Multilineage-Differentiating Stress-Enduring Cells (Muse Cells): An Easily Accessible, Pluripotent Stem Cell Niche with Unique and Powerful Properties for Multiple Regenerative Medicine Applications
Abstract
1. Introduction
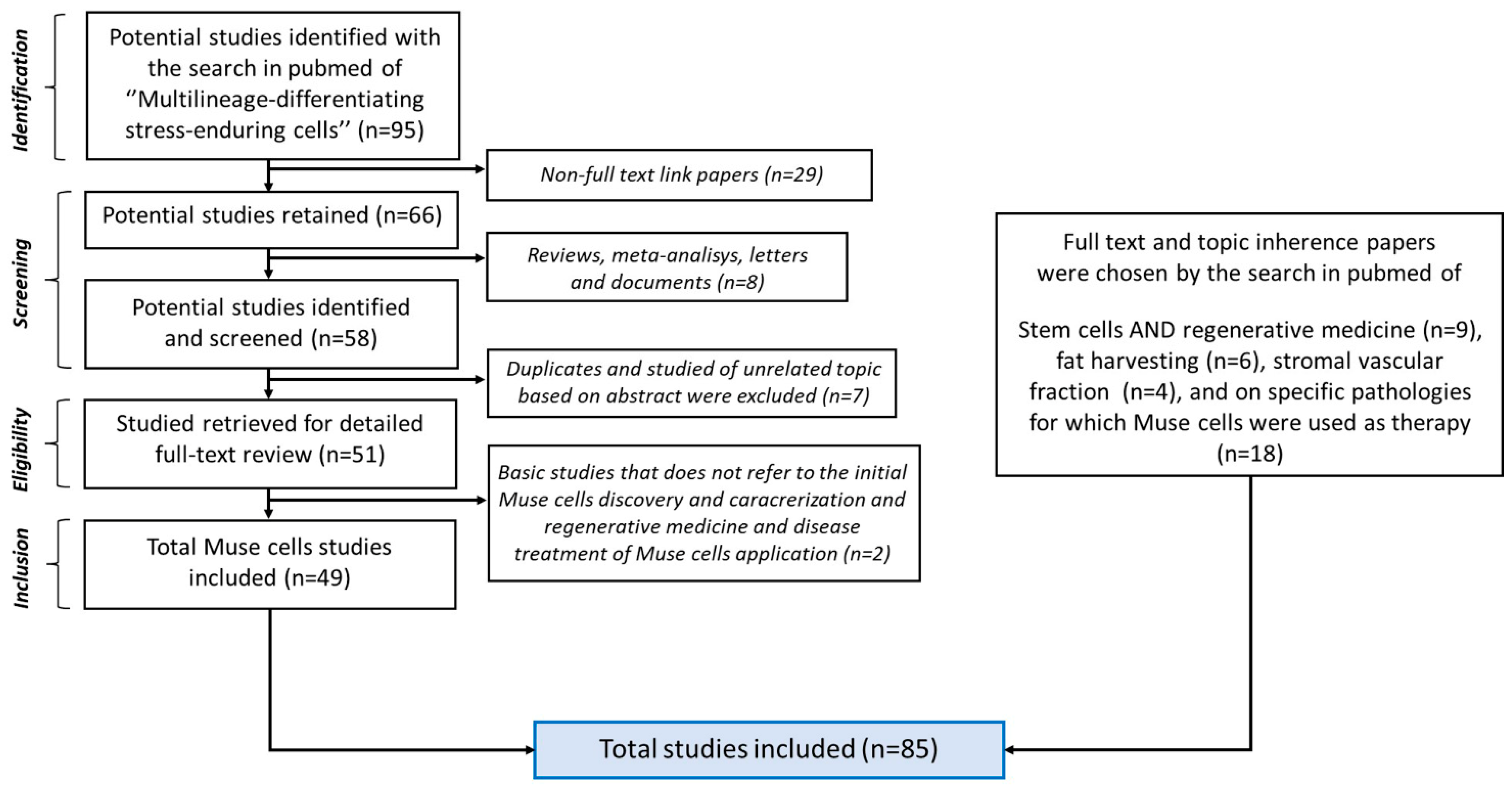
2. Bibliographic Research
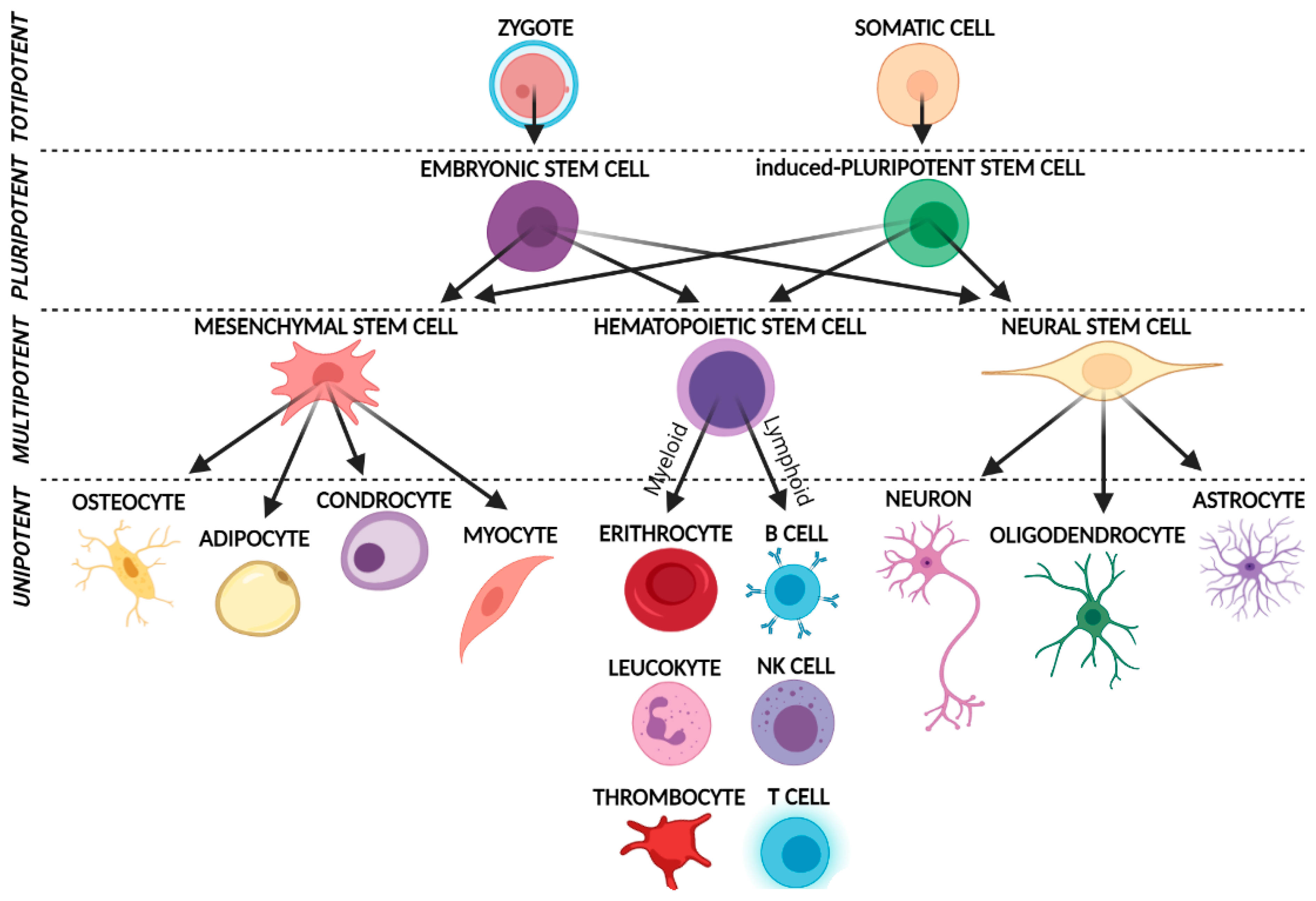
3. Muse Cells
3.1. Basic Characteristics and Isolation Methods
3.2. Muse Cells Pluripotency and Their Unique Regenerative Features
