The Effect of Diosmin, Escin, and Bromelain on Human Endothelial Cells Derived from the Umbilical Vein and the Varicose Vein—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. The Cytotoxicity Test
2.4. Cell Treatment Conditions
2.5. Measurement of Reactive Oxygen and Nitrogen Species
2.6. Measurement of Membrane Fluidity
2.7. Statistical Analysis
3. Results
3.1. Cytotoxicity of the Investigated Compounds
3.2. Reactive Oxygen and Nitrogen Species
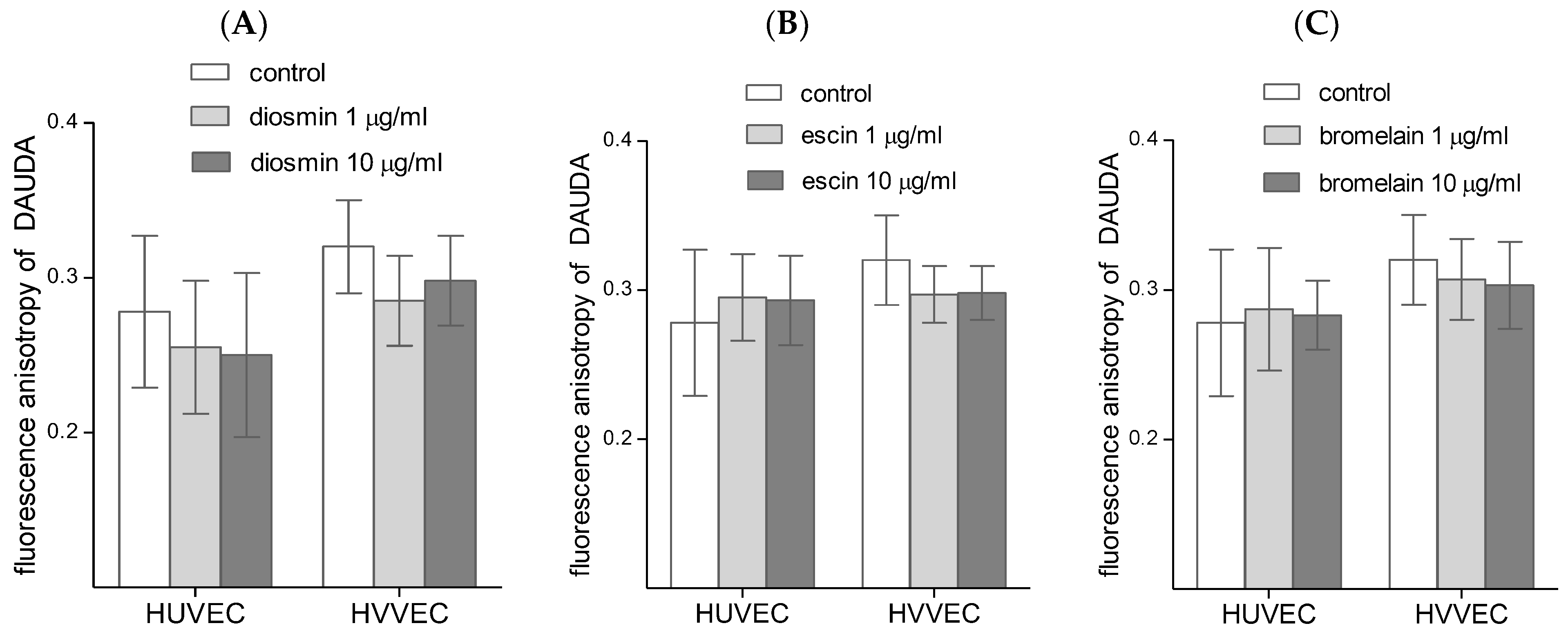
3.3. Cell Membrane Fluidity
4. Discussion
4.1. Endothelial Cells Viability
4.2. Reactive Oxygen and Nitrogen Species Generation
4.3. Cell Membrane Fluidity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-DS | 5-doxylstearic acid |
| AChE | acetylcholinesterase |
| CAT | catalase |
| CVD | chronic venous disease |
| CVI | chronic venous insufficiency |
| DAF-FM | diaminofluorescein-FM |
| DAUDA | 11-(dansylamino)undecanoic acid |
| DHEt | dihydroethidium |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DVT | deep venous thrombosis |
| ECM | extracellular matrix |
| ED | endothelial dysfunction |
| EDTA | ethylenediaminetetraacetic acid sodium salt |
| ERK | extracellular signal-regulated kinase |
| FRAP | ferric reducing activity power |
| GSH | glutathione |
| H2DCFDA | 2′,7′-dichlorofluorescin diacetate |
| HIF | hypoxia inducible factors |
| HUVEC | human umbilical vein endothelial cells |
| HVVEC | human varicose vein endothelial cells |
| ICAM | intercellular adhesion molecules |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MMP | matrix metalloproteinase |
| MPO | myeloperoxidase |
| O2•− | superoxide |
| PBS | phosphate buffered saline |
| PMN | polymorphonuclear cells |
| RBC | Red blood cell (erythrocyte) |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid reactive substances |
| TMA-DPH | 1-(4-(trimethylamino)phenyl)-6-phenylhexa-1,3,5-triene |
| VCAM | vascular cell adhesion molecules |
| VEGF | vascular endothelial growth factor |
| VV | varicose veins |
| XD | xanthine dehydrogenase |
| XO | xanthine oxidase |
| XOR | xanthine oxidoreductase |
References
- Gawas, M.; Bains, A.; Janghu, S.; Kamat, P.; Chawla, P. A Comprehensive Review on Varicose Veins: Preventive Measures and Different Treatments. J. Am. Coll. Nutr. 2022, 41, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Lumley, E.; Phillips, P.; Aber, A.; Buckley-Woods, H.; Jones, G.L.; Michaels, J.A. Experiences of living with varicose veins: A systematic review of qualitative research. J. Clin. Nurs. 2019, 28, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.A.; Evans, C.J.; Lee, A.J.; Allan, P.L.; Ruckley, C.V.; Fowkes, F.G.R. Incidence and risk factors for venous reflux in the general population: Edinburgh Vein Study. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr. Vasc. Pharmacol. 2008, 6, 158–172. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Gohel, M.S.; Shepherd, A.C.; Paleolog, E.; Davies, A.H. Venous hypoxia: A poorly studied etiological factor of varicose veins. J. Vasc. Res. 2011, 48, 185–194. [Google Scholar] [CrossRef]
