Comparison of Two Nuclear Magnetic Resonance Spectroscopy Methods for the Measurement of Lipoprotein Particle Concentrations
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Blood Sampling
2.3. Specimen Material
2.4. Storage of Samples and Shipping
2.5. Laboratory Measurements
2.6. Statistical Methods
2.7. Ethical and Regulatory Aspects
3. Results
3.1. Raw Data
3.2. Standard Lipid Measured with ß-Quantification, Lipofit NMR and Lipoprofile NMR
3.3. Correlations among Lipids and Lipoprotein Particles within the Lipofit NMR Method and within the Lipoprofile NMR Method
3.4. Lipoprotein Particles Measured with Lipofit NMR and Lipoprofile NMR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Austin, M.A.; Breslow, J.L.; Hennekens, G.H.; Buring, J.E.; Willett, W.C.; Krauss, R.M. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Kwiterovich, P.O., Jr. Small, dense low-density lipoprotein particles and coronary heart disease risk. A clear association with uncertain implications. JAMA 1996, 276, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Collins, D.; Freedman, D.S.; Shalaurova, I.; Schaefer, E.J.; McNamara, J.R.; Robins, S.J. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006, 113, 1556–1563. [Google Scholar] [CrossRef]
- Mora, S.; Otvos, J.D.; Rifai, N.; Rosenson, R.S.; Buring, J.E.; Ridker, P.M. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009, 119, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Melander, O.; Shiffman, D.; Caulfield, M.P.; Louie, J.Z.; Rowland, C.M.; Devlin, J.J.; Krauss, R.M. Low-Density Lipoprotein Particle Number Is Associated With Cardiovascular Events Among Those Not Classified Into Statin Benefit Groups. J. Am. Coll. Cardiol. 