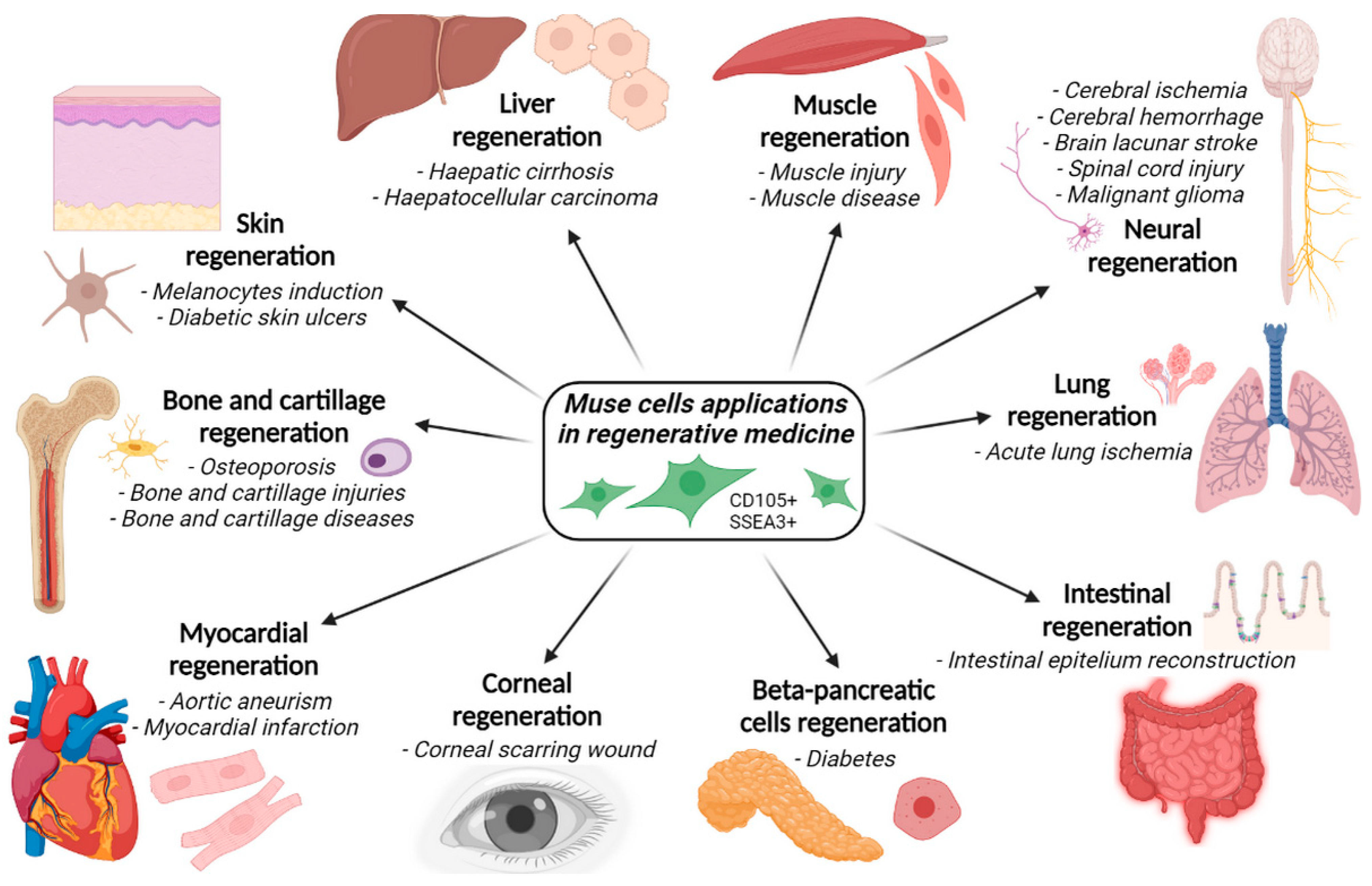
4. Applications of Muse Cells in Regenerative Medicine and Disorders Healing
- Acute lung ischemia;
- Aortic aneurysm;
- Bladder inflammation;
- Brain lacunar stroke;
- Cerebral ischemia;
- Corneal scarring;
- Diabetes;
- Intestinal epithelium injuries;
- Intracerebral hemorrhage;
- Liver dysfunction;
- Malignant gliomas;
- Myocardial infarction;
- Osteochondral lesions;
- Skin damage;
- Skin ulcers;
- Spinal cord injuries.
