Clinical Results of Transarterial Radioembolization (TARE) with Holmium-166 Microspheres in the Multidisciplinary Oncologic Treatment of Patients with Primary and Secondary Liver Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. 166Ho-TARE Procedures
2.3. Follow-Up
2.4. Outcome Evaluation and Statistics
3. Results
3.1. Patient Characteristics and Pre-TARE Treatments
3.2. 166Ho-TARE Interventional Procedures
3.3. Patients with HCC
3.4. Patients with mCRC
3.5. Patients with ICC or Hemangioendothelioma of the Liver
4. Discussion
4.1. Procedural Characteristics and Technique
4.2. Clinical Outcomes
4.3. Adverse Events during Follow-Up
4.4. Achievement of Treatment-Free Intervals
4.5. Standard and Personalized Dosimetry
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bester, L.; Meteling, B.; Boshell, D.; Chua, T.C.; Morris, D.L. Transarterial chemoembolisation and radioembolisation for the treatment of primary liver cancer and secondary liver cancer: A review of the literature. J. Med. Imaging Radiat. Oncol. 2014, 58, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, T.; Ruf, C.; Kratzsch, H.C.; Ziegler, M.; Spath, G. Arterial, portal or combined arterio-portal regional chemotherapy in experimental liver tumours? J. Cancer Res. Clin. Oncol. 1992, 118, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonne, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.H.; Lopes Vendrami, C.; Gabr, A.; Horowitz, J.M.; Kelahan, L.C.; Riaz, A.; Salem, R.; Lewandowski, R.J. Evolution of Radioembolization in Treatment of Hepatocellular Carcinoma: A Pictorial Review. Radiographics 2021, 41, 1802–1818. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.F.; Mahvash, A.; Pracht, M.; Montazeri, A.H.; Bandula, S.; Martin, R.C.G., 2nd; Herrmann, K.; Brown, E.; Zuckerman, D.; Wilson, G.; et al. Radioembolization with Chemotherapy for Colorectal Liver Metastases: A Randomized, Open-Label, International, Multicenter, Phase III Trial. J. Clin. Oncol. 2021, 39, 3897–3907. [Google Scholar] [CrossRef]
- Stella, M.; Braat, A.; van Rooij, R.; de Jong, H.; Lam, M. Holmium-166 Radioembolization: Current Status and Future Prospective. Cardio Vasc. Interv. Radiol. 2022, 45, 1634–1645. [Google Scholar] [CrossRef]
- Braat, A.; Kwekkeboom, D.J.; Kam, B.L.R.; Teunissen, J.J.M.; de Herder, W.W.; Dreijerink, K.M.A.; van Rooij, R.; Krijger, G.C.; de Jong, H.; van den Bosch, M.; et al. Additional hepatic (166)Ho-radioembolization in patients with neuroendocrine tumours treated with (177)Lu-DOTATATE.; a single center, interventional, non-randomized, non-comparative, open label, phase II study (HEPAR PLUS trial). BMC Gastroenterol. 2018, 18, 84. [Google Scholar] [CrossRef]
- Prince, J.F.; van den Bosch, M.; Nijsen, J.F.W.; Smits, M.L.J.; van den Hoven, A.F.; Nikolakopoulos, S.; Wessels, F.J.; Bruijnen, R.C.G.; Braat, M.; Zonnenberg, B.A.; et al. Efficacy of Radioembolization with (166)Ho-Microspheres in Salvage Patients with Liver Metastases: A Phase 2 Study. J. Nucl. Med. 2018, 59, 582–588. [Google Scholar] [CrossRef]
- Smits, M.L.; Nijsen, J.F.; van den Bosch, M.A.; Lam, M.G.; Vente, M.A.; Mali, W.P.; van Het Schip, A.D.; Zonnenberg, B.A. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): A phase 1, dose-escalation study. Lancet Oncol. 2012, 13, 1025–1034. [Google Scholar] [CrossRef]
- van Roekel, C.; van den Hoven, A.F.; Bastiaannet, R.; Bruijnen, R.C.G.; Braat, A.; de Keizer, B.; Lam, M.; Smits, M.L.J. Use of an anti-reflux catheter to improve tumor targeting for holmium-166 radioembolization-a prospective, within-patient randomized study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1658–1668. [Google Scholar] [CrossRef]
- Reinders, M.T.M.; van Erpecum, K.J.; Smits, M.L.J.; Braat, A.; Bruijne, J.; Bruijnen, R.; Sprengers, D.; Man, R.A.; Vegt, E.; JNM, I.J.; et al. Safety and Efficacy of (166)Ho Radioembolization in Hepatocellular Carcinoma: The HEPAR Primary Study. J. Nucl. Med. 2022, 63, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Quirem Medical, B.V. Instructions for Use Ho-166-PLLA Microspheres, Version LS-1101-10; Quirem Medical B.V.: Deventer, The Netherlands, 2017. [Google Scholar]
- Reinders, M.T.M.; Smits, M.L.J.; van Roekel, C.; Braat, A. Holmium-166 Microsphere Radioembolization of Hepatic Malignancies. Semin. Nucl. Med. 2019, 49, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Quirem Medical B.V. QuiremSpheres Administration Procedure Quick Guide, Version MAN-1101-18-00. Quirem Medical B.V.: Deventer, The Netherlands, 2018. [Google Scholar]
- National Cancer Institute. Division of Cancer Treatment and Diagnosis. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 20 March 2023).
