling the Silent Danger of Childhood Obesity: Non-Invasive Biomarkers Such as Carotid Intima-Media Thickness, Arterial Stiffness Surrogate Markers, and Blood Pressure Are Useful in Detecting Early Vascular Alterations in Obese Children. Biomedicines. 2023; 11(7):1841. https://doi.org/10.3390/biomedicines11071841
Chicago/Turabian StyleMihuta, Monica Simina, Corina Paul, Andreea Borlea, Cristina Mihaela Roi, Oana-Alexandra Velea-Barta, Ioana Mozos, and Dana Stoian. 2023. "Unveiling the Silent Danger of Childhood Obesity: Non-Invasive Biomarkers Such as Carotid Intima-Media Thickness, Arterial Stiffness Surrogate Markers, and Blood Pressure Are Useful in Detecting Early Vascular Alterations in Obese Children" Biomedicines 11, no. 7: 1841. https://doi.org/10.3390/biomedicines11071841
APA StyleMihuta, M. S., Paul, C., Borlea, A., Roi, C. M., Velea-Barta, O.-A., Mozos, I., & Stoian, D. (2023). Unveiling the Silent Danger of Childhood Obesity: Non-Invasive Biomarkers Such as Carotid Intima-Media Thickness, Arterial Stiffness Surrogate Markers, and Blood Pressure Are Useful in Detecting Early Vascular Alterations in Obese Children. Biomedicines, 11(7), 1841. https://doi.org/10.3390/biomedicines11071841










