Juvenile Dermatomyositis and Infantile Cerebral Palsy: Aicardi-Gouteres Syndrome, Type 5, with a Novel Mutation in SAMHD1—A Case Report
Abstract
1. Introduction
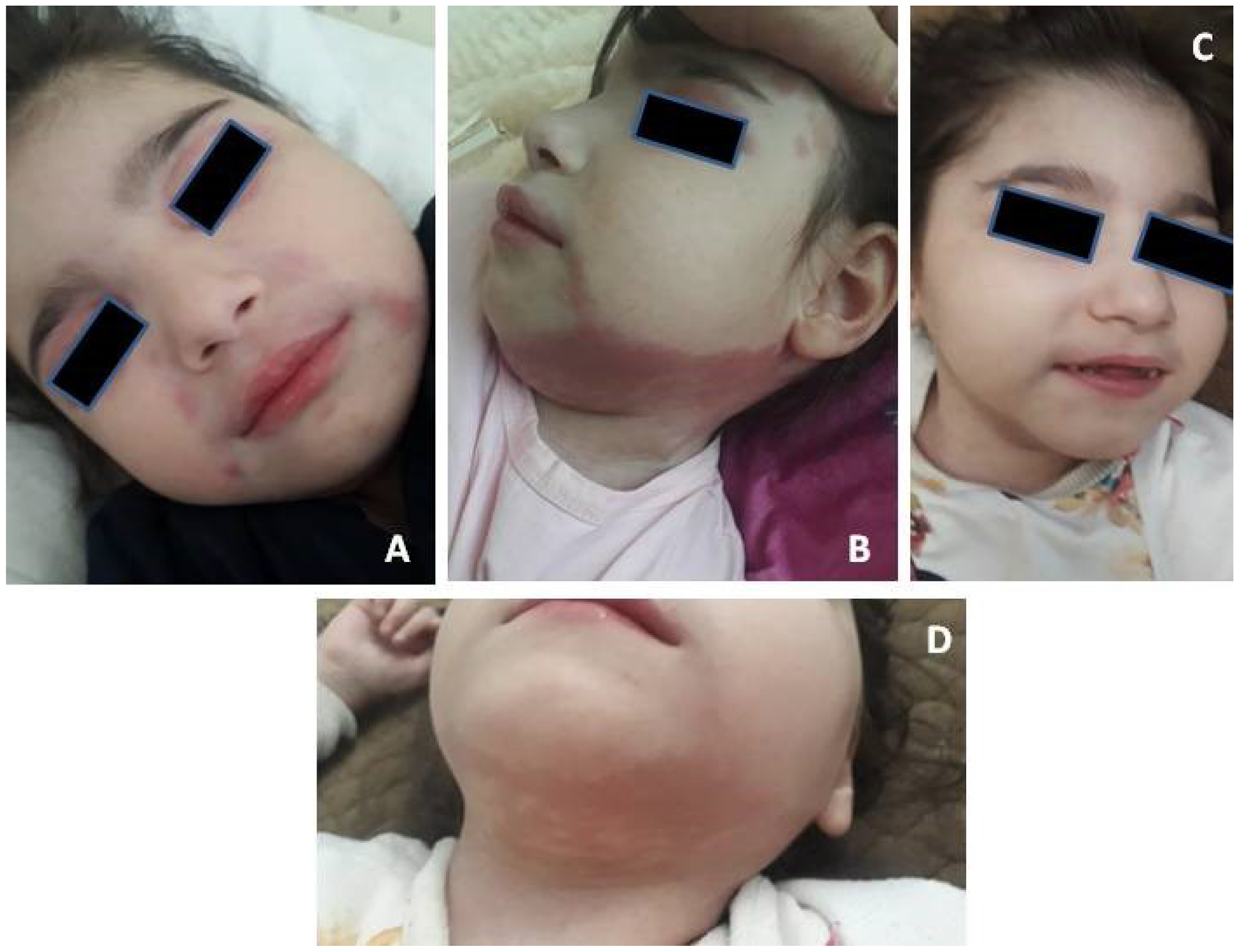
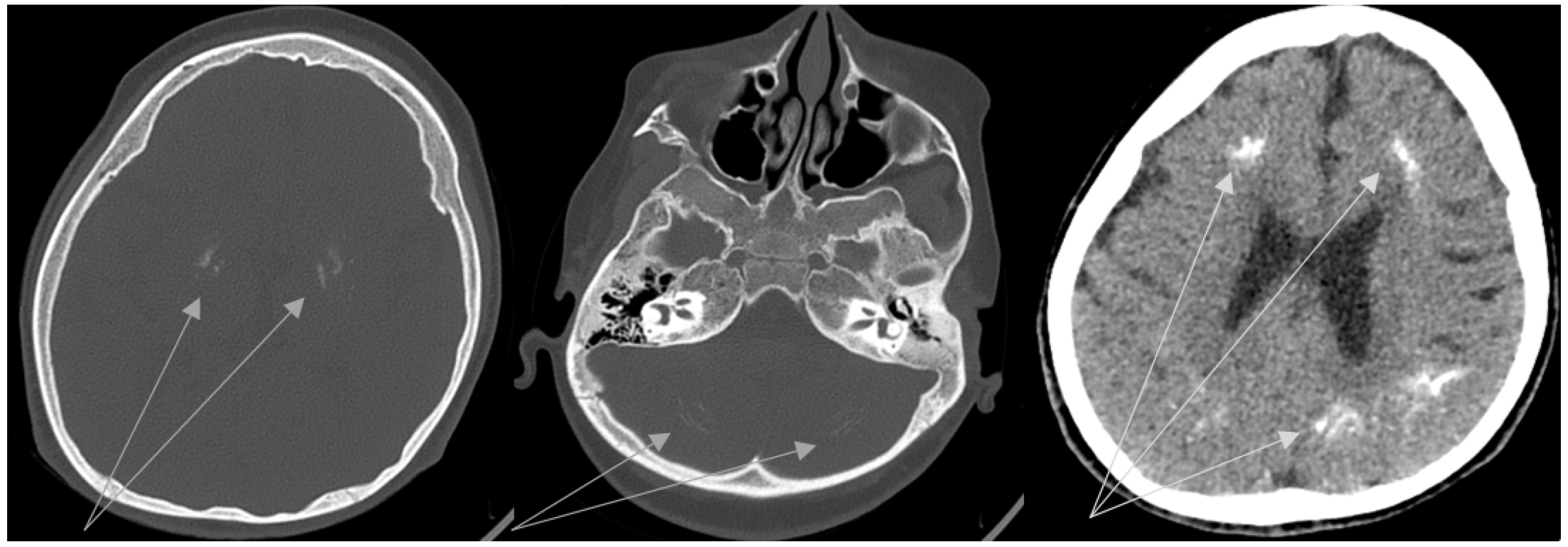
2. Case Description
3. Discussion
Treatment Strategy
4. Conclusions
- Early diagnosis of AGS is highly important to start treatment in a timely manner. Timely treatment in return can help to avoid the development/progression of end-organ damage, including severe neurological complications and early death.
- It is necessary to spread information about AGS among neurologists, neonatologists, infectious disease specialists, and pediatricians.
- Newborn screening for AGS seems to be a promising tool for early AGS diagnosis.