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Newell, P.H.; Wu, Y.; Hoen, H.; Uppal, R.; Thiesing, J.T.; Sasadeusz, K.; Cassera, M.A.; Wolf, R.F.; Hansen, P.; Hammill, C.W. Multimodal treatment of unresectable hepatocellular carcinoma to achieve complete response results in improved survival. HPB (Oxford) 2015, 17, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.; Prince, J.F.; van Rooij, R.; Bruijnen, R.C.G.; van den Bosch, M.; Lam, M. Safety analysis of holmium-166 microsphere scout dose imaging during radioembolisation work-up: A cohort study. Eur. Radiol. 2018, 28, 920–928. [Google Scholar] [CrossRef]
- Smits, M.L.J.; Dassen, M.G.; Prince, J.F.; Braat, A.; Beijst, C.; Bruijnen, R.C.G.; de Jong, H.; Lam, M. The superior predictive value of (166)Ho-scout compared with (99m)Tc-macroaggregated albumin prior to (166)Ho-microspheres radioembolization in patients with liver metastases. J. Nucl. Med. Mol. Imaging 2019, 47, 798–806. [Google Scholar] [CrossRef]
- Drescher, R.; Seifert, P.; Guhne, F.; Aschenbach, R.; Kuhnel, C.; Freesmeyer, M. Radioembolization with Holmium-166 Polylactic Acid Microspheres: Distribution of Residual Activity in the Delivery Set and Outflow Dynamics During Planning and Treatment Procedures. J. Endovasc. Ther. 2021, 28, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Radosa, C.G.; Radosa, J.C.; Grosche-Schlee, S.; Zophel, K.; Plodeck, V.; Kuhn, J.P.; Kotzerke, J.; Hoffmann, R.T. Holmium-166 Radioembolization in Hepatocellular Carcinoma: Feasibility and Safety of a New Treatment Option in Clinical Practice. Cardiovasc. Interv. Radiol. 2019, 42, 405–412. [Google Scholar] [CrossRef]
- Aliseda, D.; Marti-Cruchaga, P.; Zozaya, G.; Rodriguez-Fraile, M.; Bilbao, J.I.; Benito-Boillos, A.; Martinez De La Cuesta, A.; Lopez-Olaondo, L.; Hidalgo, F.; Ponz-Sarvise, M.; et al. Liver Resection and Transplantation Following Yttrium-90 Radioembolization for Primary Malignant Liver Tumors: A 15-Year Single-Center Experience. Cancers 2023, 15, 733. [Google Scholar] [CrossRef]