2015, 65, 2571–2573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silbernagel, G.; Pagel, P.; Pfahlert, V.; Genser, B.; Scharnagl, H.; Kleber, M.E.; Delgado, G.; Ohrui, H.; Ritsch, A.; Grammer, T.B.; et al. High-Density Lipoprotein Subclasses, Coronary Artery Disease, and Cardiovascular Mortality. Clin. Chem. 2017, 63, 1886–1896. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Lawler, P.R.; Cook, N.R.; Ridker, P.M.; Mora, S.; Pradhan, A.D. Lipoprotein Particle Profiles, Standard Lipids, and Peripheral Artery Disease Incidence. Circulation 2018, 138, 2330–2341. [Google Scholar] [CrossRef]
- Hollstein, T.; Vogt, A.; Grenkowitz, T.; Stojakovic, T.; März, W.; Laufs, U.; Bölükbasi, B.; Steinhagen-Thiessen, E.; Scharnagl, H.; Kassner, U. Treatment with PCSK9 inhibitors reduces atherogenic VLDL remnants in a real-world study. Vascul. Pharmacol. 2019, 116, 8–15. [Google Scholar] [CrossRef]
- Ensign, W.; Hill, N.; Heward, C.B.; Ensign, W.; Disparate, L.D.L. phenotypic classification among 4 different methods assessing LDL particle characteristics. Clin. Chem. 2006, 52, 1722–1727. [Google Scholar] [CrossRef]
- Williams, P.T.; Zhao, X.-Q.; Marcovina, S.M.; Otvos, J.D.; Brown, B.G.; Krauss, R.M. Comparison of four methods of analysis of lipoprotein particle subfractions for their association with angiographic progression of coronary artery disease. Atherosclerosis 2014, 233, 713–720. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef] [PubMed]
- Needham, L.L.; Smy, L.; Lee, M.A.; Kunzler, T.M.; Genzen, J.R. Phlebotomy tube interference with nuclear magnetic resonance (NMR) lipoprotein subclass analysis. Clin. Chim. Acta 2019, 488, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Li, Y.K.; Bell, G.A.; Burt, A.A.; Vaisar, T.; Hutchins, P.M.; Furlong, C.E.; Otvos, J.D.; Polak, J.F.; Arnan, M.K.; et