Interaction of Drug-Sensitive and -Resistant Human Melanoma Cells with HUVEC Cells: A Label-Free Cell-Based Impedance Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Transmission Electron Microscopy
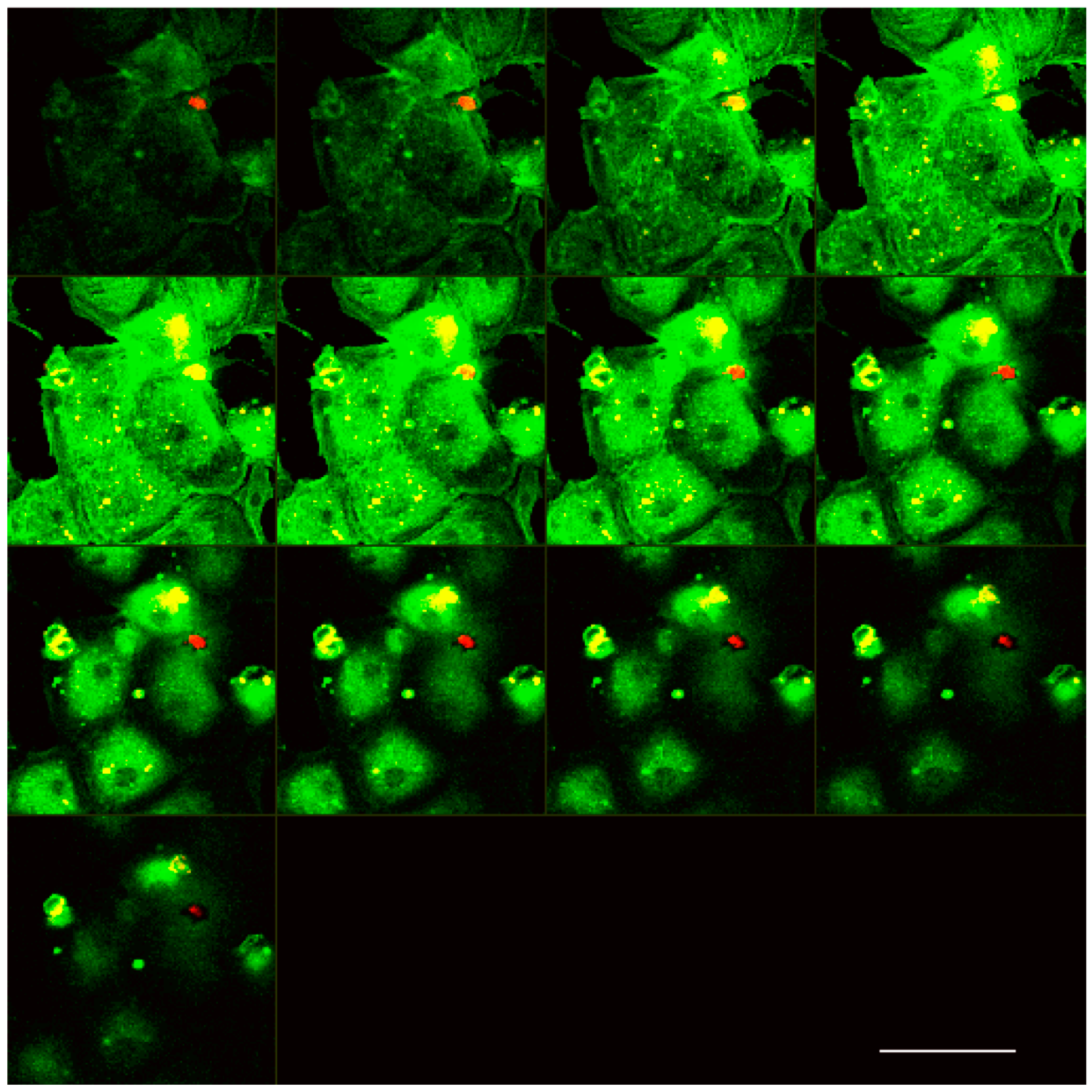
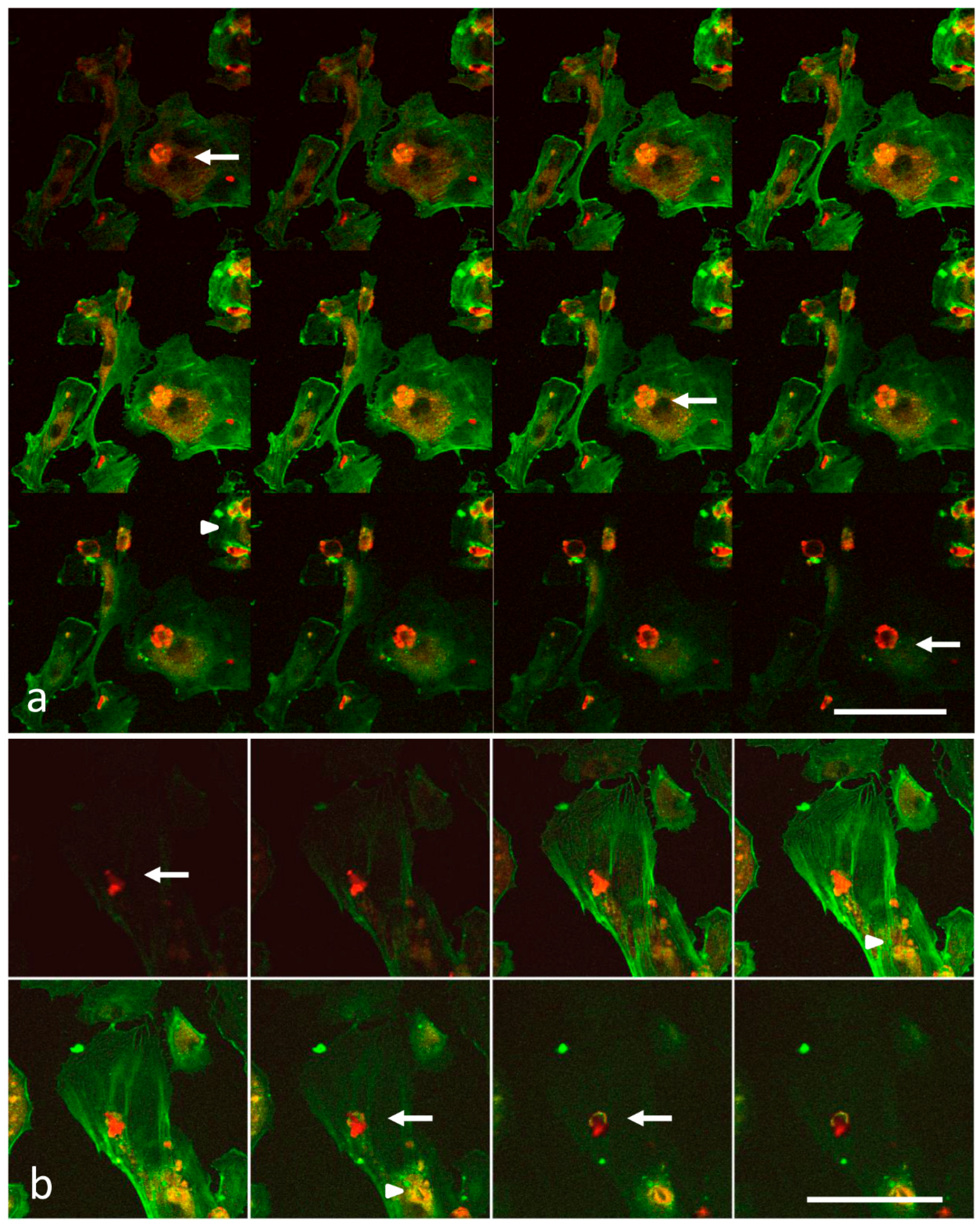
