Auxological and Metabolic Parameters of Children Undergoing the Gonadotropin-Releasing Hormone Stimulation Test: Correlations with the Final Diagnosis of Central Precocious Puberty in a Single-Center Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Characteristics
2.2. Patient Selection
2.3. Characteristics of the GnRH Stimulation Test
2.4. Statistical Analysis
3. Results
3.1. Main Results
3.2. Multivariate Analysis
4. Discussion
5. Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latronico, A.C.; Brito, V.N.; Carel, J.C. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Berberoğlu, M. Precocious puberty and normal variant puberty: Definition, etiology, diagnosis and current management. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Eugster, E.A. Central Precocious Puberty: Update on Diagnosis and Treatment. Paediatr. Drugs 2015, 17, 273–281. [Google Scholar] [CrossRef]
- Percorso Diagnostico Terapeutico SIEDP—Pubertà Precoce Centrale. Available online: http://www.siedp.it/pagina/823/pdta+puberta%27+precoce+centrale (accessed on 1 March 2023).
- Neely, E.K.; Wilson, D.M.; Lee, P.A.; Stene, M.; Hintz, R.L. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J. Pediatr. 1995, 127, 47–52. [Google Scholar] [CrossRef]
- The Italian Data Protection Authority—Authorisation no. 9/2014—General Authorisation to Process Personal Data for Scientific Research Purposes. Available online: https://www.garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/3786078 (accessed on 1 March 2023).
- Cheuiche, A.V.; da Silveira, L.G.; de Paula, L.C.P.; Lucena, I.R.S.; Silveiro, S.P. Diagnosis and management of precocious sexual maturation: An updated review. Eur. J. Pediatr. 2021, 180, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.T.; Di Maio, S.; Soliman, N.; Elsedfy, H. Long-term effects and significant Adverse Drug Reactions (ADRs) associated with the use of Gonadotropin-Releasing Hormone analogs (GnRHa) for central precocious puberty: A brief review of literature. Acta Biomed. 2019, 90, 345–359. [Google Scholar] [CrossRef]
- Yeshaya, A.; Kauschansky, A.; Orvieto, R.; Varsano, I.; Nussinovitch, M.; Ben-Rafael, Z. Prolonged vaginal bleeding during central precocious puberty therapy with a long-acting gonadotropin-releasing hormone agonist. Acta Obstet. Gynecol. Scand. 1998, 77, 327–329. [Google Scholar]