- Terada, L.S.; Guidot, D.M.; Leff, J.A.; Willingham, I.R.; Hanley, M.E.; Piermattei, D.; Repine, J.E. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc. Natl. Acad. Sci. USA 1992, 89, 3362–3366. [Google Scholar] [CrossRef] [Green Version]
- Krzyściak, W.; Kózka, M. Generation of reactive oxygen species by a sufficient, insufficient and varicose vein wall. Acta Biochim. Pol. 2011, 58, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Horecka, A.; Biernacka, J.; Hordyjewska, A.; Dąbrowski, W.; Terlecki, P.; Zubilewicz, T.; Musik, I.; Kurzepa, J. Antioxidative mechanism in the course of varicose veins. Phlebology 2018, 33, 464–469. [Google Scholar] [CrossRef]
- Saribal, D.; Kanber, E.M.; Hocaoglu-Emre, F.S.; Akyolcu, M.C. Effects of the oxidative stress and genetic changes in varicose vein patients. Phlebology 2019, 34, 406–413. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Shraddha; Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef] [PubMed]
- Chisci, G.; Fredianelli, L. Therapeutic Efficacy of Bromelain in Alveolar Ridge Preservation. Antibiotics 2022, 11, 1542. [Google Scholar] [CrossRef]
- Dudek-Makuch, M.; Studzińska-Sroka, E. Horse chestnut—Efficacy and safety in chronic venous insufficiency: An overview. Rev. Bras. Farmacogn. 2015, 25, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Youn, Y.J.; Lee, J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean J. Intern. Med. 2019, 34, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Pietrzycka, A.; Kózka, M.; Urbanek, T.; Stpniewski, M.; Kucharzewski, M. Effect of Micronized Purified Flavonoid Fraction Therapy on Endothelin-1 and TNF-α Levels in Relation to Antioxidant Enzyme Balance in the Peripheral Blood of Women with Varicose Veins. Curr. Vasc. Pharmacol. 2015, 13, 801–808. [Google Scholar] [CrossRef]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef]
- Fields, R.D.; Lancaster, M.V. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 1993, 11, 48–50. [Google Scholar]
- Owusu-Ansah, E.; Yavari, A.; Banerjee, U. A protocol for in vivo detection of reactive oxygen species. Protocol Exchange 2008, 1–7. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Fales, H.M.; Sokoloski, E.A.; Levine, R.L.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 2005, 102, 5727–5732. [Google Scholar] [CrossRef] [Green Version]
- Balcerczyk, A.; Soszynski, M.; Bartosz, G. On the specificity of 4-amino-5-methylamino-2’,7’-difluorofluorescein as a probe for nitric oxide. Free Radic. Biol. Med. 2005, 39, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Matczak, K.; Koceva-Chyła, A.; Gwoździński, K.; Jóźwiak, Z. Doxorubicin and paclitaxel cause different changes in plasma membrane fluidity of MCF-7 breast cancer cells. Post. Biol. Kom. 2009, 25, 135–152. [Google Scholar]