- Lopez-Lopez, V.; Miura, K.; Kuemmerli, C.; Capel, A.; Eshmuminov, D.; Ferreras, D.; Baroja-Mazo, A.; Cascales-Campos, P.; Jimenez-Mascunan, M.I.; Pons, J.A.; et al. Selecting the Appropriate Downstaging and Bridging Therapies for Hepatocellular Carcinoma: What Is the Role of Transarterial Radioembolization? A Pooled Analysis. Cancers 2023, 15, 2122. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Y.; Xiong, X.; Liu, A.; Zhou, R.; You, Z.; Li, F.; Cheng, N. Comparison of current guidelines and consensus on the management of patients with cholangiocarcinoma: 2022 update. Intractable Rare Dis. Res. 2022, 11, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Karaman, B.; Battal, B.; Alagoz, E.; Akgun, V.; Ince, S.; Ustunsoz, B. Complete disappearance of uptake of FDG in the multifocal liver hemangioendothelioma after radioembolization therapy using yttrium-90 microspheres. Ann. Nucl. Med. 2012, 26, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Bastiaannet, R.; van Roekel, C.; Smits, M.L.J.; Elias, S.G.; van Amsterdam, W.A.C.; Doan, D.; Prince, J.F.; Bruijnen, R.C.G.; de Jong, H.; Lam, M. First Evidence for a Dose-Response Relationship in Patients Treated with (166)Ho Radioembolization: A Prospective Study. J. Nucl. Med. 2020, 61, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, P.; Rietbergen, D.D.D.; van Erkel, A.R.; Coenraad, M.J.; Arntz, M.J.; Bennink, R.J.; Braat, A.E.; Crobach, A.; van Delden, O.M.; van der Hulle, T.; et al. Study Protocol: Adjuvant Holmium-166 Radioembolization After Radiofrequency Ablation in Early-Stage Hepatocellular Carcinoma Patients-A Dose-Finding Study (HORA EST HCC Trial). Cardiovasc. Interv. Radiol. 2022, 45, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Quirem Medical, B.V. Instructions for Use Ho-166-PLLA Microspheres, Version LC-80043 [07]; Quirem Medical, B.V.: Deventer, The Netherlands, 2022. [Google Scholar]