4.1. Acute Lung Ischemia
4.2. Aortic Aneurysm
4.3. Bladder Inflammation
4.4. Brain Lacunar Stroke
4.5. Cerebral Ischemia
4.6. Corneal Scarring
4.7. Diabetes
4.8. Intestinal Epithelium Injuries
4.9. Intracerebral Haemorrhage
4.10. Liver Dysfunction
4.11. Malignant Gliomas
4.12. Myocardial Infarction
4.13. Osteochondral Lesions
4.14. Skin Damage
4.15. Skin Ulcers
4.16. Spinal Cord Injury
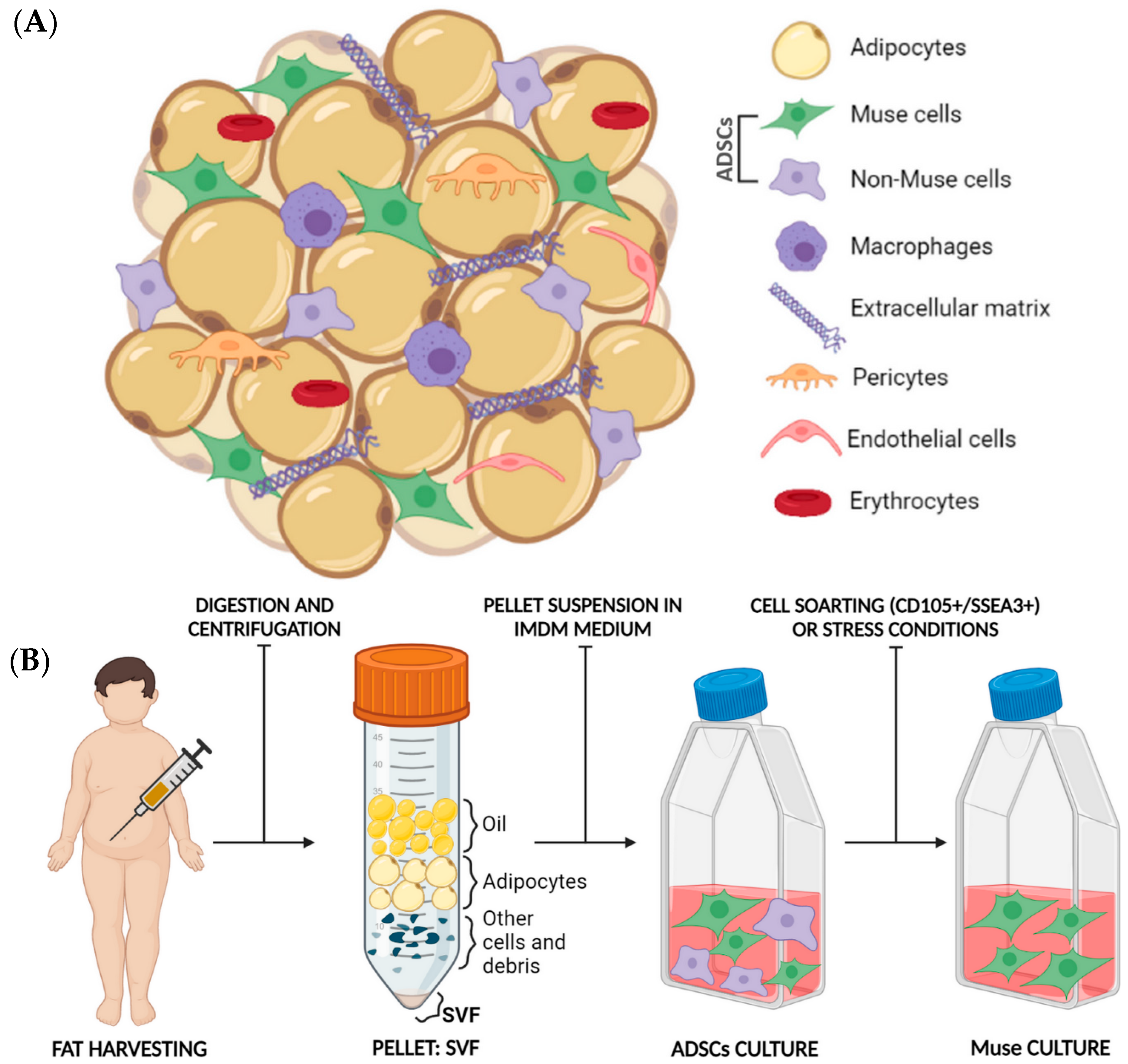
5. Fat as an Optimal Source of Muse Cells Isolation
5.1. Fat Harvesting: An Established Procedure with Many Applications
5.2. Fat Composition
5.3. Muse Cells Isolation from Adipose Tissue
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wakao, S.; Kitada, M.; Kuroda, Y.; Shigemoto, T.; Matsuse, D.; Akashi, H.; Tanimura, Y.; Tsuchiyama, K.; Kikuchi, T.; Goda, M.; et al. Multilineage-Differentiating Stress-Enduring (Muse) Cells Are a Primary Source of Induced Pluripotent Stem Cells in Human Fibroblasts. Proc. Natl. Acad. Sci. USA 2011, 108, 9875–9880. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Wakao, S.; Kitada, M.; Murakami, T.; Nojima, M.; Dezawa, M. Isolation, Culture and Evaluation of Multilineage-Differentiating Stress-Enduring (Muse) Cells. Nat. Protoc. 2013, 8, 1391–1415. [Google Scholar] [CrossRef] [PubMed]
- Fus-Kujawa, A.; Mendrek, B.; Trybus, A.; Bajdak-Rusinek, K.; Stepien, K.L.; Sieron, A.L. Potential of Induced Pluripotent Stem Cells for Use in Gene Therapy: History, Molecular Bases, and Medical Perspectives. Biomolecules 2021, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Simerman, A.A.; Perone, M.J.; Gimeno, M.L.; Dumesic, D.A.; Chazenbalk, G.D. A Mystery Unraveled: Nontumorigenic Pluripotent Stem Cells in Human Adult Tissues. Expert Opin. Biol. Ther. 2014, 14, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Scesa, G.; Adami, R.; Bottai, D. IPSC Preparation and Epigenetic Memory: Does the Tissue Origin Matter? Cells 2021, 10, 1470. [Google Scholar] [CrossRef]
- Wakao, S.; Akashi, H.; Kushida, Y.; Dezawa, M. Muse Cells, Newly Found Non-Tumorigenic Pluripotent Stem Cells, Reside in Human Mesenchymal Tissues. Pathol. Int. 2014, 64, 1–9. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Ilic, D.; Polak, J.M. Stem Cells in Regenerative Medicine: Introduction. Br. Med. Bull. 2011, 98, 117–126. [Google Scholar] [CrossRef]
- Sato, T.; Wakao, S.; Kushida, Y.; Tatsumi, K.; Kitada, M.; Abe, T.; Niizuma, K.; Tominaga, T.; Kushimoto, S.; Dezawa, M. A Novel Type of Stem Cells Double-Positive for SSEA-3 and CD45 in Human Peripheral Blood. Cell Transplant. 2020, 29, 0963689720923574. [Google Scholar] [CrossRef]
- Heneidi, S.; Simerman, A.A.; Keller, E.; Singh, P.; Li, X.; Dumesic, D.A.; Chazenbalk, G. Awakened by Cellular Stress: Isolation and Characterization of a Novel Population of Pluripotent Stem Cells Derived from Human Adipose Tissue. PLoS ONE 2013, 8, e64752. [Google Scholar] [CrossRef]
- Ogura, F.; Wakao, S.; Kuroda, Y.; Tsuchiyama, K.; Bagheri, M.; Heneidi, S.; Chazenbalk, G.; Aiba, S.; Dezawa, M. Human Adipose Tissue Possesses a Unique Population of Pluripotent Stem Cells with Nontumorigenic and Low Telomerase Activities: Potential Implications in Regenerative Medicine. Stem Cells Dev. 2014, 23, 717–728. [Google Scholar] [CrossRef]
- Quintero Sierra, L.A.; Biswas, R.; Conti, A.; Busato, A.; Ossanna, R.; Zingaretti, N.; Parodi, P.C.; Conti, G.; Riccio, M.; Sbarbati, A.; et al. Highly Pluripotent Adipose-Derived Stem Cell-Enriched Nanofat: A Novel Translational System in Stem Cell Therapy. Cell Transplant. 2023, 32, 9636897231175968. [Google Scholar] [CrossRef] [PubMed]