- Pasternak, K.; Wróbel, D.; Nowacka, O.; Pieszyński, I.; Bryszewska, M.; Kujawa, J. The effect of MLS laser radiation on cell lipid membrane. Ann. Agric. Environ. Med. 2018, 25, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.A.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jantet, G. Chronic venous insufficiency: Worldwide results of the RELIEF study. Reflux assessment and quality of life improvement with micronized flavonoids. Angiology 2002, 53, 245–256. [Google Scholar] [CrossRef]
- Ligi, D.; Croce, L.; Mannello, F. Chronic Venous Disorders: The Dangerous, the Good, and the Diverse. Int. J. Mol. Sci. 2018, 19, 2544. [Google Scholar] [CrossRef] [Green Version]
- Takase, S.; Pascarella, L.; Bergan, J.J.; Schmid-Schönbein, G.W. Hypertension-induced venous valve remodeling. J. Vasc. Surg. 2004, 39, 1329–1334. [Google Scholar] [CrossRef] [Green Version]
- Tiwary, S.K.; Kumar, A.; Mishra, S.P.; Kumar, P.; Khanna, A.K. Study of association of varicose veins and inflammation by inflammatory markers. Phlebology 2020, 35, 679–685. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxid. Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef] [Green Version]
- Berry, C.E.; Hare, J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef] [PubMed]
- Page, S.; Powell, D.; Benboubetra, M.; Stevens, C.R.; Blake, D.R.; Selase, F.; Wolstenholme, A.J.; Harrison, R. Xanthine oxidoreductase in human mammary epithelial cells: Activation in response to inflammatory cytokines. Biochim. Biophys. Acta (BBA) Gen. Subj. 1998, 1381, 191–202. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Takase, S.; Schmid-Schönbein, G.; Bergan, J.J. Leukocyte activation in patients with venous insufficiency. J. Vasc. Surg. 1999, 30, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Hua, S. Targeting sites of inflammation: Intercellular adhesion molecule-1 as a target for novel inflammatory therapies. Front. Pharmacol. 2013, 4, 127. [Google Scholar] [CrossRef] [Green Version]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Piazza, G. Varicose veins. Circulation 2014, 130, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Poredos, P.; Spirkoska, A.; Rucigaj, T.; Fareed, J.; Jezovnik, M.K. Do blood constituents in varicose veins differ from the systemic blood constituents? Eur. J. Vasc. Endovasc. Surg. 2015, 50, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Riva, N.; Donadini, M.P.; Ageno, W. Epidemiology and pathophysiology of venous thromboembolism: Similarities with atherothrombosis and the role of inflammation. Thromb. Haemost. 2015, 113, 1176–1183. [Google Scholar] [CrossRef]
- Chang, S.-L.; Huang, Y.-L.; Lee, M.-C.; Hu, S.; Hsiao, Y.-C.; Chang, S.-W.; Chang, C.J.; Chen, P.-C. Association of Varicose Veins With Incident Venous Thromboembolism and Peripheral Artery Disease. JAMA 2018, 319, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Wautier, J.-L.; Wautier, M.-P. Cellular and Molecular Aspects of Blood Cell-Endothelium Interactions in Vascular Disorders. Int. J. Mol. Sci. 2020, 21, 5315. [Google Scholar] [CrossRef]