- Ho, S.Y.; Liu, P.H.; Hsu, C.Y.; Huang, Y.H.; Liao, J.I.; Su, C.W.; Hou, M.C.; Huo, T.I. Comparison of Four Albumin-Based Liver Reserve Models (ALBI/EZ-ALBI/PALBI/PAL) against MELD for Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Cancers 2023, 15, 1925. [Google Scholar] [CrossRef]
- Park, H.J.; Jang, H.Y.; Kim, S.Y.; Lee, S.J.; Won, H.J.; Byun, J.H.; Choi, S.H.; Lee, S.S.; An, J.; Lim, Y.S. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J. Hepatol. 2020, 72, 718–724. [Google Scholar] [CrossRef]
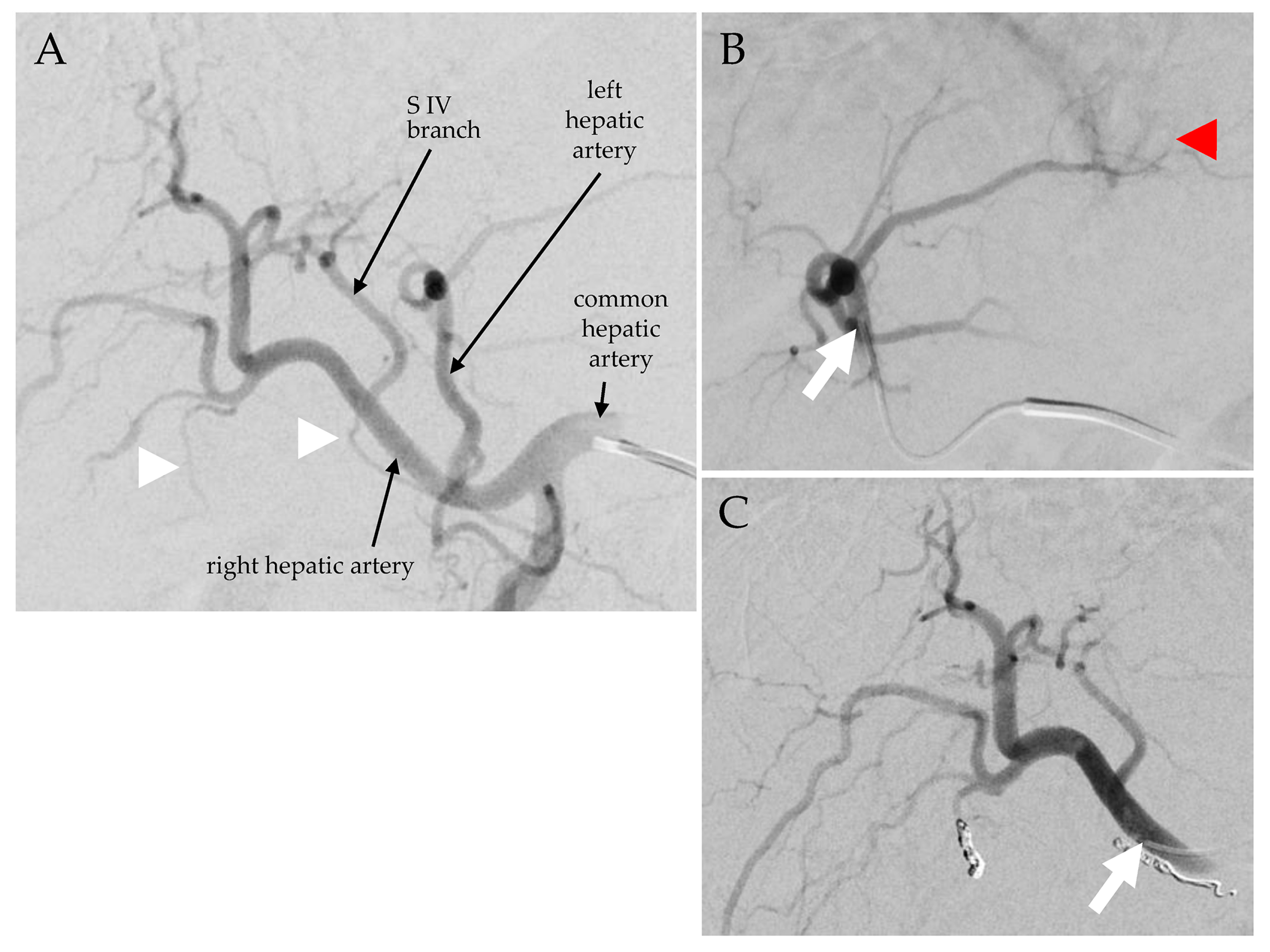

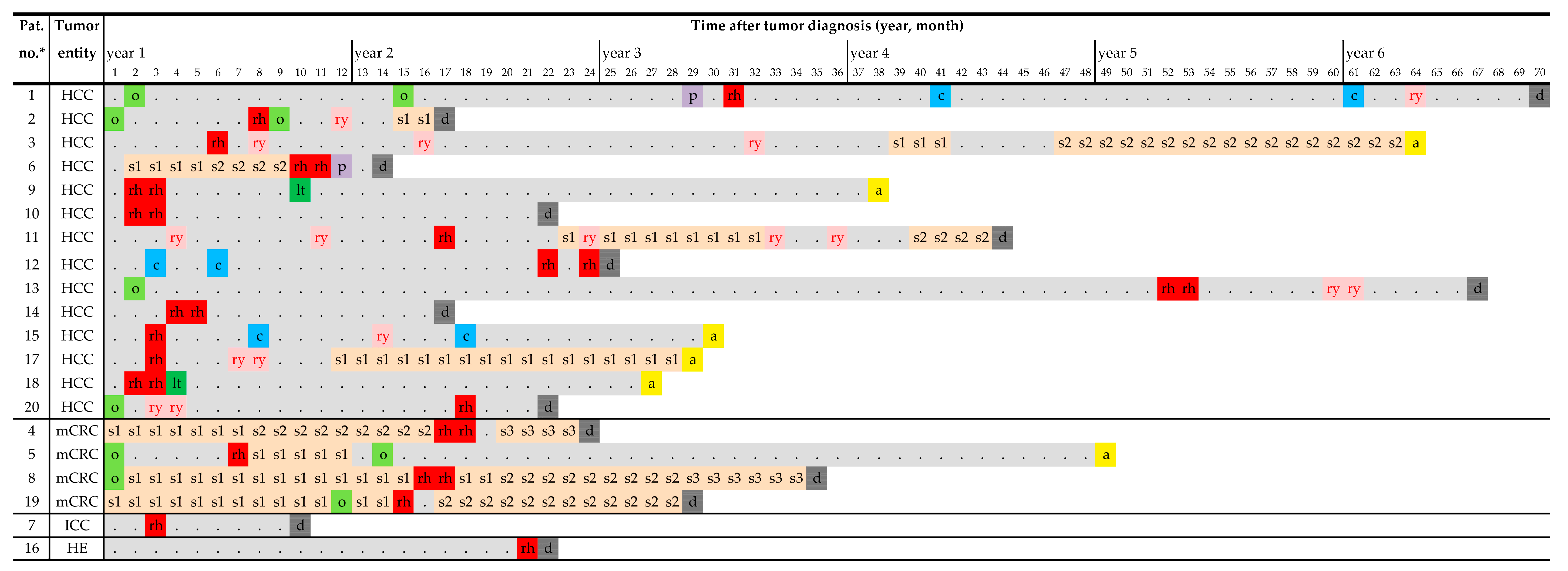


| Patient and Disease Characteristics | Status prior to 166Ho-TARE | 166Ho-TARE Procedures | Status at 3 Months Follow-Up | Progression-Free Survival after 166Ho-TARE | Treatment-Free Interval | Overall Survival | Cause of Death | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no.* | Age (yrs.), Gender | Tumor Entity | Underlying Liver Disease | Tumor Stage | Tumor Volume (mL) | Tumor Load (%) | Liver Function State (CPS) | Proportion of the Liver (%) | Prescribed Activity (GBq) | Periprocedural Adverse Events | Liver Function State (CPS) | Treatment Response | Treated Liver (mos.) | Untreated Liver (mos.) | Extrahepatic (mos.) | (mos.) | After 166Ho-TARE (mos.) | After Initial Diagnosis (mos.) | |
| 1 | 73, m | HCC | cirrhosis | II | 24 | 7 | A5 | 27 | 1.4 | none | A5 | PR | 34.1 | 9.0 | 38.7 | 35.8 | 38.7 | 69.6 | LF |
| 2 | 75, m | HCC | NAFLD | IVB | 19 | 4 | A6 | 29 | 1.8 | none | A6 | PR | 6.6 | 3.6 | 6.6 | 7.0 | 9.5 | 17.1 | prM |
| 3 | 66, m | HCC | none | IIIA | 87 | 6 | A5 | 56 | 5.6 | none | A5 | PR | 32.5 | 8.6 | 58.0 | 32.7 | 58.0 | 63.5 | - |
| 6 | 81, m | HCC | cirrhosis | IVB | 288 | 14 | A5 | 100 | 3.3, 5.0 | none | A6 | n.a. | 4.4 | - | 4.4 | n.a. | 4.4 | 14.1 | CRS |
| 9 | 58, m | HCC | cirrhosis | II | 67 | 5 | A5 | 68 | 2.5, 2.9 | none | A5 | PR | LTx | LTx | 35.6 | 7.5 | 35.6 | 37.1 | - |