al. Concentration of Smaller High-Density Lipoprotein Particle (HDL-P) Is Inversely Correlated With Carotid Intima Media Thickening After Confounder Adjustment: The Multi Ethnic Study of Atherosclerosis (MESA). J. Am. Heart Assoc. 2016, 5, e002977. [Google Scholar] [CrossRef] [PubMed]
- Delatour, V.; Clouet-Foraison, N.; Gaie-Levrel, F.; Marcovina, S.M.; Hoofnagle, A.N.; Kuklenyik, Z.; Caulfield, M.P.; Otvos, J.D.; Krauss, R.M.; Kulkarni, K.R.; et al. Comparability of Lipoprotein Particle Number Concentrations Across ES-DMA, NMR, LC-MS/MS, Immunonephelometry, and VAP: In Search of a Candidate Reference Measurement Procedure for apoB and non-HDL-P Standardization. Clin. Chem. 2018, 64, 1485–1495. [Google Scholar] [CrossRef]
- Matera, R.; Horvath, K.V.; Nair, H.; Schaefer, E.J.; Asztalos, B.F. HDL Particle Measurement: Comparison of 5 Methods. Clin. Chem. 2018, 64, 492–500. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) joint consensus initiative. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 2016, 37, 1944–1958. [Google Scholar]
- Metzner, T.; Leitner, D.R.; Mellitzer, K.; Beck, A.; Sourij, H.; Stojakovic, T.; Reishofer, G.; März, W.; Landmesser, U.; Scharnagl, H.; et al. Effects of Alirocumab on Triglyceride Metabolism: A Fat-Tolerance Test and Nuclear Magnetic Resonance Spectroscopy Study. Biomedicines 2022, 10, 193. [Google Scholar] [CrossRef]
- Remaley, A.T.; Otvos, J.D. Methodological issues egarding: “A third of nonfasting plasma cholesterol is in remnant lipoproteins: Lipoprotein subclass profiling in 9293 individuals”. Atherosclerosis 2020, 302, 55–56. [Google Scholar] [CrossRef]