- Carel, J.C.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Palmert, M.R.; ESPE-LWPES GnRH Analogs Consensus Conference Group; Antoniazzi, F.; Berenbaum, S.; Bourguignon, J.P.; Chrousos, G.P.; et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef]
- Turriziani Colonna, A.; Curatola, A.; Sodero, G.; Lazzareschi, I.; Cammisa, I.; Cipolla, C. Central precocious puberty in children after COVID-19 outbreak: A single-center retrospective study. Minerva Pediatr. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Miranda-Lora, A.L.; Torres-Tamayo, M.; Zurita-Cruz, J.N.; Aguilar-Herrera, B.E.; Calzada-León, R.; Rivera-Hernández, A.J.; Morales-Pérez, M.A.; Padrón-Martínez, M.M.; Ruiz-Reyes, M.L.; García-Morales, L.M.; et al. Diagnosis of precocious puberty: Clinical guideline for the diagnosis and treatment of precocious puberty. Bol. Med. Hosp. Infant. Mex. 2020, 77 (Suppl. 1), 7–14. [Google Scholar] [CrossRef]
- Yeh, S.N.; Ting, W.H.; Huang, C.Y.; Huang, S.K.; Lee, Y.C.; Chua, W.K.; Lin, C.H.; Cheng, B.W.; Lee, Y.J. Diagnostic evaluation of central precocious puberty in girls. Pediatr. Neonatol. 2021, 62, 187–194. [Google Scholar] [CrossRef]
- Kim, H.K.; Kee, S.J.; Seo, J.Y.; Yang, E.M.; Chae, H.J.; Kim, C.J. Gonadotropin-releasing hormone stimulation test for precocious puberty. Korean J. Lab. Med. 2011, 31, 244–249. [Google Scholar] [CrossRef]
- Sodero, G.; Mariani, F.; Caprarelli, M.; Agazzi, C.; Quarta, L.; Benacquista, L.; Rigante, D.; Cipolla, C. Growth hormone responses during arginine and clonidine stimulation test: Correlations with patients’ auxological and metabolic parameters in a single centre study. Growth Horm. IGF Res. 2022, 68, 101522. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.F.; Liang, J.F.; Zhou, X.L.; Prasad, H.C.; Jin, J.H.; Dong, G.P.; Rose, S.R. Impact of BMI on gonadorelin-stimulated LH peak in premenarcheal girls with idiopathic central precocious puberty. Obesity 2015, 23, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Yoon, J.S.; Hwang, J.S. Luteinizing hormone secretion during gonadotropin-releasing hormone stimulation tests in obese girls with central precocious puberty. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Liu, J.; Fu, P.; Zhou, Y.; Li, Z.; Liu, P. The diagnostic utility of the basal luteinizing hormone level and single 60-minute post GnRH agonist stimulation test for idiopathic central precocious puberty in girls. Front. Endocrinol. 2021, 12, 713880. [Google Scholar] [CrossRef]