| 10 | 77, m | HCC | cirrhosis | II | 17 | 2 | A5 | 63 | 1.1, 2.2 | none | A5 | CR | 19.9 | 19.9 | 19.9 | n.a. | 19.9 | 21.7 | LF |
| 11 | 65, m | HCC | cirrhosis | IIIA | 223 | 28 | A5 | 21 | 3.2 | none | A6 A | SD | 5.6 | 14.6 | 22.2 | 6.2 | 26.5 | 43.7 | - |
| 12 | 60, f | HCC | cirr., Hep. C | II | 40 | 4 | A5 | 100 | 2.6, 1.8 | none | C10 A | PD* | 3.0 | - | 3.0 | n.a. | 3.0 | 25.0 | LF/HRS |
| 13 | 81, m | HCC | none | IIIA | 365 | 22 | A5 | 100 | 5.5, 1.1 | abd. pain | A5 | PR | 7.2 | - | 15.8 | 8.4 | 15.8 | 67.8 | n.a. |
| 14 | 74, m | HCC | cirrhosis | IIIB | 151 | 8 | A5 | 100 | 3.5, 3.9 | abd. pain | A6 A | PR | 13.4 | - | 13.4 | n.a. | 13.4 | 17.2 | pneumonia |
| 15 | 65, m | HCC | NASH | II | 60 | 5 | A5 | 62 | 4.4 | none | A5 | PR | 24.0 | 3.0 | 26.5 | n.a. | 26.5 | 29.0 | - |
| 17 | 82, m | HCC | cirrhosis | IB | 323 | 32 | A5 | 49 | 4.0 | none | A5 | SD | 25.1 | 25.1 | 7.4 | 4.9 | 25.1 | 28.0 | - |
| 18 | 68, m | HCC | hemochrm. | IIIA | 160 | 10 | A5 | 89 | 3.9, 2.4 | none | A6 A | LTx | LTx | LTx | 24.3 | 2.6 | 24.3 | 26.0 | - |
| 20 | 76, m | HCC | cirrhosis | IB | 19 | 3 | B7 A | 30 | 2.5 | abd. pain | C10 A | PD * | 3.4 | 3.4 | 3.4 | n.a. | 3.4 | 21.4 | HRS |
| 4 | 58, m | mCRC | none | IVB | 127 | 9 | A6 | 100 | 3.6, 1.8 | abd. pain | A6 A | PD | 2.6 | - | 2.6 | 1.5 | 7.2 | 23.8 | prM, LF |
| 5 | 62, f | mCRC | NAFLD | IVA | 84 | 15 | A5 | 25 | 2.2 | none | A5 | PR | Res | 41.9 | 41.9 | 1.4 | 41.9 | 48.4 | - |
| 8 | 57, f | mCRC | none | IVA | 571 | 25 | A5 | 100 | 4.9, 4.2 | none | A5 | CR | 11.8 | - | 3.2 | 0 | 19.6 | 35.5 | n.a. |
| 19 | 58, m | mCRC | none | IVA | 291 | 22 | A5 | 65 | 5.3 | none | A6 A | PD | 1.5 | 1.5 | 13.7 | 0.8 | 13.9 | 28.8 | LF |
| 7 | 75, m | ICC | fibrosis | IIIB | 240 | 25 | A6 | 44 | 3.8 | none | B7 A | PR | 5.2 | 2.9 | 2.9 | n.a. | 5.2 | 8.2 | n.a. |
| 16 | 71, f | HE | cirrhosis | IIIB | 730 | 58 | A6 | 52 | 5.0 | none | B9 A | n.a. | 0.8 | 0.8 | 0.8 | n.a. | 0.8 | 22.3 | HRS |
| PFS after First 166Ho-TARE (Months) | HCC (14 Patients) | mCRC (4 Patients) |