- Balling, M.; Langsted, A.; Afzal, S.; Varbo, A.; Davey Smith, G.; Nordestgaard, B.G. A third of nonfasting plasma cholesterol is in remnant lipoproteins: Lipoprotein subclass profiling in 9293 individuals. Atherosclerosis 2019, 286, 97–104. [Google Scholar] [CrossRef]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Gaudet, D.; Brisson, D.; Tremblay, K.; Alexander, V.J.; Singleton, W.; Hughes, S.G.; Geary, R.S.; Baker, B.F.; Graham, M.J.; Crooke, R.M.; et al. Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 2014, 371, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
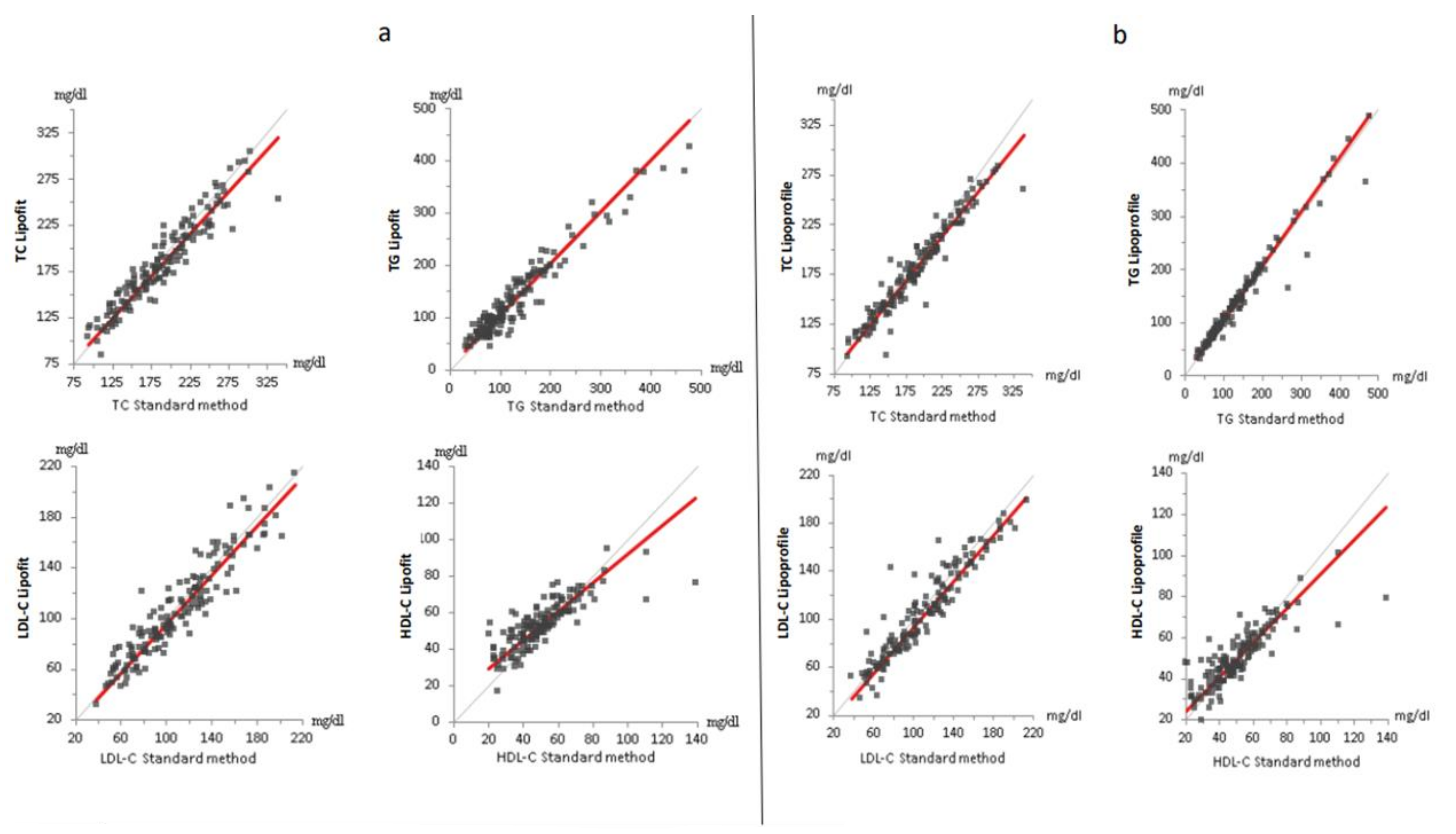
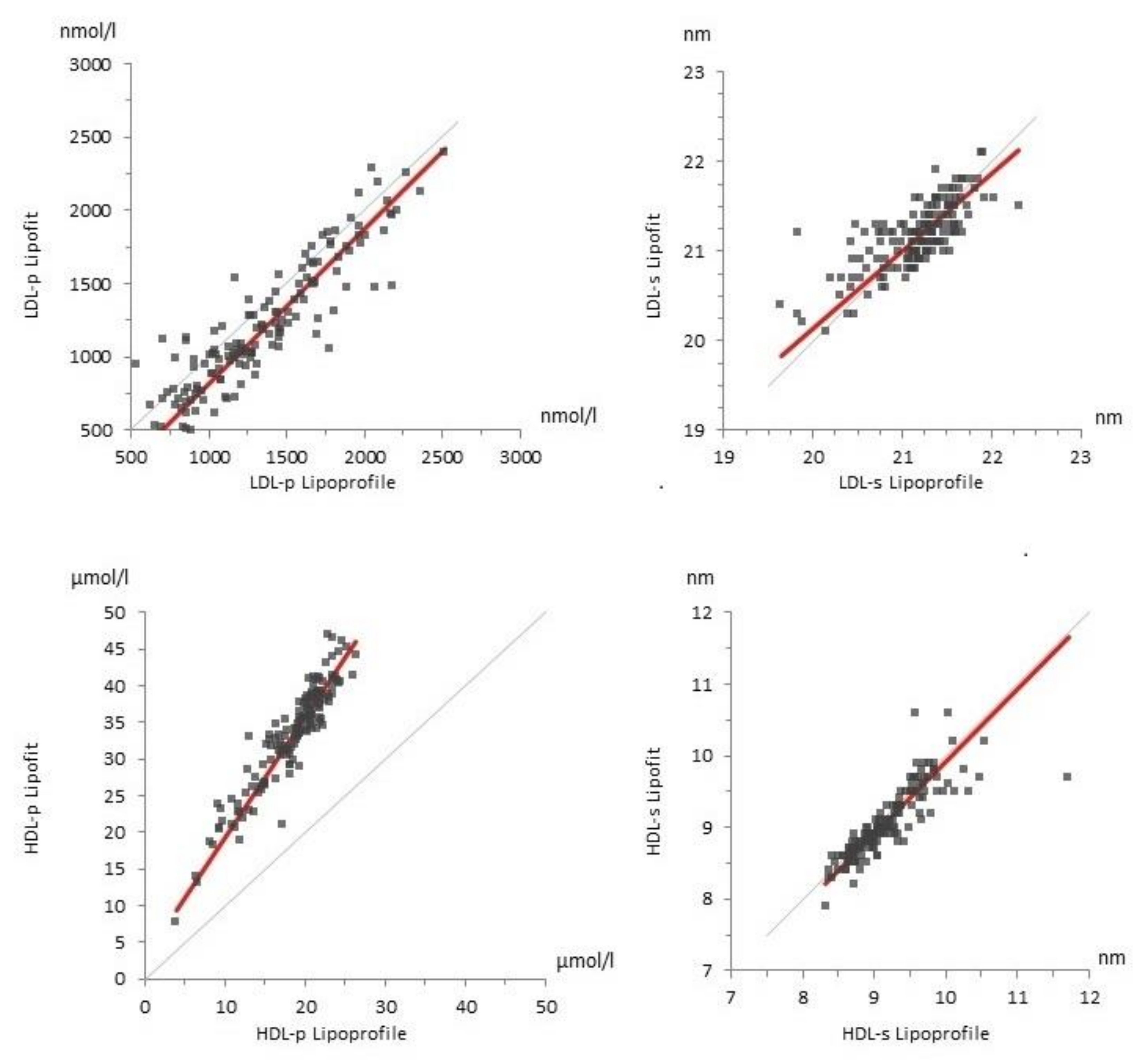
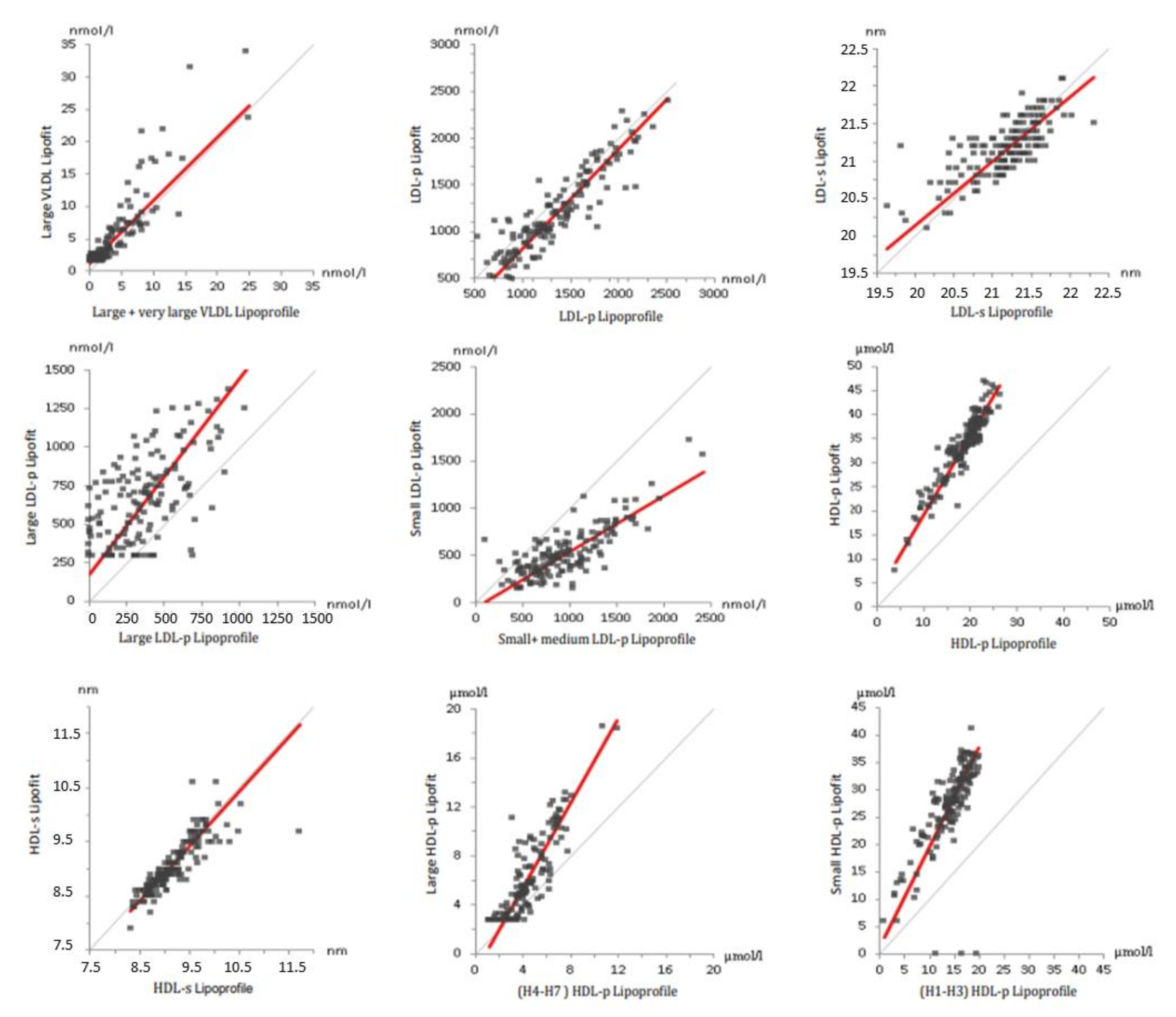
| Parameter | Units | Lipoprofile | Lipofit | Standard Method |
|---|---|---|---|---|
| TC | mg/dL | 180 (±45) | 183 (±46) | 188 (±51) |
| TG | mg/dL | 135 (±81) | 135 (±78) | 132 (±84) |
| LDL-C | mg/dL | 105 (±37) | 107 (±38) | 110 (±38) |
| HDL-C | mg/dL | 50.1 (±13.2) | 53.5 (±12.7) | 50.7 (±17.7) |
| LVLDL-p | nmol/L | 3.42 (±4.18) a | 4.94 (±5.6) | |
| LDL-p | nmol/L | 1330 (±444) | 1176 (±465) | |
| LDL-size | nm | 21,1 (±0,56) | 21.2 (±0.37) | |
| LLDL-p | nmol/L | 377 (±241) | 677 (±289) | |
| SLDL-p | nmol/L | 953 (±407) b | 528 (±270) | |
| HDL-p | µmol/L | 18.4 (±4.4) | 33.2 (±7.1) | |
| HDL-size | nm | 9.19 (±0.51) | 9.10 (±0.47) | |
| LHDL-p | µmol/L | 4.37 (±1.87) c | 6.08 (±3.31) | |
| SHDL-p | µmol/L | 14.0 (±4.0) d | 26.8 (±7.6) |
| Lipofit | LVLDL-p | p | LDL-p | p | LLDL-p a | p | SLDL-p a | p | HDL-p | p | LHDL-p | p | SHDL-p b | p | TC | p | TG | p | HDL-C | p | LDL-C | p | LDLsize | p | HDLsize | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVLDL-p | 1 | 0.34 | <0.001 | −0.01 | 0.928 | 0.56 | <0.001 | 0.07 | 0.365 | −0.29 | <0.001 | 0.23 | 0.005 | 0.15 | 0.07 | 0.9 | <0.001 | −0.28 | <0.001 | 0.05 | 0.582 | −0.47 | <0.001 | −0.34 | <0.001 | |