- Glicksman, M.A. Induced Pluripotent Stem Cells: The Most Versatile Source for Stem Cell Therapy. Clin. Ther. 2018, 40, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Germena, G.; Hinkel, R. Ipscs and Exosomes: Partners in Crime Fighting Cardiovascular Diseases. J. Pers. Med. 2021, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Kuroda, Y.; Ogura, F.; Shigemoto, T.; Dezawa, M. Regenerative Effects of Mesenchymal Stem Cells: Contribution of Muse Cells, a Novel Pluripotent Stem Cell Type That Resides in Mesenchymal Cells. Cells 2012, 1, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Kuno, S.; Ishimine, H.; Aoi, N.; Mineda, K.; Kato, H.; Doi, K.; Kanayama, K.; Feng, J.; Mashiko, T.; et al. Therapeutic Potential of Adipose-Derived SSEA-3-Positive Muse Cells for Treating Diabetic Skin Ulcers. Stem Cells Transl. Med. 2015, 4, 146–155. [Google Scholar] [CrossRef]
- Velasco, M.G.; Satué, K.; Chicharro, D.; Martins, E.; Torres-Torrillas, M.; Peláez, P.; Miguel-Pastor, L.; Del Romero, A.; Damiá, E.; Cuervo, B.; et al. Multilineage-Differentiating Stress-Enduring Cells (Muse Cells): The Future of Human and Veterinary Regenerative Medicine. Biomedicines 2023, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Oguma, Y.; Hall, K.; Dezawa, M. Endogenous reparative pluripotent Muse cells with a unique immune privilege system: Hint at a new strategy for controlling acute and chronic inflammation. Front Pharmacol. 2022, 13, 1027961. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Q.; Cheng, Y.; Zhang, R.Z.; Liu, Y.B.; Li, Y.; Ge, K.; Jin, H.L. RNA-Seq and ATAC-Seq Analyses of Multilineage Differentiating Stress Enduring Cells: Comparison with Dermal Fibroblasts. Cell Biol. Int. 2022, 46, 1480–1494. [Google Scholar] [CrossRef]
- Mao, X.-Q.; Zhang, R.-Z.; Jin, H.-L. Application Prospects of Multilineage Differentiating Stress-Enduring (Muse) Cells in the Treatment of Skin Diseases. J. Dermatol. Cosmetol. 2022, 6, 4–7. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yamasaki, K.; Tsuchiyama, K.; Koike, S.; Aiba, S. A Quantitative Analysis of Multilineage-Differentiating Stress-Enduring (Muse) Cells in Human Adipose Tissue and Efficacy of Melanocytes Induction. J. Dermatol. Sci. 2017, 86, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Marconi, S.; Pasini, A.; Galiè, M.; Rigotti, G.; Mosna, F.; Tinelli, M.; Lovato, L.; Anghileri, E.; Andreini, A.; et al. Induction of Neural-like Differentiation in Human Mesenchymal Stem Cells Derived from Bone Marrow, Fat, Spleen and Thymus. Bone 2007, 40, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Anghileri, E.; Marconi, S.; Pignatelli, A.; Cifelli, P.; Galié, M.; Sbarbati, A.; Krampera, M.; Belluzzi, O.; Bonetti, B. Neuronal Differentiation Potential of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 909–916. [Google Scholar] [CrossRef]
- Dezawa, M. Muse Cells Provide the Pluripotency of Mesenchymal Stem Cells: Direct Contribution of Muse Cells to Tissue Regeneration. Cell Transplant. 2016, 25, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Nitobe, Y.; Nagaoki, T.; Kumagai, G.; Sasaki, A.; Liu, X.; Fujita, T.; Fukutoku, T.; Wada, K.; Tanaka, T.; Kudo, H.; et al. Neurotrophic Factor Secretion and Neural Differentiation Potential of Multilineage-Differentiating Stress-Enduring (Muse) Cells Derived from Mouse Adipose Tissue. Cell Transplant. 2019, 28, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Prè, E.D.; Conti, G.; Sbarbati, A. Hyaluronic Acid (HA) Scaffolds and Multipotent Stromal Cells (MSCs) in Regenerative Medicine. Stem Cell Rev. Rep. 2016, 12, 664–681. [Google Scholar] [CrossRef] [PubMed]
- Fisch, S.C.; Gimeno, M.L.; Phan, J.D.; Simerman, A.A.; Dumesic, D.A.; Perone, M.J.; Chazenbalk, G.D. Pluripotent Nontumorigenic Multilineage Differentiating Stress Enduring Cells (Muse Cells): A Seven-Year Retrospective. Stem Cell Res. Ther. 2017, 8, 227. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Li, M.O. TGF-β Control of Adaptive Immune Tolerance: A Break From Treg Cells. Bioessays 2018, 40, 1800063. [Google Scholar] [CrossRef]