- Zhou, X.L.; Fu, J.F.; Jin, J.H.; Dong, G.P.; Jiang, Y.J.; Huang, K.; Chen, X.F.; Wu, W. Effects of obesity on peak level of luteinizing hormone in gonadotropin-releasing hormone agonist test and obesity-related hormones in girls with central precocious puberty. Zhongguo Dang Dai Er Ke Za Zhi 2015, 17, 763–768. [Google Scholar]
- Sørensen, K.; Mouritsen, A.; Mogensen, S.S.; Aksglaede, L.; Juul, A. Insulin sensitivity and lipid profiles in girls with central precocious puberty before and during gonadal suppression. J. Clin. Endocrinol. Metab. 2010, 95, 3736–3744. [Google Scholar] [CrossRef]
- Arcari, A.J.; Freire, A.V.; Escobar, M.E.; Ballerini, M.G.; Ropelato, M.G.; Bergadá, I.; Gryngarten, M.G. One-year treatment with gonadotropin-releasing hormone analogues does not affect body mass index, insulin sensitivity or lipid profile in girls with central precocious puberty. J. Pediatr. Endocrinol. Metab. 2019, 32, 181–186. [Google Scholar] [CrossRef]
- Sørensen, K.; Aksglaede, L.; Petersen, J.H.; Andersson, A.M.; Juul, A. Serum IGF1 and insulin levels in girls with normal and precocious puberty. Eur. J. Endocrinol. 2012, 166, 903–910. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.H. Change in body mass index and insulin resistance after 1-year treatment with gonadotropin-releasing hormone agonists in girls with central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Oh, S.B.; Lee, W.Y.; Kim, H.R.; Nam, H.K.; Kim, J.H.; Rhie, Y.J.; Lee, K.H. Thyroid function in girls with central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Chung, L.Y.; Kang, E.; Nam, H.K.; Rhie, Y.J.; Lee, K.H. Serum levels of thyroid stimulating hormone and luteinizing hormone are decreased in girls with central precocious puberty after 12-month GnRH agonist treatment. Tohoku J. Exp. Med. 2020, 252, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Su, P.H.; Wang, S.L.; Chen, J.Y.; Chen, S.J.; Ke, J.C. A study of anthropomorphic and biochemical characteristics in girls with central precocious puberty and thelarche variant. J. Pediatr. Endocrinol. Metab. 2008, 21, 213–220. [Google Scholar] [CrossRef]
- Juul, A.; Scheike, T.; Nielsen, C.T.; Krabbe, S.; Müller, J.; Skakkebaek, N.E. Serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 levels are increased in central precocious puberty: Effects of two different treatment regimens with gonadotropin-releasing hormone agonists, without or in combination with an antiandrogen (cyproterone acetate). J. Clin. Endocrinol. Metab. 1995, 80, 3059–3067. [Google Scholar] [CrossRef]