- Liu, W.Y.; Liou, S.-S.; Hong, T.-Y.; Liu, I.-M. The Benefits of the Citrus Flavonoid Diosmin on Human Retinal Pigment Epithelial Cells under High-Glucose Conditions. Molecules 2017, 22, 2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batchvarov, I.V.; Batselova, M.G.; Damyanov, I.I. One-year diosmin therapy (600 mg) in patients with chronic venous insufficiency—Results and analysis. J. Clin. Biomed. Res. 2010, 3, 51–54. [Google Scholar]
- Feldo, M.; Woźniak, M.; Wójciak-Kosior, M.; Sowa, I.; Kot-Waśik, A.; Aszyk, J.; Bogucki, J.; Zubilewicz, T.; Bogucka-Kocka, A. Influence of Diosmin Treatment on the Level of Oxidative Stress Markers in Patients with Chronic Venous Insufficiency. Oxid. Med. Cell. Longev. 2018, 2018, 2561705. [Google Scholar] [CrossRef] [Green Version]
- Monsalve, B.; Concha-Meyer, A.; Palomo, I.; Fuentes, E. Mechanisms of Endothelial Protection by Natural Bioactive Compounds from Fruit and Vegetables. An. Acad. Bras. Cienc. 2017, 89, 615–633. [Google Scholar] [CrossRef]
- Oak, M.-H.; Auger, C.; Belcastro, E.; Park, S.-H.; Lee, H.-H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Senthamizhselvan, O.; Manivannan, J.; Silambarasan, T.; Raja, B. Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur. J. Pharmacol. 2014, 736, 131–137. [Google Scholar] [CrossRef]
- Mahgoub, S.; Sallam, A.O.; Sarhan, H.K.A.; Ammar, A.A.A.; Soror, S.H. Role of Diosmin in protection against the oxidative stress induced damage by gamma-radiation in Wistar albino rats. Regul. Toxicol. Pharmacol. 2020, 113, 104622. [Google Scholar] [CrossRef]
- Qiao, P.; Zhang, B.; Liu, X.; Xu, J.; Li, X. Effects of Escin on Oxidative Stress and Apoptosis of H9c2 Cells Induced by H2O2. Dis. Markers 2022, 2022, 7765353. [Google Scholar] [CrossRef]
- Cengiz, M.; Kutlu, H.M.; Peker Cengiz, B.; Ayhancı, A. Escin attenuates oxidative damage, apoptosis and lipid peroxidation in a model of cyclophosphamide-induced liver damage. Drug Chem. Toxicol. 2022, 45, 1180–1187. [Google Scholar] [CrossRef]
- Jebur, A.B.; El-Demerdash, F.M.; Kang, W. Bromelain from Ananas comosus stem attenuates oxidative toxicity and testicular dysfunction caused by aluminum in rats. J. Trace Elem. Med. Biol. 2020, 62, 126631. [Google Scholar] [CrossRef]
- Feldo, M.; Wójciak-Kosior, M.; Sowa, I.; Kocki, J.; Bogucki, J.; Zubilewicz, T.; Kęsik, J.; Bogucka-Kocka, A. Effect of Diosmin Administration in Patients with Chronic Venous Disorders on Selected Factors Affecting Angiogenesis. Molecules 2019, 24, 3316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddhan, R.; Manoharan, S. Diosmin reduces cell viability of A431 skin cancer cells through apoptotic induction. J. Cancer Res. Ther. 2017, 13, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, L. Daflon and the protection of venous valves. Phlebolymphology 2016, 23, 20–30. [Google Scholar]
- Wang, X.-H.; Xu, B.; Liu, J.-T.; Cui, J.-R. Effect of beta-escin sodium on endothelial cells proliferation, migration and apoptosis. Vascul. Pharmacol. 2008, 49, 158–165. [Google Scholar] [CrossRef]