- Alessio, N.; Özcan, S.; Tatsumi, K.; Murat, A.; Peluso, G.; Dezawa, M.; Galderisi, U. The Secretome of MUSE Cells Contains Factors That May Play a Role in Regulation of Stemness, Apoptosis and Immunomodulation. Cell Cycle 2017, 16, 33–44. [Google Scholar] [CrossRef]
- Beckers, P.A.J.; Gielis, J.F.; van Schil, P.E.; Adriaensen, D. Lung Ischemia Reperfusion Injury: The Therapeutic Role of Dipeptidyl Peptidase 4 Inhibition. Ann. Transl. Med. 2017, 5, 129. [Google Scholar] [CrossRef]
- Yabuki, H.; Wakao, S.; Kushida, Y.; Dezawa, M.; Okada, Y. Human Multilineage-Differentiating Stress-Enduring Cells Exert Pleiotropic Effects to Ameliorate Acute Lung Ischemia–Reperfusion Injury in a Rat Model. Cell Transplant. 2018, 27, 979–993. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Michel, J.B.; Katsargyris, A.; Kuivaniemi, H.; Defraigne, J.O.; Nchimi, A.; Powell, J.T.; Yoshimura, K.; Hultgren, R. Abdominal Aortic Aneurysms. Nat. Rev. Dis. Prim. 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, K.; Wakao, S.; Kushida, Y.; Ogura, F.; Maeda, K.; Adachi, O.; Kawamoto, S.; Dezawa, M.; Saiki, Y. Intravenously Injected Human Multilineage-Differentiating Stress-Enduring Cells Selectively Engraft into Mouse Aortic Aneurysms and Attenuate Dilatation by Differentiating into Multiple Cell Types. J. Thorac. Cardiovasc. Surg. 2018, 155, 2301–2313.e4. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Akiyama, Y.; Tomoe, H.; Furuta, A.; Ueda, T.; Maeda, D.; Lin, A.T.L.; Kuo, H.C.; Lee, M.H.; Oh, S.J.; et al. Clinical Guidelines for Interstitial Cystitis/Bladder Pain Syndrome. Int. J. Urol. 2020, 27, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Kuroda, Y.; Yamamoto, T.; Egawa, S.; Dezawa, M.; Yoshimura, N. Effects of Human Muse Cells on Bladder Inflammation, Overactivity, and Nociception in a Chemically Induced Hunner-Type Interstitial Cystitis-like Rat Model. Int. Urogynecol. J. 2022, 33, 1293–1301. [Google Scholar] [CrossRef]
- Regenhardt, R.W.; Das, A.S.; Lo, E.H.; Caplan, L.R. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA Neurol. 2018, 75, 1273–1281. [Google Scholar] [CrossRef]
- Abe, T.; Aburakawa, D.; Niizuma, K.; Iwabuchi, N.; Kajitani, T.; Wakao, S.; Kushida, Y.; Dezawa, M.; Borlongan, C.V.; Tominaga, T. Intravenously Transplanted Human Multilineage-Differentiating Stress-Enduring Cells Afford Brain Repair in a Mouse Lacunar Stroke Model. Stroke 2020, 51, 601–611. [Google Scholar] [CrossRef]
- Uchida, H.; Niizuma, K.; Kushida, Y.; Wakao, S.; Tominaga, T.; Borlongan, C.V.; Dezawa, M. Human Muse Cells Reconstruct Neuronal Circuitry in Subacute Lacunar Stroke Model. Stroke 2017, 48, 428–435. [Google Scholar] [CrossRef]
- Shin, T.H.; Lee, D.Y.; Basith, S.; Manavalan, B.; Paik, M.J.; Rybinnik, I.; Mouradian, M.M.; Ahn, J.H.; Lee, G. Metabolome Changes in Cerebral Ischemia. Cells 2020, 9, 1630. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kuroda, Y.; Morita, T.; Shichinohe, H.; Houkin, K.; Dezawa, M.; Kuroda, S. Therapeutic Effects of Human Multilineage-Differentiating Stress Enduring (Muse) Cell Transplantation into Infarct Brain of Mice. PLoS ONE 2015, 10, e0116009. [Google Scholar] [CrossRef]
