Topical Application of No-Ozone Cold Plasma in Combination with Vitamin C Reduced Skin Redness and Pigmentation of UV-Irradiated Mice
Abstract
1. Introduction
2. Materials and Methods
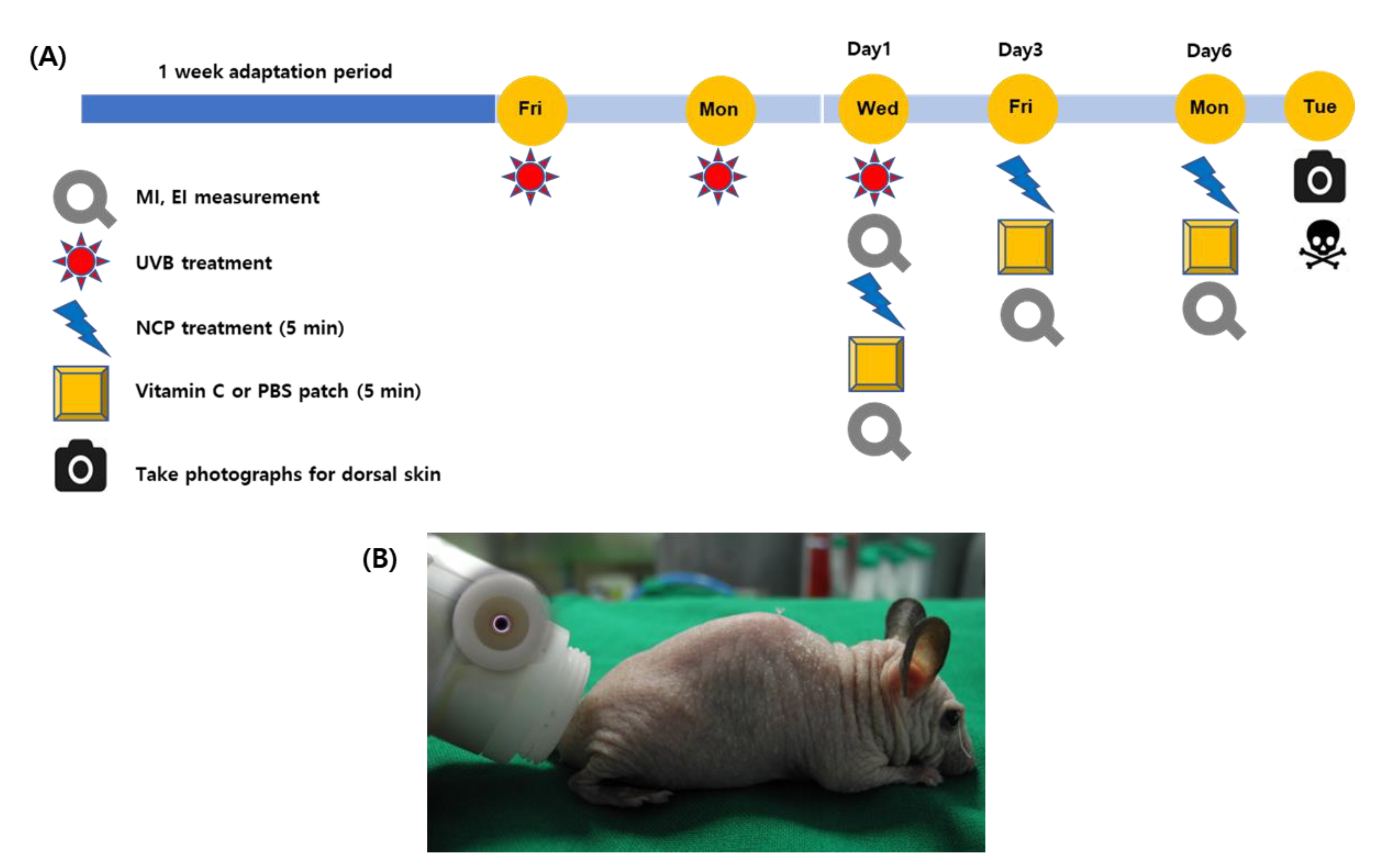
2.1. NCP Device for the Animal Experiments
2.2. Animal Model and Skin-Whitening Procedure
2.3. Measurement of Skin Conditions
2.4. Histological and Immunohistochemical Analysis
2.5. Statistical Analysis
3. Results
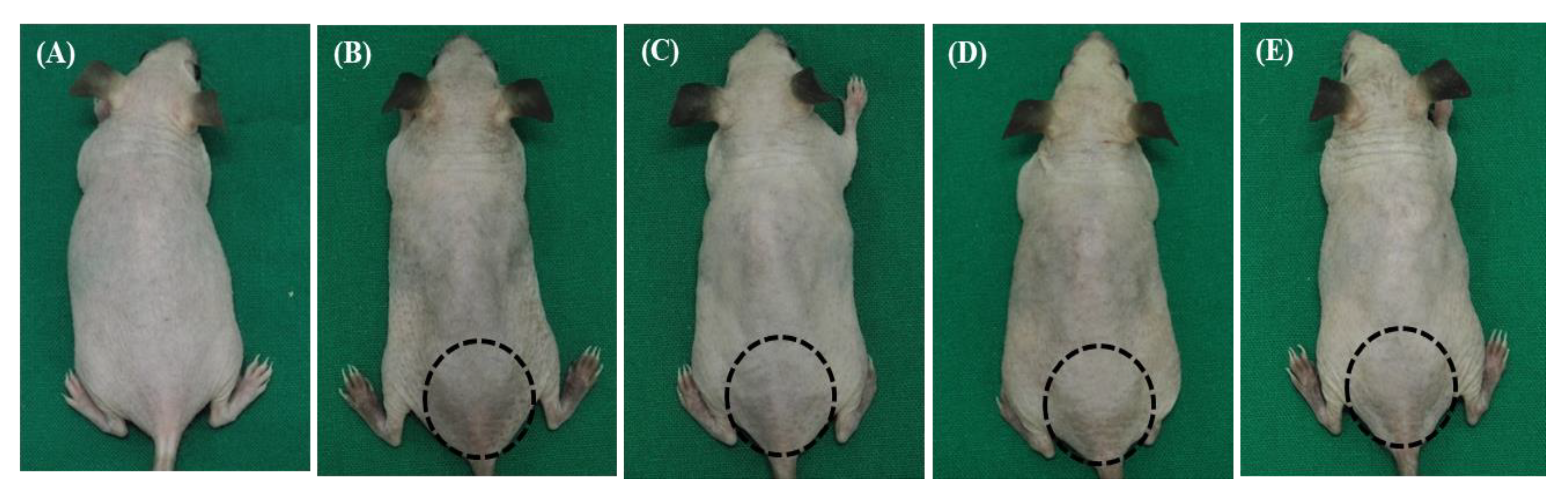
3.1. The Combined Treatment of NCP and Vitamin C Revealed the Fastest Restoring Activity in the Appearance of the UVB-Irradiated Skin
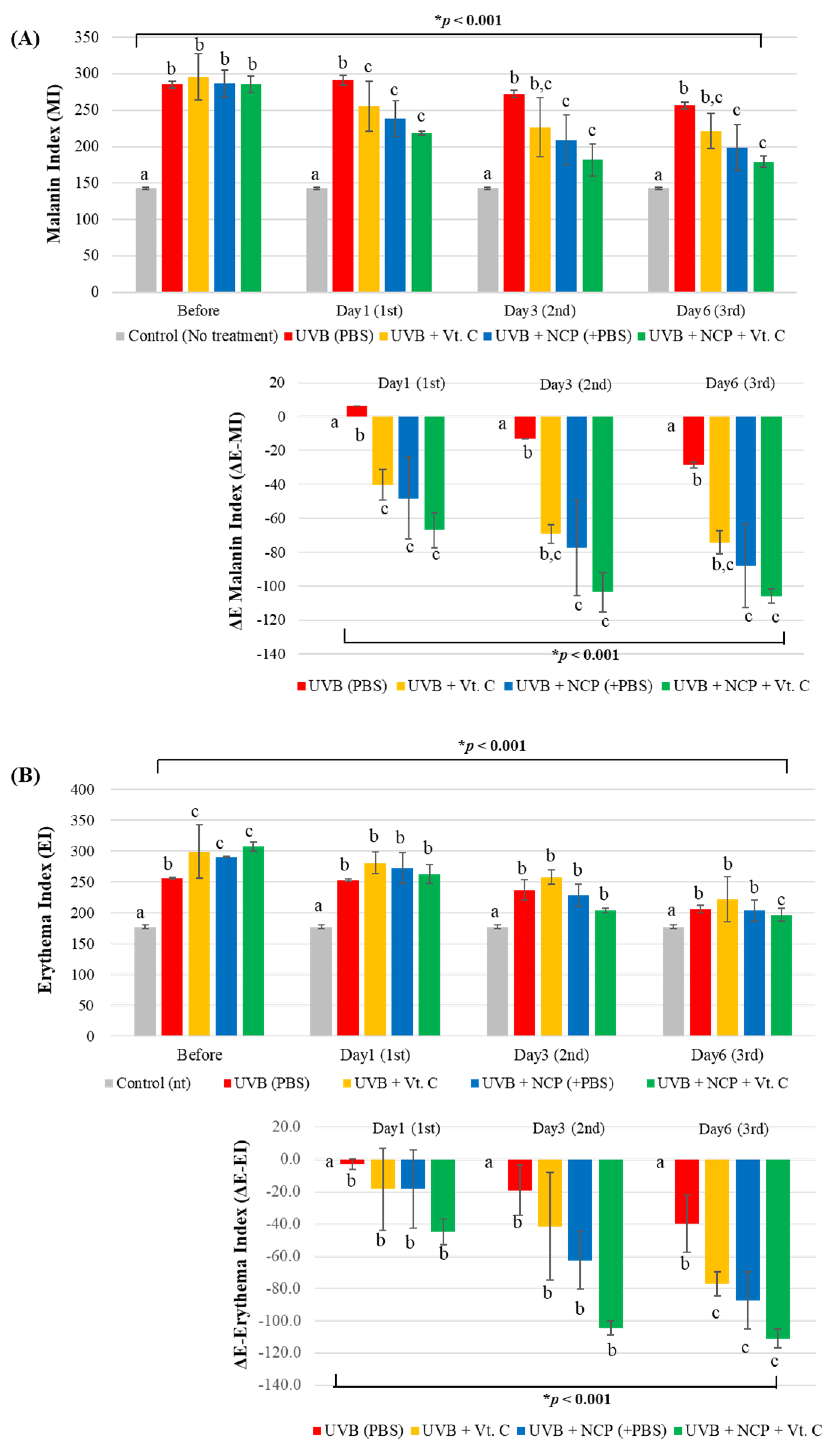
3.2. Assessment of Melanin Index (MI) and Erythema Index (EI) Value
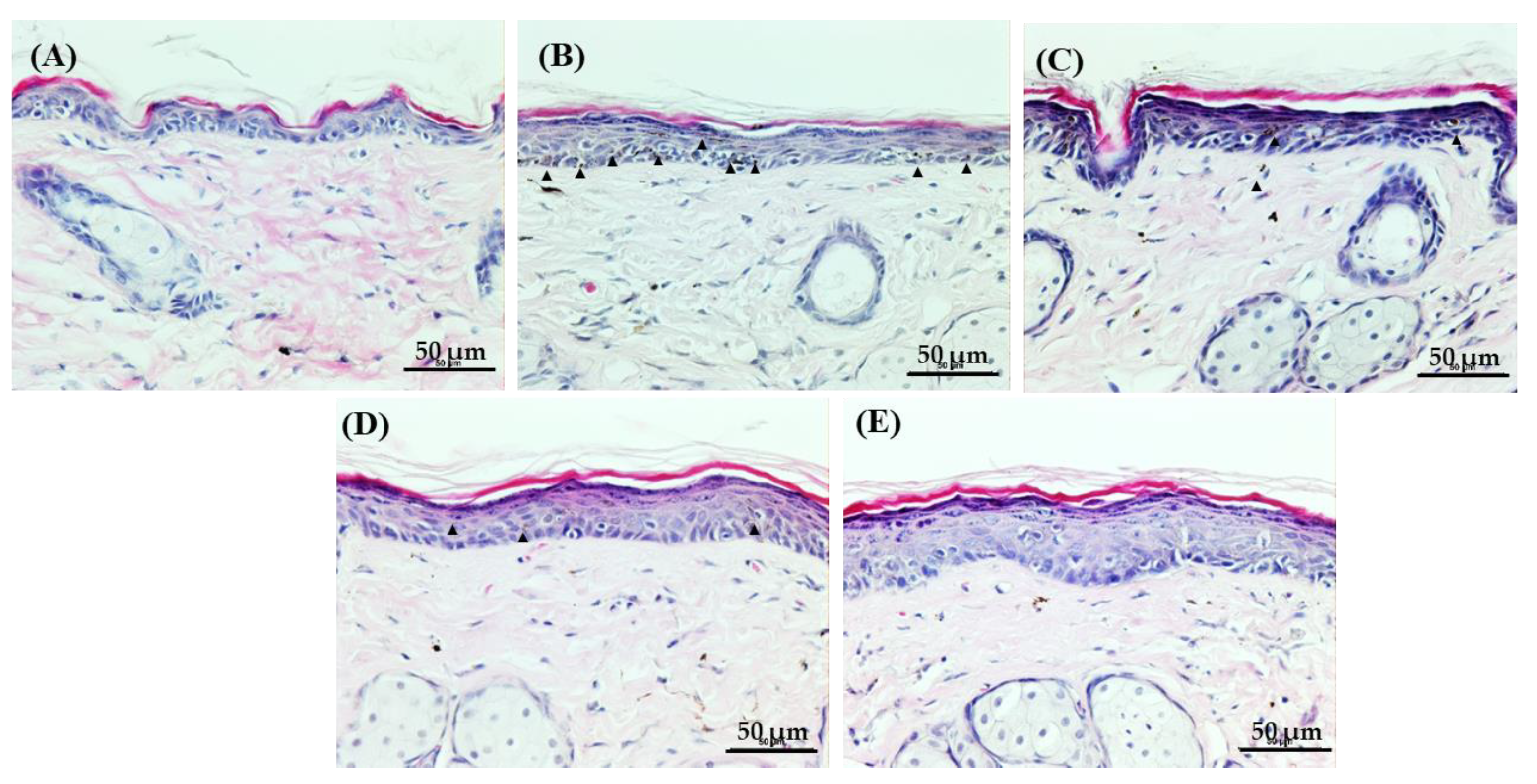
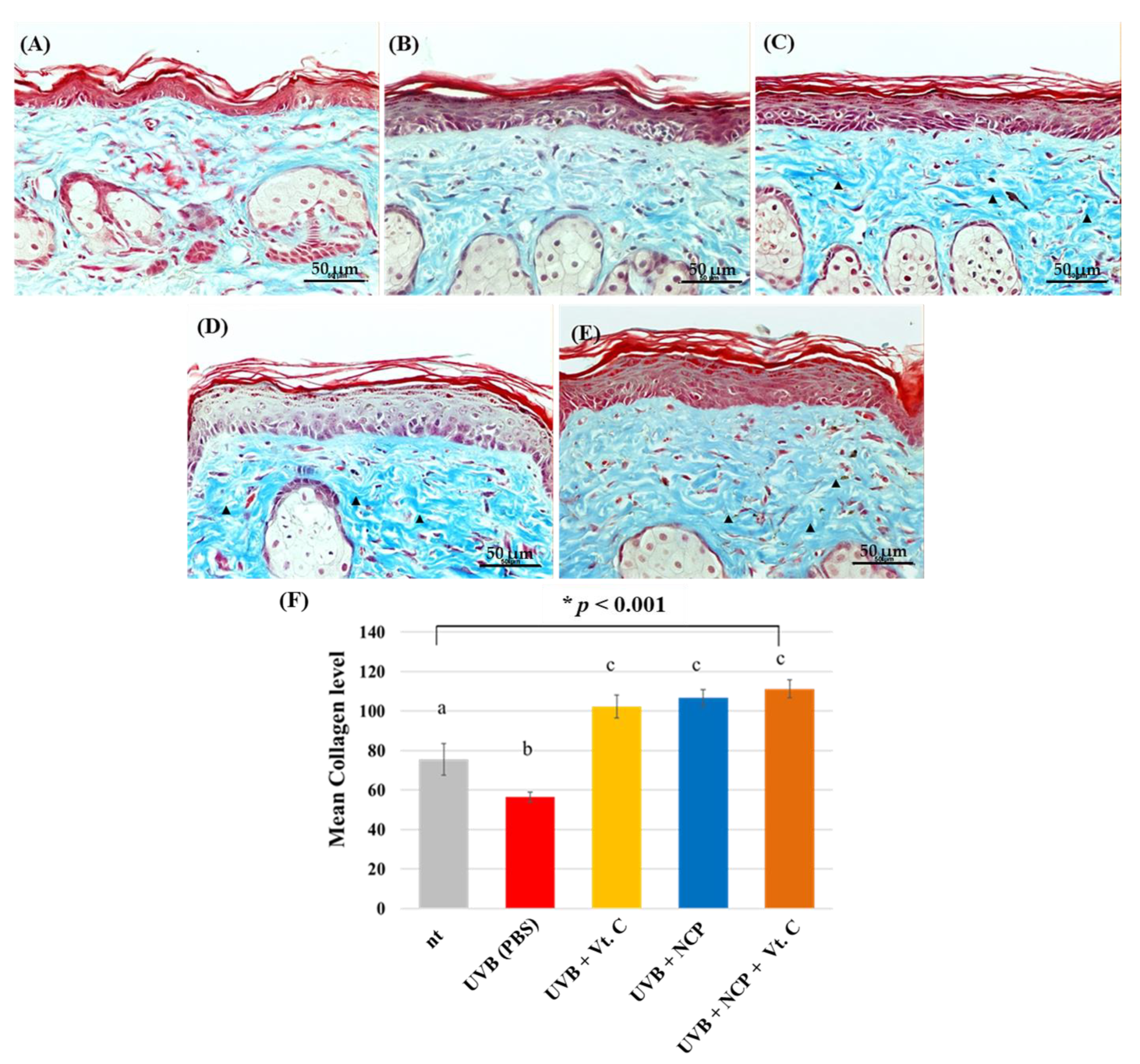
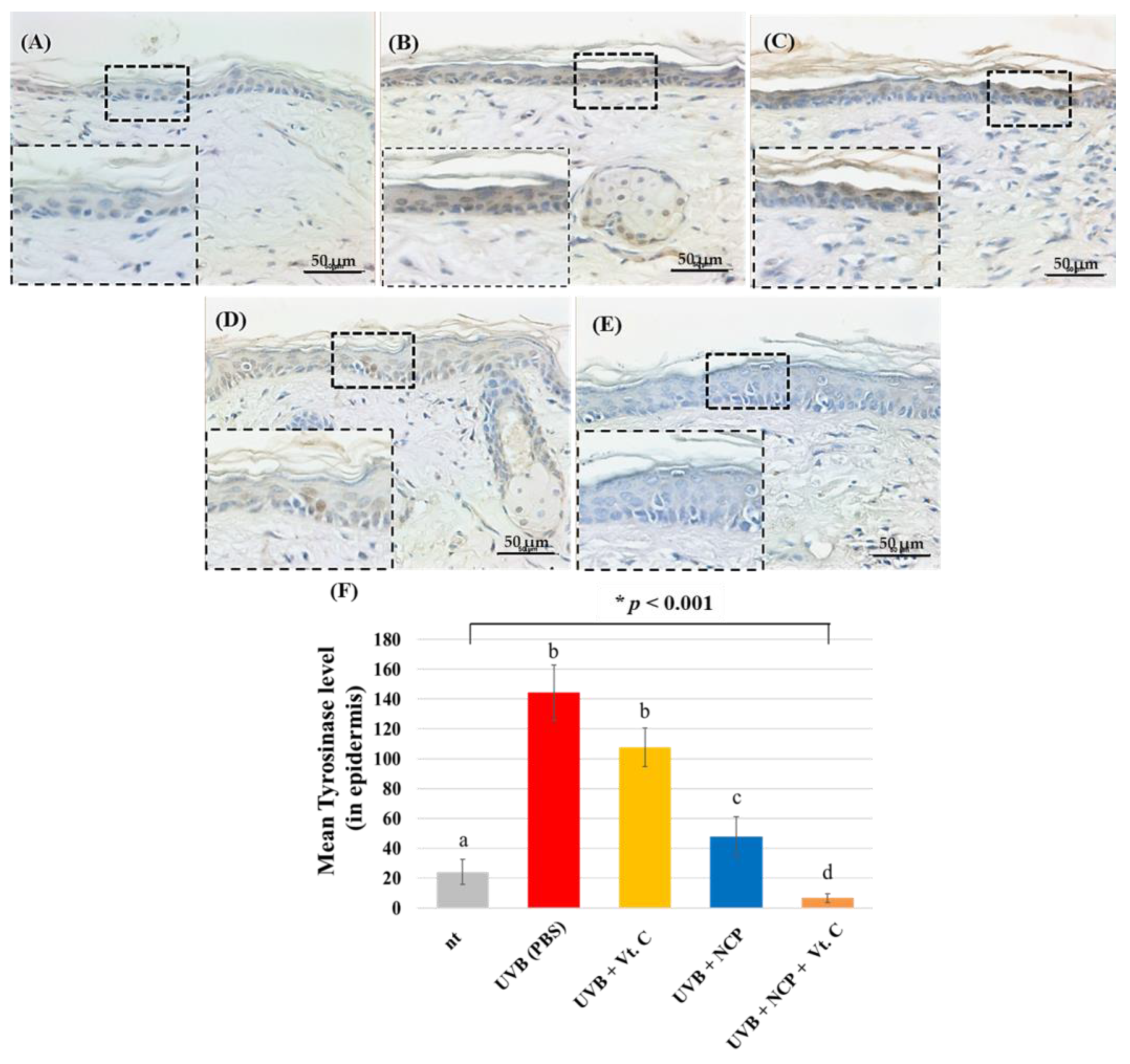
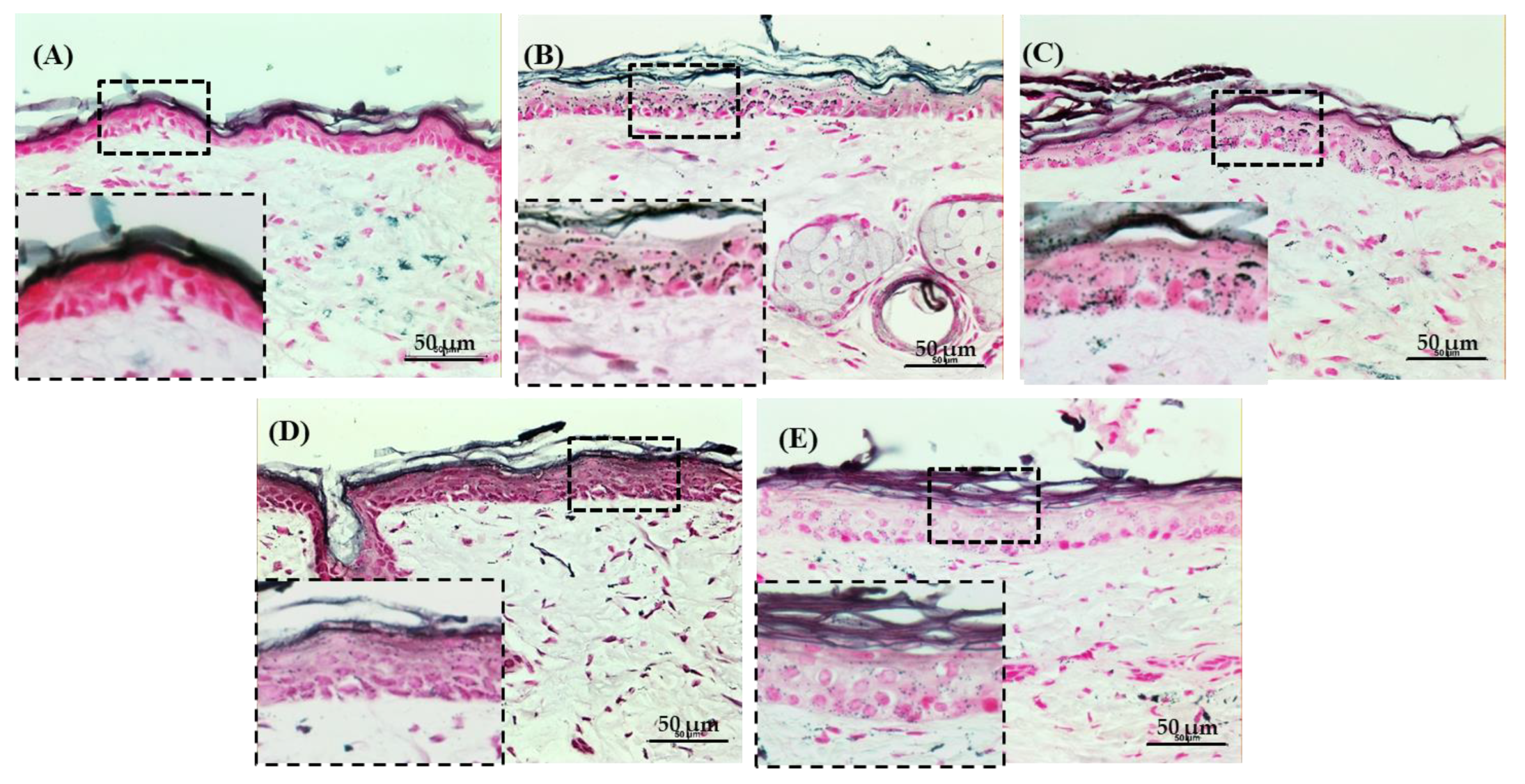
3.3. Histological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Martens, M.C.; Emmert, S.; Boeckmann, L. Sunlight, Vitamin D, and Xeroderma Pigmentosum. Adv. Exp. Med. Biol. 2020, 1268, 319–331. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wang, S.Q. Skin cancer: Role of ultraviolet radiation in carcinogenesis. Rev. Environ. Health 2014, 29, 265–273. [Google Scholar] [CrossRef]
- Olson, R.L.; Gaylor, J.; Everett, M.A. Skin color, melanin, and erythema. Arch. Derm. 1973, 108, 541–544. [Google Scholar] [CrossRef]
- Moreiras, H.; O’Connor, C.; Bell, M.; Tobin, D.J. Visible light and human skin pigmentation: The importance of skin phototype. Exp. Derm. 2021, 30, 1324–1331. [Google Scholar] [CrossRef]
- Adameyko, I.; Lallemend, F.; Aquino, J.B.; Pereira, J.A.; Topilko, P.; Muller, T.; Fritz, N.; Beljajeva, A.; Mochii, M.; Liste, I.; et al. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 2009, 139, 366–379. [Google Scholar] [CrossRef]
- Yazici, K.; Baz, K.; Yazici, A.E.; Kokturk, A.; Tot, S.; Demirseren, D.; Buturak, V. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J. Eur. Acad. Derm. Venereol. 2004, 18, 435–439. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lee, E.H.; Kang, T.H.; Ha, S.K.; Oh, M.S.; Kim, S.M.; Yoon, T.J.; Kang, C.; Park, J.H.; Kim, S.Y. Inhibitory effects of arbutin on melanin biosynthesis of alpha-melanocyte stimulating hormone-induced hyperpigmentation in cultured brownish guinea pig skin tissues. Arch. Pharm. Res. 2009, 32, 367–373. [Google Scholar] [CrossRef]
- Lutsenko, E.A.; Cárcamo, J.M.; Golde, D.W. Vitamin C prevents DNA mutation induced by oxidative stress. J. Biol. Chem. 2002, 277, 16895–16899. [Google Scholar] [CrossRef]
- Sanadi, R.M.; Deshmukh, R.S. The effect of Vitamin C on melanin pigmentation—A systematic review. J. Oral Maxillofac. Pathol. 2020, 24, 374–382. [Google Scholar] [CrossRef]
- Miao, F.; Su, M.Y.; Jiang, S.; Luo, L.F.; Shi, Y.; Lei, T.C. Intramelanocytic acidification plays a role in the antimelanogenic and antioxidative properties of vitamin C and its derivatives. Oxid. Med. Cell. Longev. 2019, 2019, 2084805. [Google Scholar] [CrossRef]
- Gref, R.; Delomenie, C.; Maksimenko, A.; Gouadon, E.; Percoco, G.; Lati, E.; Desmaele, D.; Zouhiri, F.; Couvreur, P. Vitamin C-squalene bioconjugate promotes epidermal thickening and collagen production in human skin. Sci. Rep. 2020, 10, 16883. [Google Scholar] [CrossRef]