- Domanski, D.; Zegrocka-Stendel, O.; Perzanowska, A.; Dutkiewicz, M.; Kowalewska, M.; Grabowska, I.; Maciejko, D.; Fogtman, A.; Dadlez, M.; Koziak, K. Molecular Mechanism for Cellular Response to β-Escin and Its Therapeutic Implications. PLoS ONE 2016, 11, e0164365. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Lao, Y.; Liu, Y.; Niu, J.; Xiao, Z.; Arulselvan, P.; Shen, J. Escin induces apoptosis in ovarian cancer cell line by triggering S-phase cell cycle arrest and p38 MAPK/ERK pathway inhibition. J. King Saud Univ. Sci. 2022, 34, 101644. [Google Scholar] [CrossRef]
- Gallelli, L. Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug Des. Devel. Ther. 2019, 13, 3425–3437. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Jiang, Z.; Ning, X.; Yu, X.; Xu, J.; Buzzacott, P.; Xu, W. Endothelia-Targeting Protection by Escin in Decompression Sickness Rats. Sci. Rep. 2017, 7, 41288. [Google Scholar] [CrossRef] [Green Version]
- Varinská, L.; Fáber, L.; Kello, M.; Petrovová, E.; Balážová, Ľ.; Solár, P.; Čoma, M.; Urdzík, P.; Mojžiš, J.; Švajdlenka, E.; et al. β-Escin Effectively Modulates HUVECS Proliferation and Tube Formation. Molecules 2018, 23, 197. [Google Scholar] [CrossRef] [Green Version]
- Çiftçi, G.A.; Işcan, A.; Kutlu, M. Escin reduces cell proliferation and induces apoptosis on glioma and lung adenocarcinoma cell lines. Cytotechnology 2015, 67, 893–904. [Google Scholar] [CrossRef] [Green Version]
- Insuan, O.; Janchai, P.; Thongchuai, B.; Chaiwongsa, R.; Khamchun, S.; Saoin, S.; Insuan, W.; Pothacharoen, P.; Apiwatanapiwat, W.; Boondaeng, A.; et al. Anti-Inflammatory Effect of Pineapple Rhizome Bromelain through Downregulation of the NF-κB- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells. Curr. Issues Mol. Biol. 2021, 43, 93–106. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.-T.; Park, H.-R.; Kim, J.-B. The potential use of bromelain as a natural oral medicine having anticarcinogenic activities. Food Sci. Nutr. 2019, 7, 1656–1667. [Google Scholar] [CrossRef] [Green Version]
- Zavadova, E.; Desser, L.; Mohr, T. Stimulation of reactive oxygen species production and cytotoxicity in human neutrophils in vitro and after oral administration of a polyenzyme preparation. Cancer Biother. 1995, 10, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Yu, W.; Liu, B.; Wang, Y.; Shao, J.; Wang, J.; Xia, K.; Liang, C.; Fang, W.; Zhou, C.; et al. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2017, 8, e3113. [Google Scholar] [CrossRef] [Green Version]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017, 265, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Siwak, J.; Rzeszutek, I.; Wnuk, M. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol. In Vitro 2015, 29, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-Y.; Cheng, C.-L.; Wang, S.-S.; Ho, H.-C.; Chiu, K.-Y.; Chen, C.-S.; Chen, C.-C.; Shiau, M.-Y.; Ou, Y.-C. Escin induces apoptosis in human renal cancer cells through G2/M arrest and reactive oxygen species-modulated mitochondrial pathways. Oncol. Rep. 2017, 37, 1002–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.-L.; Chao, W.-T.; Li, Y.-H.; Ou, Y.-C.; Wang, S.-S.; Chiu, K.-Y.; Yuan, S.-Y. Escin induces apoptosis in human bladder cancer cells: An in vitro and in vivo study. Eur. J. Pharmacol. 2018, 840, 79–88. [Google Scholar] [CrossRef]