- A standardized treatment strategy should be developed according to AGS type and previous experience. A multi-disciplinary team is required to provide optimal care in the context of multiorgan system involvement.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shiari, R. Neurologic manifestations of childhood rheumatic diseases. Iran. J. Child Neurol. 2012, 6, 1–7. [Google Scholar] [PubMed]
- Crow, Y.; Livingston, J. Neurologic Phenotypes Associated with Mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi–Goutières Syndrome and Beyond. Neuropediatrics 2016, 47, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Uggenti, C.; Lepelley, A.; Depp, M.; Badrock, A.P.; Rodero, M.P.; El-Daher, M.-T.; Dhir, A.; Wheeler, A.P.; Dhir, S.; Albawardi, W.; et al. cGAS-mediated induction of type I interferon due to inborn errors of histone pre-mRNA processing. Nat. Genet. 2020, 52, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Aicardi, J.; Goutières, F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann. Neurol. 1984, 15, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Suspitsin, E.N.; Raupov, R.K.; Kuchinskaya, E.M.; Kostik, M.M. Analysis of interferon type I signature for differential diagnosis of diseases of the immune system (review of literature). Klin. Lab. Diagn. 2021, 66, 279–284. [Google Scholar] [CrossRef]
- Crow, Y.J.; Shetty, J.; Livingston, J.H. Treatments in Aicardi-Goutières syndrome. Dev. Med. Child Neurol. 2020, 62, 42–47. [Google Scholar] [CrossRef]
- Ramesh, V.; Abinun, M.; Mitchell, P.; Mitra, D.; Friswell, M.; Rice, G.L.; Gornall, H.; Crow, Y.J. P270 Moyamoya syndrome and peripheral vascular disease due to mutation in newly described Aicardi Goutieres syndrome 5 gene SAMHD1. Eur. J. Paediatr. Neurol. 2009, 13, S105. [Google Scholar] [CrossRef]
- Rice, G.I.; Bond, J.; Asipu, A.; Brunette, R.L.; Manfield, I.W.; Carr, I.M.; Fuller, J.C.; Jackson, R.M.; Lamb, T.; Briggs, T.A.; et al. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 2009, 41, 829–832. [Google Scholar] [CrossRef]
- Xin, B.; Jones, S.; Puffenberger, E.G.; Hinze, C.; Bright, A.; Tan, H.; Zhou, A.; Wu, G.; Vargus-Adams, J.; Agamanolis, D.; et al. Homozygous mutation in SAMHD1 gene causes cerebral vasculopathy and early onset stroke. Proc. Natl. Acad. Sci. USA 2011, 108, 5372–5377. [Google Scholar] [CrossRef]
- Coggins, S.A.; Mahboubi, B.; Schinazi, R.F.; Kim, B. SAMHD1 Functions and Human Diseases. Viruses 2020, 12, 382. [Google Scholar] [CrossRef]
- Roers, A.; Hiller, B.; Hornung, V. Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity 2016, 44, 739–754. [Google Scholar] [CrossRef]
- Schumann, T.; Ramon, S.C.; Schubert, N.; Mayo, M.A.; Hega, M.; Maser, K.I.; Ada, S.R.; Sydow, L.; Hajikazemi, M.; Badstübner, M.; et al. Deficiency for SAMHD1 activates MDA5 in a cGAS/STING-dependent manner. J. Exp. Med. 2023, 220, e20220829. [Google Scholar] [CrossRef]
- Lebon, P.; Badoual, J.; Ponsot, G.; Goutieres, F.; Hemeury-Cukier, F.; Aicardi, J. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J. Neurol. Sci. 1988, 84, 201–208. [Google Scholar] [CrossRef]