| LDL-p | 0.34 | <0.001 | 1 | 0.8 | <0.001 | 0.74 | <0.001 | 0.32 | <0.001 | −0.15 | 0.069 | 0.42 | <0.001 | 0.87 | <0.001 | 0.54 | <0.001 | 0.06 | 0.485 | 0.91 | <0.001 | −0.11 | 0.188 | −0.34 | <0.001 | |
| LLDL-p a | −0.01 | 0.928 | 0.8 | <0.001 | 1 | 0.21 | <0.011 | 0.42 | <0.001 | 0.26 | <0.002 | 0.31 | <0.001 | 0.85 | <0.001 | 0.18 | 0.029 | 0.41 | <0.001 | 0.83 | <0.001 | 0.35 | <0.001 | 0.04 | 0.634 | |
| SLDL-p a | 0.56 | <0.001 | 0.74 | <0.001 | 0.21 | 0.011 | 1 | 0.02 | 0.859 | −0.61 | <0.001 | 0.36 | <0.001 | 0.49 | <0.001 | 0.69 | <0.001 | −0.4 | <0.001 | 0.56 | <0.001 | −0.56 | <0.001 | −0.69 | <0.001 | |
| HDL-p | 0.07 | 0.365 | 0.32 | <0.001 | 0.42 | <0.001 | 0.02 | 0.859 | 1 | 0.3 | <0.001 | 0.86 | <0.001 | 0.49 | <0.001 | 0.11 | 0.17 | 0.7 | <0.001 | 0.32 | <0.001 | 0.04 | 0.661 | −0.01 | 0.877 | |
| LHDL-p | −0.29 | <0.001 | −0.15 | 0.069 | 0.26 | 0.002 | −0.61 | <0.001 | 0.3 | <0.001 | 1 | −0.22 | 0.009 | 0.15 | 0.069 | −0.38 | <0.001 | 0.81 | <0.001 | −0.02 | 0.841 | 0.51 | <0.001 | 0.92 | <0.001 | |
| SHDL-p b | 0.23 | 0.005 | 0.42 | <0.001 | 0.31 | <0.001 | 0.36 | <0.001 | 0.86 | <0.001 | −0.22 | 0.009 | 1 | 0.42 | <0.001 | 0.33 | <0.001 | 0.29 | <0.001 | 0.35 | <0.001 | −0.23 | 0.005 | −0.52 | <0.001 | |
| TC | 0.15 | 0.07 | 0.87 | <0.001 | 0.85 | <0.001 | 0.49 | <0.001 | 0.49 | <0.001 | 0.15 | 0.069 | 0.42 | <0.001 | 1 | 0.36 | <0.001 | 0.44 | <0.001 | 0.96 | <0.001 | 0.25 | 0.003 | −0.03 | 0.717 | |
| TG | 0.9 | <0.001 | 0.54 | <0.001 | 0.18 | 0.029 | 0.69 | <0.001 | 0.11 | 0.17 | −0.38 | <0.001 | 0.33 | <0.001 | 0.36 | <0.001 | 1 | −0.29 | <0.001 | 0.28 | <0.001 | −0.42 | <0.001 | −0.5 | <0.001 | |