- Gwozdzinski, L.; Bernasinska-Slomczewska, J.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Pieniazek, A. Diosmin and Bromelain Stimulate Glutathione and Total Thiols Production in Red Blood Cells. Molecules 2023, 28, 2291. [Google Scholar] [CrossRef] [PubMed]
- Metzig, C.; Grabowska, E.; Eckert, K.; Rehse, K.; Maurer, H.R. Bromelain proteases reduce human platelet aggregation in vitro, adhesion to bovine endothelial cells and thrombus formation in rat vessels in vivo. In Vivo 1999, 13, 7–12. [Google Scholar]
- Musfiroh, F.F.; Setiasih, S.; Handayani, S.; Hudiyono, S.; Ilyas, N.M. In Vivo antiplatelet activity aggregation assay of bromelain fractionate by ethanol from extract pineapple core (Ananas comosus [L.] merr.). IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 12017. [Google Scholar] [CrossRef]
- Azarkan, M.; González, M.M.; Esposito, R.C.; Errasti, M.E. Stem Bromelain Proteolytic Machinery: Study of the Effects of its Components on Fibrin (ogen) and Blood Coagulation. Protein Pept. Lett. 2020, 27, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Engwerda, C.R.; Andrew, D.; Ladhams, A.; Mynott, T.L. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell. Immunol. 2001, 210, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Engwerda, C.R.; Andrew, D.; Murphy, M.; Mynott, T.L. Bromelain activates murine macrophages and natural killer cells in vitro. Cell. Immunol. 2001, 210, 5–10. [Google Scholar] [CrossRef]
- Barth, H.; Guseo, A.; Klein, R. In vitro study on the immunological effect of bromelain and trypsin on mononuclear cells from humans. Eur. J. Med. Res. 2005, 10, 325–331. [Google Scholar] [PubMed]
- Onken, J.E.; Greer, P.K.; Calingaert, B.; Hale, L.P. Bromelain treatment decreases secretion of pro-inflammatory cytokines and chemokines by colon biopsies in vitro. Clin. Immunol. 2008, 126, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Hale, L.P.; Greer, P.K.; Sempowski, G.D. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin. Immunol. 2002, 104, 183–190. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A. Physical properties of lipid bilayer membranes: Relevance to membrane biological functions. Acta Biochim. Pol. 2000, 47, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.; Vaz, W.L.C. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Lande, M.B.; Donovan, J.M.; Zeidel, M.L. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J. Gen. Physiol. 1995, 106, 67–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guţu, M.; Rusu, V.; Ştefănescu, C. Fluiditatea membranară--parametru biofizic in relaţie cu procesele de transport membranare. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 153–162. [Google Scholar] [PubMed]
- Gwozdzinski, L.; Pieniazek, A.; Bernasinska, J.; Grabowski, M.; Kowalczyk, E.; Gwozdzinski, K. Erythrocytes properties in varicose veins patients. Microvasc. Res. 2017, 111, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gwozdzinski, L.; Pieniazek, A.; Bernasinska-Slomczewska, J.; Hikisz, P.; Gwozdzinski, K. Alterations in the Plasma and Red Blood Cell Properties in Patients with Varicose Vein: A Pilot Study. Cardiol. Res. Pract. 2021, 2021, 5569961. [Google Scholar] [CrossRef]