- Matsuyama, N.; Shimizu, S.; Ueda, K.; Suzuki, T.; Suzuki, S.; Miura, R.; Katayama, A.; Ando, M.; Mizuno, M.; Hirakawa, A.; et al. Safety and Tolerability of a Multilineage-Differentiating Stress-Enduring Cell-Based Product in Neonatal Hypoxic-Ischaemic Encephalopathy with Therapeutic Hypothermia (SHIELD Trial): A Clinical Trial Protocol Open-Label, Non-Randomised, Dose-Escalation Trial. BMJ Open 2022, 12, e057073. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Wilson, S.E. Fibrocytes, Wound Healing, and Corneal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 28. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xue, Y.; Wang, P.; Cui, Z.; Cao, J.; Liu, S.; Yu, Q.; Zeng, Q.; Zhu, D.; Xie, M.; et al. Muse Cell Spheroids Have Therapeutic Effect on Corneal Scarring Wound in Mice and Tree Shrews. Sci. Transl. Med. 2020, 12, eaaw1120. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.R.; Lajtha, A.; Lambris, J.D.; Paoletti, R.; Rezaei, N. Muse Cells; Advances in Experimental Medicine and Biology; Dezawa, M., Ed.; Springer: Tokyo, Japan, 2018; Volume 1103, ISBN 978-4-431-56845-2. [Google Scholar]
- Fouad, A.M.; Gabr, M.M.; Abdelhady, E.K.; Zakaria, M.M.; Khater, S.M.; Ismail, A.M.; Refaie, A.F. In Vitro Differentiation of Human Multilineage Differentiating Stress-Enduring (Muse) Cells into Insulin Producing Cells. J. Genet. Eng. Biotechnol. 2018, 16, 433–440. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.G. Intestinal Epithelial Plasticity and Regeneration via Cell Dedifferentiation. Cell Regen. 2020, 9, 14. [Google Scholar] [CrossRef]
- Sun, D.; Yang, L.; Cao, H.; Shen, Z.Y.; Song, H.L. Study of the Protective Effect on Damaged Intestinal Epithelial Cells of Rat Multilineage-Differentiating Stress-Enduring (Muse) Cells. Cell Biol. Int. 2020, 44, 549–559. [Google Scholar] [CrossRef]
- Lan, X.; Han, X.; Li, Q.; Yang, Q.W.; Wang, J. Modulators of Microglial Activation and Polarization after Intracerebral Haemorrhage. Nat. Rev. Neurol. 2017, 13, 420–433. [Google Scholar] [CrossRef]
- Shimamura, N.; Kakuta, K.; Wang, L.; Naraoka, M.; Uchida, H.; Wakao, S.; Dezawa, M.; Ohkuma, H. Neuro-Regeneration Therapy Using Human Muse Cells Is Highly Effective in a Mouse Intracerebral Hemorrhage Model. Exp. Brain Res. 2017, 235, 565–572. [Google Scholar] [CrossRef]
- Zhou, W.C.; Zhang, Q.B.; Qiao, L. Pathogenesis of Liver Cirrhosis. World J. Gastroenterol. 2014, 20, 7312–7324. [Google Scholar] [CrossRef]
- Katagiri, H.; Kushida, Y.; Nojima, M.; Kuroda, Y.; Wakao, S.; Ishida, K.; Endo, F.; Kume, K.; Takahara, T.; Nitta, H.; et al. A Distinct Subpopulation of Bone Marrow Mesenchymal Stem Cells, Muse Cells, Directly Commit to the Replacement of Liver Components. Am. J. Transplant. 2016, 16, 468–483. [Google Scholar] [CrossRef]
- Iseki, M.; Mizuma, M.; Wakao, S.; Kushida, Y.; Kudo, K.; Fukase, M.; Ishida, M.; Ono, T.; Shimura, M.; Ise, I.; et al. The Evaluation of the Safety and Efficacy of Intravenously Administered Allogeneic Multilineage-Differentiating Stress-Enduring Cells in a Swine Hepatectomy Model. Surg. Today 2021, 51, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Sampetrean, O.; Saya, H. Modeling Phenotypes of Malignant Gliomas. Cancer Sci. 2018, 109, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Wakao, S.; Kawaji, H.; Koizumi, S.; Sameshima, T.; Dezawa, M.; Namba, H. Genetically Engineered Multilineage-Differentiating Stress-Enduring Cells as Cellular Vehicles against Malignant Gliomas. Mol. Ther. Oncolytics 2017, 6, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Ambrose, J.A. Understanding Myocardial Infarction [Version 1; Referees: 2 Approved]. F1000Res 2018, 7, 1378. [Google Scholar] [CrossRef]
- Yamada, Y.; Minatoguchi, S.; Kanamori, H.; Mikami, A.; Okura, H.; Dezawa, M.; Minatoguchi, S. Stem Cell Therapy for Acute Myocardial Infarction—Focusing on the Comparison between Muse Cells and Mesenchymal Stem Cells. J. Cardiol. 2022, 80, 80–87. [Google Scholar] [CrossRef]
- Noda, T.; Nishigaki, K.; Minatoguchi, S. Safety and Efficacy of Human Muse Cell-Based Product for Acute Myocardial Infarction in a First-in-Human Trial. Circ. J. 2020, 84, 1189–1192. [Google Scholar] [CrossRef]