| HDL-C | −0.28 | <0.001 | 0.06 | 0.485 | 0.41 | <0.001 | −0.4 | <0.001 | 0.7 | <0.001 | 0.81 | <0.001 | 0.29 | <0.001 | 0.44 | <0.001 | −0.29 | <0.001 | 1 | 0.25 | 0.002 | 0.51 | <0.001 | 0.64 | <0.001 | |
| LDL-C | 0.05 | 0.582 | 0.91 | <0.001 | 0.83 | <0.001 | 0.56 | <0.001 | 0.32 | <0.001 | −0.2 | 0.841 | 0.35 | <0.001 | 0.96 | <0.001 | 0.28 | <0.001 | 0.25 | 0.002 | 1 | 0.19 | 0.021 | −0.16 | 0.053 | |
| LDL size | −0.47 | <0.001 | −0.11 | 0.188 | 0.35 | <0.001 | −0.56 | <0.001 | 0.04 | 0.661 | 0.51 | <0.001 | −0.23 | 0.005 | 0.25 | 0.003 | −0.42 | <0.001 | 0.51 | <0.001 | 0.19 | 0.021 | 1 | 0.56 | <0.001 | |
| HDL size | −0.36 | 0.1 | −0.34 | <0.001 | 0.04 | 0.634 | −0.69 | <0.001 | −0.01 | 0.877 | 0.92 | <0.001 | −0.52 | <0.001 | −0.03 | 0.717 | −0.52 | <0.001 | 0.65 | <0.001 | −0.16 | 0.034 | 0.56 | <0.001 | 1 |
| Lipoprofile | LVLDL-p | p | LDL-p | p | LLDL-p | p | SLDL-p | p | HDL-p | p | LHDL-p | p | SHDL-p | p | TC | p | TG | p | HDL-C | p | LDL-C | p | LDL-s | p | HDL-s | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVLDL-p | 1 | 0.18 | 0.031 | −0.35 | <0.001 | 0.4 | <0.001 | 0.09 | 0.267 | −0.35 | <0.001 | 0.23 | 0.003 | 0.2 | 0.016 | 0.81 | <0.001 | −0.32 | <0.001 | 0.06 | 0.451 | −0.46 | <0.001 | −0.42 | <0.001 | |
| LDL-p | 0.18 | 0.031 | 1 | 0.42 | <0.001 | 0.31 | <0.001 | 0.3 | <0.001 | −0.12 | 0.14 | 0.3 | <0.001 | 0.9 | <0.001 | 0.35 | <0.001 | 0.07 | 0.368 | 0.92 | <0.001 | 0.08 | 0.345 | −0.32 | <0.001 | |
| LLDL-p | −0.35 | <0.001 | 0.42 | <0.001 | 1 | −0.13 | 0.106 | 0.01 | 0.902 | 0.25 | 0.002 | −0.11 | 0.198 | 0.52 | <0.001 | −0.28 | 0.001 | 0.32 | <0.001 | 0.61 | <0.001 | 0.79 | <0.001 | 0.3 | <0.001 | |