- Lebon, P.; Meritet, J.F.; Krivine, A.; Rozenberg, F. Interferon and Aicardi-Goutières syndrome. Eur. J. Paediatr. Neurol. 2002, 6, A47–A53; discussion A55–A58, A77–A86. [Google Scholar] [CrossRef]
- Senju, C.; Nakazawa, Y.; Shimada, M.; Iwata, D.; Matsuse, M.; Tanaka, K.; Miyazaki, Y.; Moriwaki, S.; Mitsutake, N.; Ogi, T. Aicardi-Goutières syndrome with SAMHD1 deficiency can be diagnosed by unscheduled DNA synthesis test. Front. Pediatr. 2022, 10, 1048002. [Google Scholar] [CrossRef]
- Armangue, T.; Orsini, J.J.; Takanohashi, A.; Gavazzi, F.; Conant, A.; Ulrick, N.; Morrissey, M.A.; Nahhas, N.; Helman, G.; Gordish-Dressman, H.; et al. Neonatal detection of Aicardi Goutières Syndrome by increased C26:0 lysophosphatidylcholine and interferon signature on newborn screening blood spots. Mol. Genet. Metab. 2017, 122, 134–139, Erratum in Mol. Genet. Metab. 2022, 136, 80. [Google Scholar] [CrossRef] [PubMed]
- Adang, L.A.; Gavazzi, F.; Jawad, A.F.; Cusack, S.V.; Kopin, K.; Peer, K.; Besnier, C.; De Simone, M.; De Giorgis, V.; Orcesi, S.; et al. Development of a neurologic severity scale for Aicardi Goutières Syndrome. Mol. Genet. Metab. 2020, 130, 153–160, Erratum in Mol. Genet. Metab. 2022, 136, 81. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.I.; Meyzer, C.; Bouazza, N.; Hully, M.; Boddaert, N.; Semeraro, M.; Zeef, L.A.; Rozenberg, F.; Bondet, V.; Duffy, D.; et al. Reverse-Transcriptase Inhibitors in the Aicardi–Goutières Syndrome. N. Engl. J. Med. 2018, 379, 2275–2277. [Google Scholar] [CrossRef] [PubMed]
- Crow, Y.J.; Rehwinkel, J. Aicardi-Goutieres syndrome and related phenotypes: Linking nucleic acid metabolism with autoimmunity. Hum. Mol. Genet. 2009, 18, R130–R136. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Arias, P.J.; Gómez-García, F.; Hernández-Parada, J.; Montilla-López, A.M.; Ruano, J.; Parra-Peralbo, E. Efficacy and Safety of Janus Kinase Inhibitors in Type I Interferon-Mediated Monogenic Autoinflammatory Disorders: A Scoping Review. Dermatol. Ther. 2021, 11, 733–750. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Yang, Y.; Miao, H.; Yang, L.; Liu, Y.; Zhang, X.; Liu, Y.; Wang, T. Type I interferonopathies with novel compound heterozygous TREX1 mutations in two siblings with different symptoms responded to tofacitinib. Pediatr. Rheumatol. 2021, 19, 1. [Google Scholar] [CrossRef]
- Briand, C.; Frémond, M.L.; Bessis, D.; Carbasse, A.; Rice, G.I.; Bondet, V.; Duffy, D.; Chatenoud, L.; Blanche, S.; Crow, Y.J.; et al. Efficacy of JAK1/2 inhibition in the treatment of chilblain lupus due to TREX1 deficiency. Ann. Rheum. Dis. 2019, 78, 431–433. [Google Scholar] [CrossRef]
- Vanderver, A.; Adang, L.; Gavazzi, F.; McDonald, K.; Helman, G.; Frank, D.B.; Jaffe, N.; Yum, S.W.; Collins, A.; Keller, S.R.; et al. Janus Kinase Inhibition in the Aicardi-Goutières Syndrome. N. Engl. J. Med. 2020, 383, 986–989. [Google Scholar] [CrossRef]