- Yamada, Y.; Minatoguchi, S.; Baba, S.; Shibata, S.; Takashima, S.; Wakao, S.; Okura, H.; Dezawa, M.; Minatoguchi, S. Human Muse Cells Reduce Myocardial Infarct Size and Improve Cardiac Function without Causing Arrythmias in a Swine Model of Acute Myocardial Infarction. PLoS ONE 2022, 17, e0265347. [Google Scholar] [CrossRef]
- Looze, C.A.; Capo, J.; Ryan, M.K.; Begly, J.P.; Chapman, C.; Swanson, D.; Singh, B.C.; Strauss, E.J. Evaluation and Management of Osteochondral Lesions of the Talus. Cartilage 2017, 8, 19–30. [Google Scholar] [CrossRef]
- Mahmoud, E.E.; Kamei, N.; Shimizu, R.; Wakao, S.; Dezawa, M.; Adachi, N.; Ochi, M. Therapeutic Potential of Multilineage-Differentiating Stress-Enduring Cells for Osteochondral Repair in a Rat Model. Stem Cells Int. 2017, 2017, 8154569. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.S.; Longaker, M.T. A MUSE for Skin Regeneration. J. Investig. Dermatol. 2017, 137, 2471–2472. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.D.; Wu, J.L.; Gao, M.D.; Wang, Q.; Zhao, Y.Y.; Shan, C.L.; Shen, Y.; Chen, G. Multilineage-Differentiating Stress-Enduring Cells Alleviate Atopic Dermatitis-Associated Behaviors in Mice. Stem Cell Res. Ther. 2021, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.M.; Roda, Â.; Pimenta, R.; Filipe, P.L.; Freitas, J.P. Cutaneous Manifestations of Diabetes Mellitus and Prediabetes. Acta Med. Port. 2019, 32, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury—Repair and Regeneration. Clin. Neurosurg. 2017, 80, S22–S90. [Google Scholar] [CrossRef]
- Kajitani, T.; Endo, T.; Iwabuchi, N.; Inoue, T.; Takahashi, Y.; Abe, T.; Niizuma, K.; Tominaga, T. Association of Intravenous Administration of Human Muse Cells with Deficit Amelioration in a Rat Model of Spinal Cord Injury. J. Neurosurg. Spine 2021, 34, 648–655. [Google Scholar] [CrossRef]
- Veronese, S.; Dai Prè, E.; Conti, G.; Busato, A.; Mannucci, S.; Sbarbati, A. Comparative Technical Analysis of Lipoaspirate Mechanical Processing Devices. J. Tissue Eng. Regen. Med. 2020, 14, 1213–1226. [Google Scholar] [CrossRef]
- Constantin, G.; Marconi, S.; Rossi, B.; Angiari, S.; Calderan, L.; Anghileri, E.; Gini, B.; Dorothea Bach, S.; Martinello, M.; Bifari, F.; et al. Adipose-Derived Mesenchymal Stem Cells Ameliorate Chronic Experimental Autoimmune Encephalomyelitis. Stem Cells 2009, 27, 2624–2635. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Sbarbati, A. Adipose-Derived Mesenchymal Stem Cells: Past, Present, and Future. Aesthetic Plast. Surg. 2009, 33, 271–273. [Google Scholar] [CrossRef]
- Gentile, P.; Sterodimas, A.; Pizzicannella, J.; Dionisi, L.; De Fazio, D.; Calabrese, C.; Garcovich, S. Systematic Review: Allogenic Use of Stromal Vascular Fraction (SVF) and Decellularized Extracellular Matrices (ECM) as Advanced Therapy Medicinal Products (ATMP) in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 4982. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galie, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical Treatment of Radiotherapy Tissue Damage by Lipoaspirate Transplant: A Healing Process Mediated by Adipose-Derived Adult Stem Cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Drochioi, C.I.; Sulea, D.; Timofte, D.; Mocanu, V.; Popescu, E.; Costan, V.V. Autologous Fat Grafting for Craniofacial Reconstruction in Oncologic Patients. Medicina 2019, 55, 655. [Google Scholar] [CrossRef] [PubMed]
- Shukla, L.; Yuan, Y.; Shayan, R.; Greening, D.W.; Karnezis, T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front. Pharmacol. 2020, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghname, A.; Perdanasari, A.T.; Reece, E.M. Principles and Applications of Fat Grafting in Plastic Surgery. Semin. Plast. Surg. 2019, 33, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Scala, J.; Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Peric-Hajzler, Z.; Matovic, D.; Dimitrijevic, S.; Vojvodic, J.; Sijan, G.; Stepic, N.; et al. Autologous Fat Graft: Not Only an Aesthetic Solution. Open Access Maced. J. Med. Sci. 2019, 7, 2961–2963. [Google Scholar] [CrossRef]