| SLDL-p | 0.4 | <0.001 | 0.31 | <0.001 | −0.13 | 0.11 | 1 | 0.33 | <0.001 | −0.26 | 0.001 | 0.48 | <0.001 | 0.67 | <0.001 | 0.54 | <0.001 | −0.11 | 0.198 | 0.64 | <0.001 | −0.67 | <0.001 | −0.29 | <0.001 | |
| HDL-p | 0.09 | 0.267 | 0.3 | <0.001 | 0.01 | 0.902 | 0.33 | <0.001 | 1 | 0.41 | <0.001 | 0.91 | <0.001 | 0.48 | <0.001 | 0.07 | 0.375 | 0.64 | <0.001 | 0.32 | <0.001 | 0.05 | 0.527 | −0.35 | <0.001 | |
| LHDL-p | −0.35 | <0.001 | −0.12 | 0.14 | 0.25 | 0.002 | −0.26 | 0.001 | 0.41 | <0.001 | 1 | −0.02 | 0.812 | 0.14 | 0.091 | −0.31 | <0.001 | 0.86 | <0.001 | −0.02 | 0.799 | 0.32 | <0.001 | 0.73 | <0.001 | |
| SHDL-p | 0.23 | 0.003 | 0.3 | <0.001 | −0.11 | 0.198 | 0.48 | <0.001 | 0.91 | <0.001 | −0.02 | 0.812 | 1 | 0.46 | <0.001 | 0.22 | 0.006 | 0.3 | <0.001 | 0.36 | <0.001 | −0.13 | 0.122 | −0.6 | <0.001 | |
| TC | 0.2 | 0.016 | 0.9 | <0.001 | 0.52 | <0.001 | 0.67 | <0.001 | 0.48 | <0.001 | 0.14 | 0.091 | 0.46 | <0.001 | 1 | 0.35 | <0.001 | 0.34 | <0.001 | 0.95 | <0.001 | 0.25 | 0.002 | −0.16 | 0.048 | |
| TG | 0.81 | <0.001 | 0.35 | <0.001 | −0.28 | 0.001 | 0.54 | <0.001 | 0.07 | 0.375 | −0.31 | <0.001 | 0.22 | 0.006 | 0.35 | <0.001 | 1 | −0.35 | <0.001 | 0.19 | 0.021 | −0.5 | <0.001 | −0.46 | <0.001 | |
| HDL-C | −0.32 | <0.001 | 0.07 | 0.368 | 0.32 | <0.001 | −0.11 | 0.198 | 0.64 | <0.001 | 0.86 | <0.001 | 0.3 | <0.001 | 0.34 | <0.001 | −0.35 | <0.001 | 1 | 0.18 | 0.026 | 0.38 | <0.001 | 0.4 | <0.001 | |
| LDL-C | 0.06 | 0.451 | 0.92 | <0.001 | 0.61 | <0.001 | 0.64 | <0.001 | 0.32 | <0.001 | −0.02 | 0.799 | 0.36 | <0.001 | 0.95 | <0.001 | 0.19 | 0.021 | 0.18 | 0.026 | 1 | 0.34 | <0.001 | −0.17 | 0.039 | |
| LDL-s | −0.46 | <0.001 | 0.08 | 0.345 | 0.79 | <0.001 | −0.67 | <0.001 | 0.05 | 0.527 | 0.32 | <0.001 | −0.13 | 0.122 | 0.25 | 0.002 | −0.5 | <0.001 | 0.38 | <0.001 | 0.34 | <0.001 | 1 | 0.38 | <0.001 | |