- Lee, W.R.; Shen, S.C.; Kuo-Hsien, W.; Hu, C.H.; Fang, J.Y. Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J. Investig. Derm. 2003, 121, 1118–1125. [Google Scholar] [CrossRef]
- Lee, H.Y.; Choi, J.H.; Hong, J.W.; Kim, G.C.; Lee, H.J. Comparative study of the Ar and He atmospheric pressure plasmas on E-cadherin protein regulation for plasma-mediated transdermal drug deliver. J. Phys. D Appl. Phys. 2018, 51, 11. [Google Scholar] [CrossRef]
- Choi, J.H.; Nam, S.H.; Song, Y.S.; Lee, H.W.; Lee, H.J.; Song, K.; Hong, J.W.; Kim, G.C. Treatment with low-temperature atmospheric pressure plasma enhances cutaneous delivery of epidermal growth factor by regulating E-cadherin-mediated cell junctions. Arch. Derm. Res. 2014, 306, 635–643. [Google Scholar] [CrossRef]
- Nam, S.H.; Choi, B.B.R.; Kim, G.C. The Whitening Effect and histological safety of nonthermal atmospheric plasma inducing tooth bleaching. Int. J. Environ. Res. Public Health 2021, 18, 4714. [Google Scholar] [CrossRef]
- Park, N.S.; Yun, S.E.; Lee, H.Y.; Lee, H.J.; Choi, J.H.; Kim, G.C. No-ozone cold plasma can kill oral pathogenic microbes in H2O2-dependent and independent manner. Sci. Rep. 2022, 12, 7597. [Google Scholar] [CrossRef]
- Tan, F.; Wang, Y.; Zhang, S.; Shui, R.; Chen, J. Plasma dermatology: Skin therapy using cold atmospheric plasma. Front. Oncol. 2022, 12, 918484. [Google Scholar] [CrossRef]
- Choi, J.H.; Song, Y.S.; Song, K.; Lee, H.J.; Hong, J.W.; Kim, G.C. Skin renewal activity of non-thermal plasma through the activation of beta-catenin in keratinocytes. Sci. Rep. 2017, 7, 6146. [Google Scholar] [CrossRef]
- Choi, J.H.; Song, Y.S.; Lee, H.J.; Hong, J.W.; Kim, G.C. Inhibition of inflammatory reactions in 2,4-dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Sci. Rep. 2016, 6, 27376. [Google Scholar] [CrossRef]
- You, Y.J.; Wu, P.Y.; Liu, Y.J.; Hou, C.W.; Wu, C.S.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. Sesamol inhibited ultraviolet radiation-induced hyperpigmentation and damage in C57BL/6 mouse skin. Antioxidants 2019, 8, 207. [Google Scholar] [CrossRef]