| Study Population (N = 37) | |
|---|---|
| Females, n (%) | 35 (94.6) |
| Age (years) | 7.18 (2.38–7.72) |
| Height (cm) | 124 (88.00–131.35) |
| Z-score height | 0.5 (−1.33–1.18) |
| Weight (kg) | 26.65 (15.67) |
| Z-score weight | 0.42 (−1.41–1.34) |
| BMI (kg/m2) | 16.89 (14.88–19.53) |
| Z-score BMI | 0.3 (2.34) |
| Adrenarche, n (%) | 5 (13.5) |
| Familiar history of CPP, n (%) | 6 (16.2) |
| TSH (mU/L) | 1.90 (1.35–4.41) |
| fT3 (pg/mL) | 3.82 (1.39) |
| fT4 (ng/dL) | 12.14 (1.68) |
| Abnormal thyroid profile, n (%) | 10 (27.0) |
| Glucose (mg/dL) | 76.59 (7.15) |
| Hb (g/dL) | 12.99 (0.98) |
| Platelet count (×109/L) | 317.51 (84.08) |
| Abnormal blood count, n (%) | 6 (16.2) |
| GOT (IU/L) Increased GOT, n (%) | 17 (14.00–19.50) 2 (5.4) |
| Triglycerides (mg/dL) | 61 (48.5–73.5) |
| Cholesterol (mg/dL) | 155.35 (25.13) |
| LDL (mg/dL) | 89.11 (19.55) |
| HDL (mg/dL) | 52.76 (13.40) |
| Abnormal lipid profile, n (%) | 14 (37.8) |
| FSH (IU/mL) | 3.4 (1.35–5.95) |
| Increased baseline FSH, n (%) | 24 (64.9) |
| LH (mIU/mL) | 0.1 (0.1–0.45) |
| Increased baseline LH, n (%) | 5 (13.5) |
| SHBG (nmol/L) | 87 (57.50–119.50) |
| 17BE2 (pg/mL) | 19.00 (15.00–28.00) |
| Prolactin (ng/mL) | 7.6 (5.3–12.6) |
| Increased prolactin, n (%) | 13 (35.1) |
| Increased bone age ≥ 1, n (%) | 17 (45.9) |
| Pathological MRI *, n (%) | 3 (8.3) |
| Uterus length ≥ 3.6 cm **, n (%) | 6 (17.1) |
| Uterus antero–posterior diameter > 1 cm **, n (%) | 6 (17.1) |
| Uterine body/cervix ratio > 1, n (%) | 8 (22.9) |
| Ovarian volume ≥ 2 mL, n (%) | 15 (42.9) |
| LH peak | 3.4 (1.4–8.4) |
| FSH peak | 14.40 (8.55–23.45) |
| LH/FSH ratio | 0.11 (0.007–0.25) |
| LH/FSH ratio > 1, n (%) | 7 (18.9) |
| LH/FSH ratio > 0.6, n (%) | 7 (18.9) |
| CPP, n (%) | 18 (48.6) |
| CPP etiology #, n (%) Idiopathic Organic | |
| 16 (88.9) | |
| 2 (11.1) |
| Non-PPC Group (N = 19) | PPC Group (N = 18) | p | |
|---|---|---|---|
| Female, n (%) | 17 (89.5) | 18 (100) | 0.49 |
| Age (years), median (IQR) | 7.32 (5.7–7.8) | 7.02 (1.3–7.7) | 0.37 |
| Height (cm), median (IQR) | 127.2 (110.0–133.0) | 123.0 (76.5–130.3) | 0.28 |
| Z-score height, median (IQR) | 0.5 (−2.1–1.3) | 0.26 (−0.96–1.04) | 0.73 |
| Weight (kg), median (IQR) | 30.0 (15.0–40.0) | 24.5 (9.4–30.4) | 0.13 |