| HDL-s | −0.42 | <0.001 | −0.32 | <0.001 | 0.3 | <0.001 | −0.29 | <0.001 | −0.35 | <0.001 | 0.73 | <0.001 | −0.6 | <0.001 | −0.16 | 0.048 | −0.46 | <0.001 | 0.4 | <0.001 | −0.17 | 0.039 | 0.38 | <0.001 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rief, M.; Raggam, R.; Rief, P.; Metnitz, P.; Stojakovic, T.; Reinthaler, M.; Brodmann, M.; März, W.; Scharnagl, H.; Silbernagel, G. Comparison of Two Nuclear Magnetic Resonance Spectroscopy Methods for the Measurement of Lipoprotein Particle Concentrations. Biomedicines 2022, 10, 1766. https://doi.org/10.3390/biomedicines10071766
Rief M, Raggam R, Rief P, Metnitz P, Stojakovic T, Reinthaler M, Brodmann M, März W, Scharnagl H, Silbernagel G. Comparison of Two Nuclear Magnetic Resonance Spectroscopy Methods for the Measurement of Lipoprotein Particle Concentrations. Biomedicines. 2022; 10(7):1766. https://doi.org/10.3390/biomedicines10071766
Chicago/Turabian StyleRief, Martin, Reinhard Raggam, Peter Rief, Philipp Metnitz, Tatjana Stojakovic, Markus Reinthaler, Marianne Brodmann, Winfried März, Hubert Scharnagl, and Günther Silbernagel. 2022. "Comparison of Two Nuclear Magnetic Resonance Spectroscopy Methods for the Measurement of Lipoprotein Particle Concentrations" Biomedicines 10, no. 7: 1766. https://doi.org/10.3390/biomedicines10071766
APA StyleRief, M., Raggam, R., Rief, P., Metnitz, P., Stojakovic, T., Reinthaler, M., Brodmann, M., März, W., Scharnagl, H., & Silbernagel, G. (2022). Comparison of Two Nuclear Magnetic Resonance Spectroscopy Methods for the Measurement of Lipoprotein Particle Concentrations. Biomedicines, 10(7), 1766. https://doi.org/10.3390/biomedicines10071766