- Conti, G.; Calderan, L.; Quintero Sierra, L.A.; Conti, A.; Ossanna, R.; Boschi, F.; Marzola, P.; Ferrarini, F.; Governa, M.; Lievens, P.M.-J.; et al. Tumor and Peritumoral Adipose Tissue Crosstalk: De-Differentiated Adipocytes Influence Spread of Colon Carcinoma Cells. Tissue Cell 2023, 80, 101990. [Google Scholar] [CrossRef]
- Francesco, F.D.; Mannucci, S.; Conti, G.; Prè, E.D.; Sbarbati, A.; Riccio, M. A Non-Enzymatic Method to Obtain a Fat Tissue Derivative Highly Enriched in Adipose Stem Cells (ASCs) from Human Lipoaspirates: Preliminary Results. Int. J. Mol. Sci. 2018, 19, 2061. [Google Scholar] [CrossRef]
- Xue, E.Y.; Narvaez, L.; Chu, C.K.; Hanson, S.E. Fat Processing Techniques. Semin. Plast. Surg. 2020, 34, 11–16. [Google Scholar] [CrossRef]
- Rosa, I.; Romano, E.; Fioretto, B.S.; Matucci-Cerinic, M.; Manetti, M. Adipose-Derived Stem Cells: Pathophysiologic Implications vs Therapeutic Potential in Systemic Sclerosis. World J. Stem Cells 2021, 13, 30–48. [Google Scholar] [CrossRef]
- Doornaert, M.; Colle, J.; De Maere, E.; Declercq, H.; Blondeel, P. Autologous Fat Grafting: Latest Insights. Ann. Med. Surg. 2019, 37, 47–53. [Google Scholar] [CrossRef]
- Deptuła, M.; Brzezicka, A.; Skoniecka, A.; Zieliński, J.; Pikuła, M. Adipose-Derived Stromal Cells for Nonhealing Wounds: Emerging Opportunities and Challenges. Med. Res. Rev. 2021, 41, 2130–2171. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Majumdar, A.S. Adipose Tissue-Derived Stromal Vascular Fraction in Regenerative Medicine: A Brief Review on Biology and Translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Luck, J.; Smith, O.J.; Malik, D.; Mosahebi, A. Protocol for a Systematic Review of Autologous Fat Grafting for Wound Healing. Syst. Rev. 2018, 7, 99. [Google Scholar] [CrossRef]
- Conti, G.; Bertossi, D.; Dai Prè, E.; Cavallini, C.; Scupoli, M.T.; Ricciardi, G.; Parnigotto, P.; Saban, Y.; Sbarbati, A.; Nocini, P. Regenerative Potential of the Bichat Fat Pad Determined by the Quantification of Multilineage Differentiating Stress Enduring Cells, 1–7. Eur. J. Histochem. 2018, 62, 2900. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossanna, R.; Veronese, S.; Quintero Sierra, L.A.; Conti, A.; Conti, G.; Sbarbati, A. Multilineage-Differentiating Stress-Enduring Cells (Muse Cells): An Easily Accessible, Pluripotent Stem Cell Niche with Unique and Powerful Properties for Multiple Regenerative Medicine Applications. Biomedicines 2023, 11, 1587. https://doi.org/10.3390/biomedicines11061587
Ossanna R, Veronese S, Quintero Sierra LA, Conti A, Conti G, Sbarbati A. Multilineage-Differentiating Stress-Enduring Cells (Muse Cells): An Easily Accessible, Pluripotent Stem Cell Niche with Unique and Powerful Properties for Multiple Regenerative Medicine Applications. Biomedicines. 2023; 11(6):1587. https://doi.org/10.3390/biomedicines11061587
Chicago/Turabian StyleOssanna, Riccardo, Sheila Veronese, Lindsey Alejandra Quintero Sierra, Anita Conti, Giamaica Conti, and Andrea Sbarbati. 2023. "Multilineage-Differentiating Stress-Enduring Cells (Muse Cells): An Easily Accessible, Pluripotent Stem Cell Niche with Unique and Powerful Properties for Multiple Regenerative Medicine Applications" Biomedicines 11, no. 6: 1587. https://doi.org/10.3390/biomedicines11061587
APA StyleOssanna, R., Veronese, S., Quintero Sierra, L. A., Conti, A., Conti, G., & Sbarbati, A. (2023). Multilineage-Differentiating Stress-Enduring Cells (Muse Cells): An Easily Accessible, Pluripotent Stem Cell Niche with Unique and Powerful Properties for Multiple Regenerative Medicine Applications. Biomedicines, 11(6), 1587. https://doi.org/10.3390/biomedicines11061587