- Choi, W.; Yin, L.; Smuda, C.; Batzer, J.; Hearing, V.J.; Kolbe, L. Molecular and histological characterization of age spots. Exp. Derm. 2017, 26, 242–248. [Google Scholar] [CrossRef]
- Cohen, M.; Austin, E.; Masub, N.; Kurtti, A.; George, C.; Jagdeo, J. Home-based devices in dermatology: A systematic review of safety and efficacy. Arch. Derm. Res. 2022, 314, 239–246. [Google Scholar] [CrossRef]
- Mazzoni, D.; Lin, M.J.; Dubin, D.P.; Khorasani, H. Review of non-invasive body contouring devices for fat reduction, skin tightening and muscle definition. Australas J. Derm. 2019, 60, 278–283. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Rozman, B.; Gasperlin, M. Stability of vitamins C and E in topical microemulsions for combined antioxidant therapy. Drug Deliv. 2007, 14, 235–245. [Google Scholar] [CrossRef]
- Traikovich, S.S. Use of topical ascorbic acid and its effects on photodamaged skin topography. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 1091–1098. [Google Scholar] [CrossRef]
- Kim, H.Y.; Agrahari, G.; Lee, M.J.; Tak, L.J.; Ham, W.K.; Kim, T.Y. Low-temperature argon plasma regulates skin moisturizing and melanogenesis-regulating markers through yes-associated protein. Int. J. Mol. Sci. 2021, 22, 1895. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Yokota, T.; Nishio, H.; Kubota, Y.; Mizoguchi, M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998, 11, 355–361. [Google Scholar] [CrossRef]
- Maranduca, M.A.; Branisteanu, D.; Serban, D.N.; Branisteanu, D.C.; Stoleriu, G.; Manolache, N.; Serban, I.L. Synthesis and physiological implications of melanic pigments. Oncol. Lett. 2019, 17, 4183–4187. [Google Scholar] [CrossRef] [PubMed]
- Urabe, K.; Aroca, P.; Tsukamoto, K.; Mascagna, D.; Palumbo, A.; Prota, G.; Hearing, V.J. The inherent cytotoxicity of melanin precursors: A revision. Biochim. Biophys. Acta 1994, 1221, 272–278. [Google Scholar] [CrossRef]