|---|---|---|
| hepatic, treated liver | * 14.9 ± 11.6 (10.3, 8.8–21.0) | * 5.3 ± 5.7 (2.6, 2.4–8.3) |
| hepatic, untreated liver | * 10.9 ± 8.3 (8.8, 6.6–15.2) | 21.7 ± 28.6 (21.7, 6.8–36.7) |
| hepatic, whole liver | 8.9 ± 7.2 (6.4, 5.1–12.6) | 14.5 ± 18.9 (7.2, 4.6–24.4) |
| extrahepatic | 19.9 ± 16.0 (17.9, 11.6–28.3) | 15.4 ± 18.4 (8.5, 5.7–25.0) |
| whole body | 10.6 ± 9.7 (7.3, 5.5–15.7) | 12.3 ± 19.8 (2.9, 2.0–22.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drescher, R.; Köhler, A.; Seifert, P.; Aschenbach, R.; Ernst, T.; Rauchfuß, F.; Freesmeyer, M. Clinical Results of Transarterial Radioembolization (TARE) with Holmium-166 Microspheres in the Multidisciplinary Oncologic Treatment of Patients with Primary and Secondary Liver Cancer. Biomedicines 2023, 11, 1831. https://doi.org/10.3390/biomedicines11071831
Drescher R, Köhler A, Seifert P, Aschenbach R, Ernst T, Rauchfuß F, Freesmeyer M. Clinical Results of Transarterial Radioembolization (TARE) with Holmium-166 Microspheres in the Multidisciplinary Oncologic Treatment of Patients with Primary and Secondary Liver Cancer. Biomedicines. 2023; 11(7):1831. https://doi.org/10.3390/biomedicines11071831
Chicago/Turabian StyleDrescher, Robert, Alexander Köhler, Philipp Seifert, René Aschenbach, Thomas Ernst, Falk Rauchfuß, and Martin Freesmeyer. 2023. "Clinical Results of Transarterial Radioembolization (TARE) with Holmium-166 Microspheres in the Multidisciplinary Oncologic Treatment of Patients with Primary and Secondary Liver Cancer" Biomedicines 11, no. 7: 1831. https://doi.org/10.3390/biomedicines11071831
APA StyleDrescher, R., Köhler, A., Seifert, P., Aschenbach, R., Ernst, T., Rauchfuß, F., & Freesmeyer, M. (2023). Clinical Results of Transarterial Radioembolization (TARE) with Holmium-166 Microspheres in the Multidisciplinary Oncologic Treatment of Patients with Primary and Secondary Liver Cancer. Biomedicines, 11(7), 1831. https://doi.org/10.3390/biomedicines11071831