| Z-score weight, median (IQR) | 1.05 (−1.1–1.81) | 0.4 (−1.9–1.1) | 0.27 |
| BMI, median (IQR) | 17.5 (13.5–23.3) | 16.7 (15.1–18.2) | 0.40 |
| Z-score BMI, mean (+/−SD) | 0.70 (+/−1.5) | −0.007 (+/−1.26) | 0.13 |
| Adrenarche, n (%) | 3 (15.8) | 2 (11.1) | 1 |
| Familiar history of CPP, n (%) | 2 (10.5) | 4 (22.2) | 0.40 |
| TSH, median (IQR) | 1.8 (1.6–5.4) | 1.95 (1.3–3.1) | 0.73 |
| fT3, mean (+/−SD) | 3.7 (+/−1.3) | 3.98 (+/−1.5) | 0.5 |
| fT4, mean (+/−SD) | 12.6 (+/−1.6) | 11.6 (+/−1.6) | 0.07 |
| Abnormal thyroid profile, n (%) | 5 (26.3) | 5 (27.8) | 1 |
| Glycemia (mg/dl), mean (+/−SD) | 78.2 (+/- 6.6) | 74.9 (+/−7.5) | 0.16 |
| Hb, median (IQR) | 12.9 (12.5–13.6) | 13.0 (12.4–13.6) | 0.62 |
| Platelet count, mean (+/−SD) | 324.8 (+/−91.5) | 309.8 (+/−77.4) | 0.59 |
| Abnormal blood cell count, n (%) | 4 (21.1) | 2 (11.1) | 0.66 |
| GOT, median (IQR) | 17.0 (14.0–20.0) | 16.0 (13.7–19.0) | 0.54 |
| Increased GOT, n (%) | 1 (5.3) | 1 (5.6) | 1 |
| Triglycerides, median (IQR) | 61.0 (45.0–75.0) | 60.0 (55.5–69.0) | 0.66 |
| Cholesterol, mean (+/−SD) | 158.0 (+/−28.0) | 152.5 (+/−22.2) | 0.51 |
| LDL, mean (+/−SD) | 90.16 (+/−22.82) | 88. 0 (+/−15.99) | 0.74 |
| HDL, mean (+/−SD) | 53.11 (+/−14.11) | 52.3 (+/−13.01) | 0.87 |
| Abnormal lipid profile, n (%) | 10 (52.6) | 4 (22.2) | 0.09 |
| FSH, median (IQR) | 1.6 (1.0–3.4) | 5.5 (3.1–8.2) | <0.001 |
| Increased baseline FSH, n (%) | 8 (42.1) | 16 (88.9) | 0.005 |
| LH, median (IQR) | 0.1 (0.1–0.1) | 0.15 (0.1–1.5) | 0.03 |
| Increased baseline LH, n (%) | 0 (0) | 5 (27.8) | 0.02 |
| SHBG, median (IQR) | 65.0 (42.0–99.0) | 102.0 (75.2–128.5) | 0.02 |
| 17BE2, median (IQR) | 15.0 (15.0–20.0) | 23.0 (18.7–44.2) | 0.001 |
| Prolactin, median (IQR) | 8.0 (5.3–15.0) | 7.5 (5.0–12.4) | 0.68 |
| Increased prolactin, n (%) | 7 (36.8) | 6 (33.3) | 1 |
| Advanced bone age ≥ 1 year, n (%) | 12 (63.2) | 8 (44.4) | 0.25 |
| Pathological brain MRI, n (%) | 1 (5.3) | 2 (11.8) | 0.59 |
| Uterus length ≥ 3.6 cm **, n (%) | 2 (11.8) | 4 (22.2) | 0.66 |
| Uterus antero–posterior diameter > 1 cm **, n (%) | 2 (11.8) | 4 (22.2) | 0.66 |
| Uterine body/cervix ratio > 1, n (%) | 3 (17.6) | 5 (27.8) | 0.69 |
| Ovarian volume ≥ 2 mL, n (%) | 7 (41.2) | 8 (44.4) | 0.84 |
| LH peak, median (IQR) | 1.4 (0.7–2.2) | 8.4 (6.7–26.8) | <0.001 |
| FSH peak, median (IQR) | 10.5 (5.1–19.0) | 18.9 (10.2–44.6) | 0.01 |
| LH/FSH ratio, median (IQR) | 0.008 (0.005–0.048) | 0.109 (0.01–0.76) | 0.006 |