- Shim, J.H. Inhibitory Effects of cycloheterophyllin on melanin synthesis. Molecules 2021, 26, 2526. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, S.-H.; Choi, J.-H.; Kim, G.-C. Topical Application of No-Ozone Cold Plasma in Combination with Vitamin C Reduced Skin Redness and Pigmentation of UV-Irradiated Mice. Biomedicines 2023, 11, 1563. https://doi.org/10.3390/biomedicines11061563
Nam S-H, Choi J-H, Kim G-C. Topical Application of No-Ozone Cold Plasma in Combination with Vitamin C Reduced Skin Redness and Pigmentation of UV-Irradiated Mice. Biomedicines. 2023; 11(6):1563. https://doi.org/10.3390/biomedicines11061563
Chicago/Turabian StyleNam, Seoul-Hee, Jeong-Hae Choi, and Gyoo-Cheon Kim. 2023. "Topical Application of No-Ozone Cold Plasma in Combination with Vitamin C Reduced Skin Redness and Pigmentation of UV-Irradiated Mice" Biomedicines 11, no. 6: 1563. https://doi.org/10.3390/biomedicines11061563
APA StyleNam, S.-H., Choi, J.-H., & Kim, G.-C. (2023). Topical Application of No-Ozone Cold Plasma in Combination with Vitamin C Reduced Skin Redness and Pigmentation of UV-Irradiated Mice. Biomedicines, 11(6), 1563. https://doi.org/10.3390/biomedicines11061563