- Juliano, R.L.; Gagalang, E. The effect of membrane-fluidizing agents on the adhesion of CHO cells. J. Cell. Physiol. 1979, 98, 483–489. [Google Scholar] [CrossRef]
- Schaeffer, B.E.; Curtis, A.S. Effects on cell adhesion and membrane fluidity of changes in plasmalemmal lipids in mouse L929 cells. J. Cell Sci. 1977, 26, 47–55. [Google Scholar] [CrossRef]
- Salaita, K.; Nair, P.M.; Petit, R.S.; Neve, R.M.; Das, D.; Gray, J.W.; Groves, J.T. Restriction of receptor movement alters cellular response: Physical force sensing by EphA2. Science 2010, 327, 1380–1385. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Mao, H.; Joddar, B.; Umeki, N.; Sako, Y.; Wada, K.-I.; Nishioka, C.; Takahashi, E.; Wang, Y.; Ito, Y. The significance of membrane fluidity of feeder cell-derived substrates for maintenance of iPS cell stemness. Sci. Rep. 2015, 5, 11386. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, M.K.; Chi, E.Y.; Frey, S.L.; Cao, K.D.; Luther, L.M.; Lee, K.Y.C.; Majewski, J.; Kjaer, K. Ordered nanoclusters in lipid-cholesterol membranes. Phys. Rev. Lett. 2009, 103, 28103. [Google Scholar] [CrossRef]
- Zeisig, R.; Koklic, T.; Wiesner, B.; Fichtner, I.; Sentjurc, M. Increase in fluidity in the membrane of MT3 breast cancer cells correlates with enhanced cell adhesion in vitro and increased lung metastasis in NOD/SCID mice. Arch. Biochem. Biophys. 2007, 459, 98–106. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwozdzinski, L.; Bernasinska-Slomczewska, J.; Hikisz, P.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Pieniazek, A. The Effect of Diosmin, Escin, and Bromelain on Human Endothelial Cells Derived from the Umbilical Vein and the Varicose Vein—A Preliminary Study. Biomedicines 2023, 11, 1702. https://doi.org/10.3390/biomedicines11061702
Gwozdzinski L, Bernasinska-Slomczewska J, Hikisz P, Wiktorowska-Owczarek A, Kowalczyk E, Pieniazek A. The Effect of Diosmin, Escin, and Bromelain on Human Endothelial Cells Derived from the Umbilical Vein and the Varicose Vein—A Preliminary Study. Biomedicines. 2023; 11(6):1702. https://doi.org/10.3390/biomedicines11061702
Chicago/Turabian StyleGwozdzinski, Lukasz, Joanna Bernasinska-Slomczewska, Pawel Hikisz, Anna Wiktorowska-Owczarek, Edward Kowalczyk, and Anna Pieniazek. 2023. "The Effect of Diosmin, Escin, and Bromelain on Human Endothelial Cells Derived from the Umbilical Vein and the Varicose Vein—A Preliminary Study" Biomedicines 11, no. 6: 1702. https://doi.org/10.3390/biomedicines11061702
APA StyleGwozdzinski, L., Bernasinska-Slomczewska, J., Hikisz, P., Wiktorowska-Owczarek, A., Kowalczyk, E., & Pieniazek, A. (2023). The Effect of Diosmin, Escin, and Bromelain on Human Endothelial Cells Derived from the Umbilical Vein and the Varicose Vein—A Preliminary Study. Biomedicines, 11(6), 1702. https://doi.org/10.3390/biomedicines11061702