| LH/FSH ratio > 1, n (%) | 0 (0) | 7 (38.9) | 0.003 |
| Variable | F-Test | p |
|---|---|---|
| fT4 | 2.247 | 0.101 |
| Abnormal lipid profile | 1.590 | 0.210 |
| FSH | 0.952 | 0.427 |
| LH | 4.354 | 0.011 |
| SHBG | 1.166 | 0.337 |
| 17BE2 | 4.968 | 0.006 |
| LH peak | 5.425 | 0.004 |
| FSH peak | 23.457 | <0.001 |
| LH/FSH ratio | 4.801 | 0.007 |
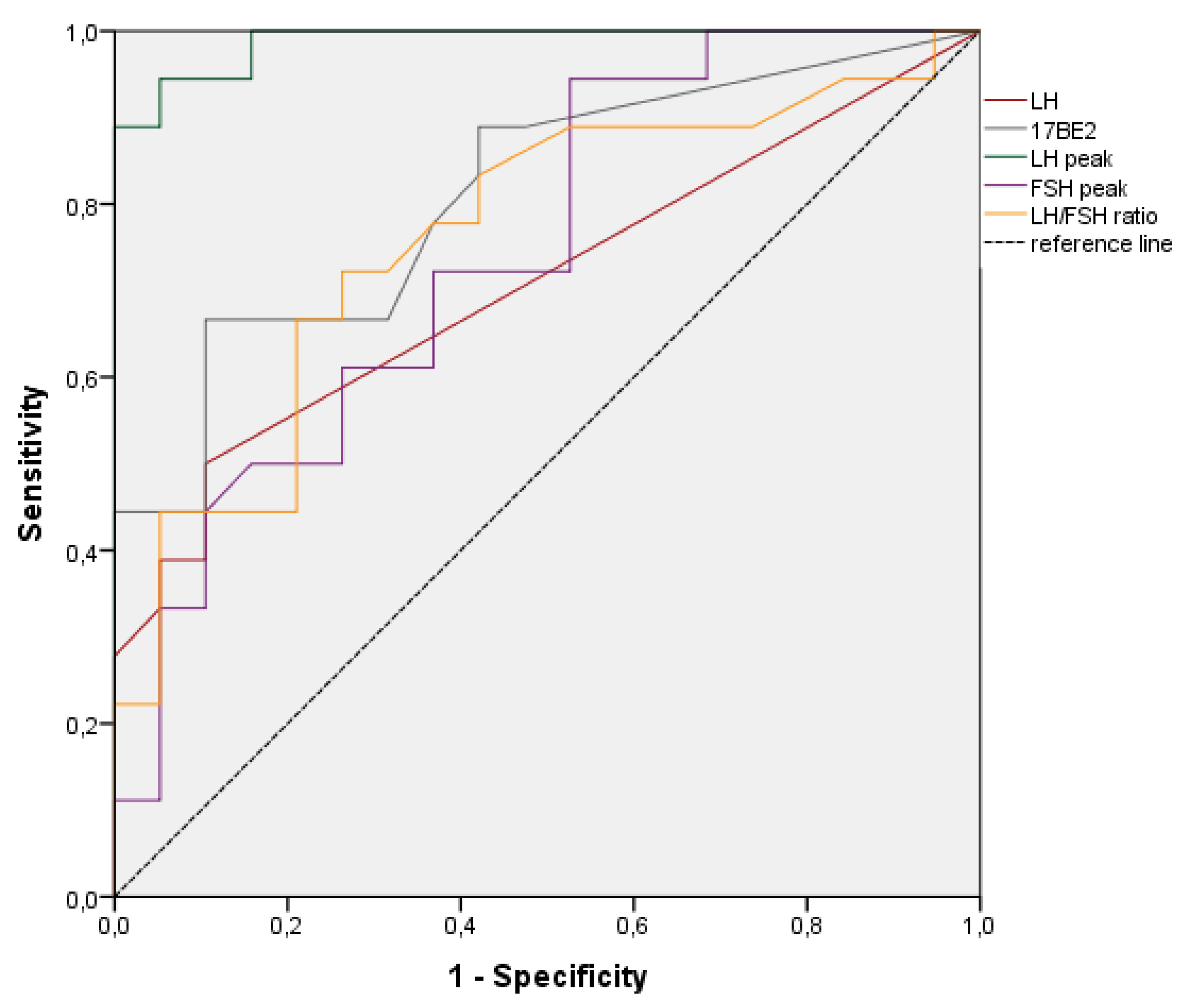
| Variable | Accuracy | 95%CI | p | Best Cut-off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| LH | 0.708 | 0.536−0.879 | 0.031 | 0.15 | 50 | 90 |
| 17BE2 | 0.811 | 0.671–0.952 | 0.001 | 21.5 | 67 | 90 |
| LH peak | 0.988 | 0.964–1.000 | 0.001 | 4.10 | 94 | 95 |
| FSH peak | 0.744 | 0.586–0.902 | 0.010 | 21.5 | 44 | 90 |
| LH/FSH | 0.762 | 0.603–0.907 | 0.007 | 0.023 | 72 | 74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipolla, C.; Sodero, G.; Pane, L.C.; Mariani, F.; Di Sarno, L.; Rigante, D.; Candelli, M. Auxological and Metabolic Parameters of Children Undergoing the Gonadotropin-Releasing Hormone Stimulation Test: Correlations with the Final Diagnosis of Central Precocious Puberty in a Single-Center Study. Biomedicines 2023, 11, 1678. https://doi.org/10.3390/biomedicines11061678
Cipolla C, Sodero G, Pane LC, Mariani F, Di Sarno L, Rigante D, Candelli M. Auxological and Metabolic Parameters of Children Undergoing the Gonadotropin-Releasing Hormone Stimulation Test: Correlations with the Final Diagnosis of Central Precocious Puberty in a Single-Center Study. Biomedicines. 2023; 11(6):1678. https://doi.org/10.3390/biomedicines11061678
Chicago/Turabian StyleCipolla, Clelia, Giorgio Sodero, Lucia Celeste Pane, Francesco Mariani, Lorenzo Di Sarno, Donato Rigante, and Marcello Candelli. 2023. "Auxological and Metabolic Parameters of Children Undergoing the Gonadotropin-Releasing Hormone Stimulation Test: Correlations with the Final Diagnosis of Central Precocious Puberty in a Single-Center Study" Biomedicines 11, no. 6: 1678. https://doi.org/10.3390/biomedicines11061678
APA StyleCipolla, C., Sodero, G., Pane, L. C., Mariani, F., Di Sarno, L., Rigante, D., & Candelli, M. (2023). Auxological and Metabolic Parameters of Children Undergoing the Gonadotropin-Releasing Hormone Stimulation Test: Correlations with the Final Diagnosis of Central Precocious Puberty in a Single-Center Study. Biomedicines, 11(6), 1678. https://doi.org/10.3390/biomedicines11061678