- Han, V.X.; Mohammad, S.S.; Jones, H.F.; Bandodkar, S.; Crow, Y.J.; Dale, R.C.; AGS-JAKi Study Group. Cerebrospinal fluid neopterin as a biomarker of treatment response to Janus kinase inhibition in Aicardi-Goutières syndrome. Dev. Med. Child Neurol. 2022, 64, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Cetin Gedik, K.; Lamot, L.; Romano, M.; Demirkaya, E.; Piskin, D.; Torreggiani, S.; Adang, L.A.; Armangue, T.; Barchus, K.; Cordova, D.R.; et al. The 2021 European Alliance of Associations for Rheumatology/American College of Rheumatology points to consider for diagnosis and management of autoinflammatory type I interferonopathies: CANDLE/PRAAS, SAVI and AGS. Ann. Rheum. Dis. 2022, 81, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.R.; Amanat, M.; Garshasbi, M.; Kameli, R.; Nilipour, Y.; Heidari, M.; Rezaei, Z.; Tavasoli, A.R. An update on clinical, pathological, diagnostic, and therapeutic perspectives of childhood leukodystrophies. Expert Rev. Neurother. 2020, 20, 65–84. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Patient | Normal Value |
|---|---|---|
| Hemoglobin, g/L | 110 | 120–160 |
| White blood cells, ×109/L | 8.1 | 4–9 |
| Platelets, ×109/L | 318 | 150–400 |
| Erythrocyte sedimentation rate, mm/h | 23 | 1–20 |
| Fibrinogen, g/L | 3.05 | 2.0–4.0 |
| Lupus anticoagulant * | 0.96 | 0.9–1.2 |
| International normalization ratio | 0.97 | 0.9–1.1 |
| Complement C3, g/L | 0.92 | 0.82–1.73 |
| Complement C4, g/L | 0.22 | 0.13–0.46 |
| Creatine kinase, U/L | 90 | 29–169 |
| Lactate dehydrogenase, U/L | 669 | 125–220 |
| Ferritin, ng/mL | 115 | 15–120 |
| Total protein, g/L | 69 | 60–80 |
| Gamma-globulins, g/L | 17.5 | 8.4–11.2 |
| Alanine aminotransferase, U/L | 51 | 0–55 |
| Aspartate aminotransferase, U/L | 107 | 5–34 |
| Creatinine, mmol/L | 0.047 | 0.027–0.062 |
| Urea, mmol/L | 6.6 | 2.5–6.0 |
| C-reactive protein, mg/L * | 1.9 | 0–5 |
| Antinuclear antibodies * | 1:160 | <1:160 |
| Antinuclear antibodies (nRNP/Sm, Sm, SS-A, Ro-52, SS-B, Scl-70, PM-Scl, Jo-1, CENP B, PCNA, dsDNA, Nucleosomes, Histones, Rib P-protein, AMA M2) * | Not detected | Not detected |
| Polymyositis antibodies (Mi-2, Ku, PM-Scl100, PM-Scl75, SRP, Jo-1, PL-7, PL-12, EJ, OJ, SSA) * | Not detected | Not detected |
| Interferon signature, Units | 24 | 0–2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokina, L.S.; Raupov, R.K.; Kostik, M.M. Juvenile Dermatomyositis and Infantile Cerebral Palsy: Aicardi-Gouteres Syndrome, Type 5, with a Novel Mutation in SAMHD1—A Case Report. Biomedicines 2023, 11, 1693. https://doi.org/10.3390/biomedicines11061693
Sorokina LS, Raupov RK, Kostik MM. Juvenile Dermatomyositis and Infantile Cerebral Palsy: Aicardi-Gouteres Syndrome, Type 5, with a Novel Mutation in SAMHD1—A Case Report. Biomedicines. 2023; 11(6):1693. https://doi.org/10.3390/biomedicines11061693
Chicago/Turabian StyleSorokina, Lubov S., Rinat K. Raupov, and Mikhail M. Kostik. 2023. "Juvenile Dermatomyositis and Infantile Cerebral Palsy: Aicardi-Gouteres Syndrome, Type 5, with a Novel Mutation in SAMHD1—A Case Report" Biomedicines 11, no. 6: 1693. https://doi.org/10.3390/biomedicines11061693
APA StyleSorokina, L. S., Raupov, R. K., & Kostik, M. M. (2023). Juvenile Dermatomyositis and Infantile Cerebral Palsy: Aicardi-Gouteres Syndrome, Type 5, with a Novel Mutation in SAMHD1—A Case Report. Biomedicines, 11(6), 1693. https://doi.org/10.3390/biomedicines11061693