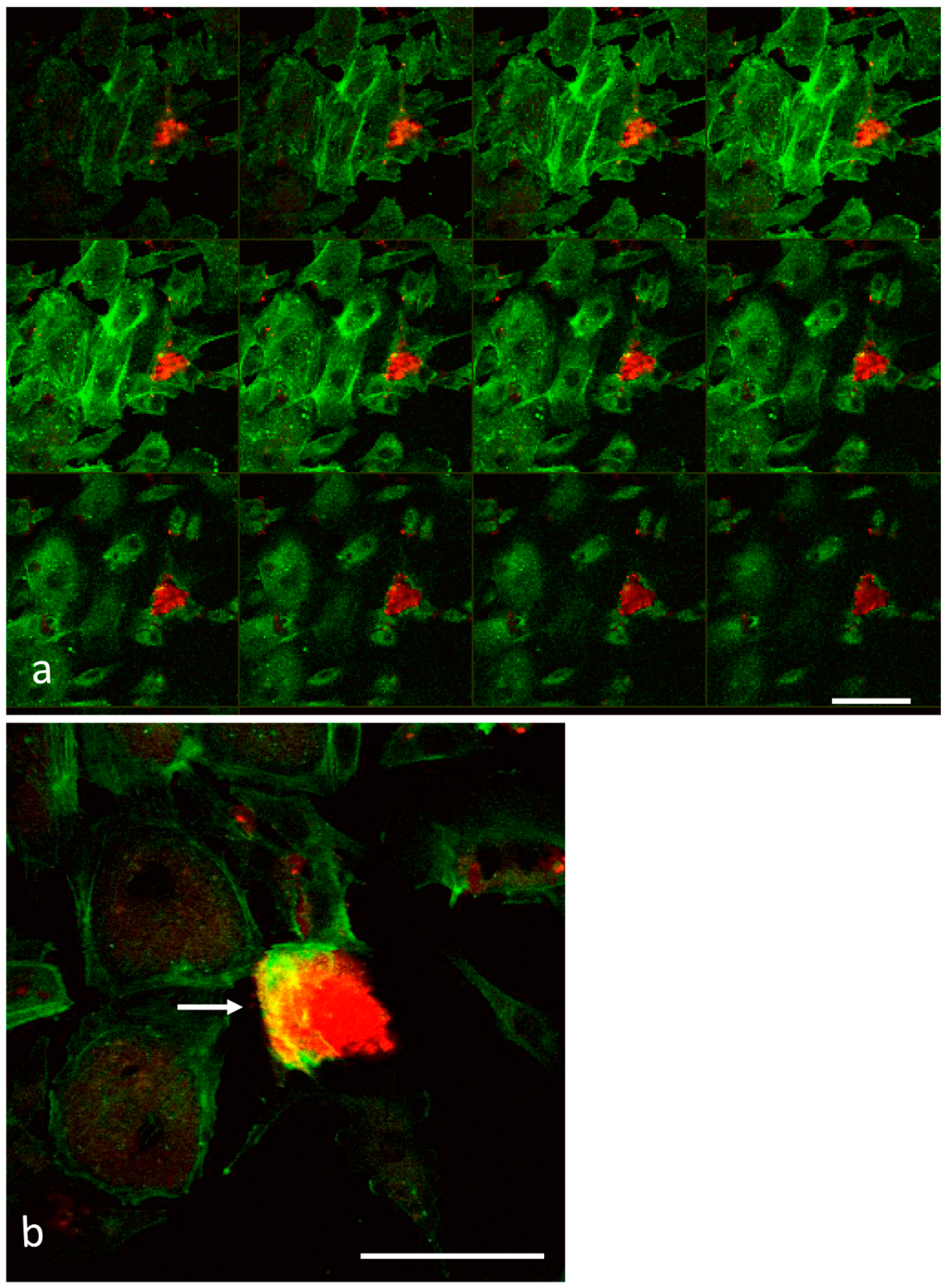
2.3. Laser Scanning Confocal Microscopy
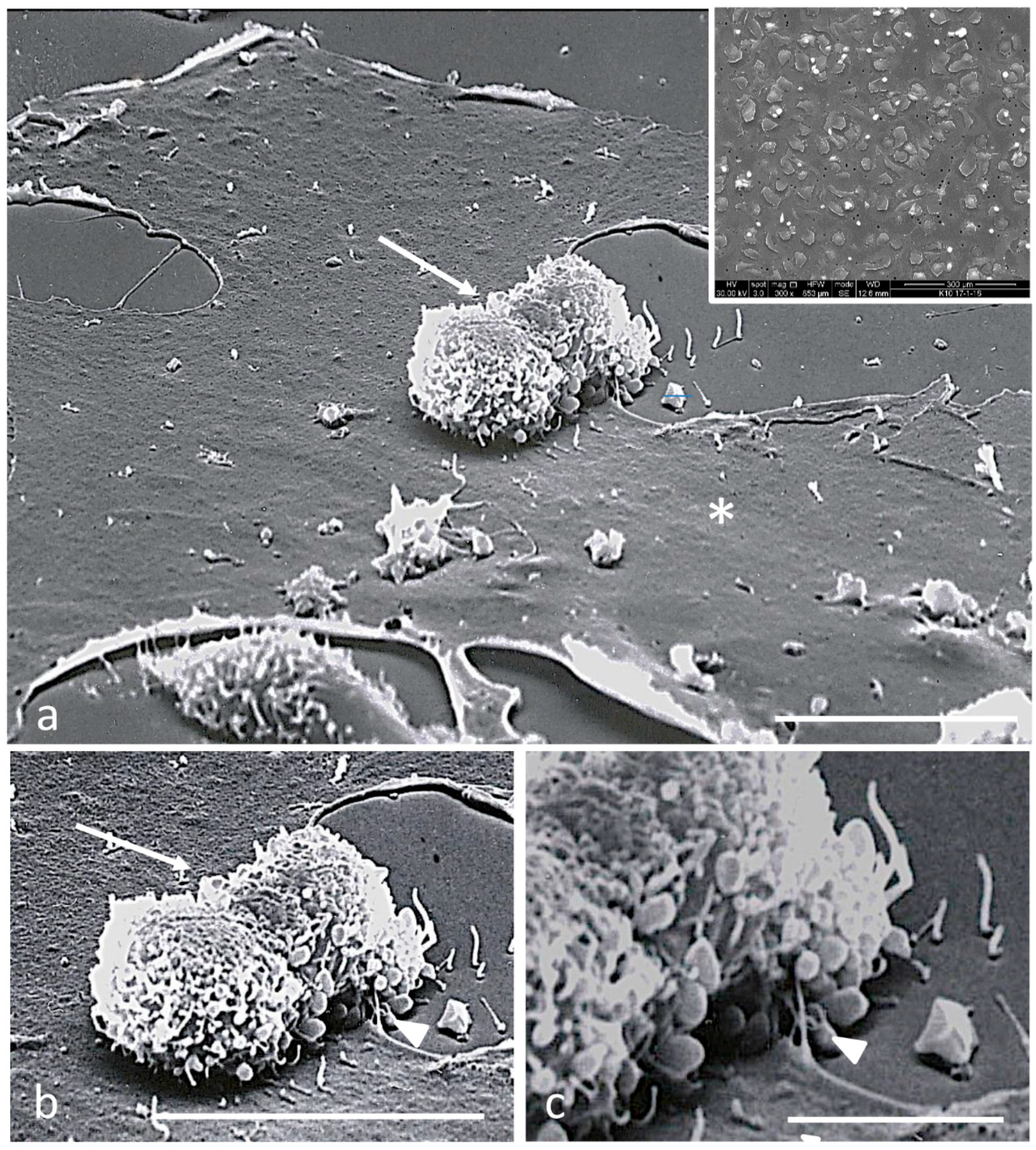
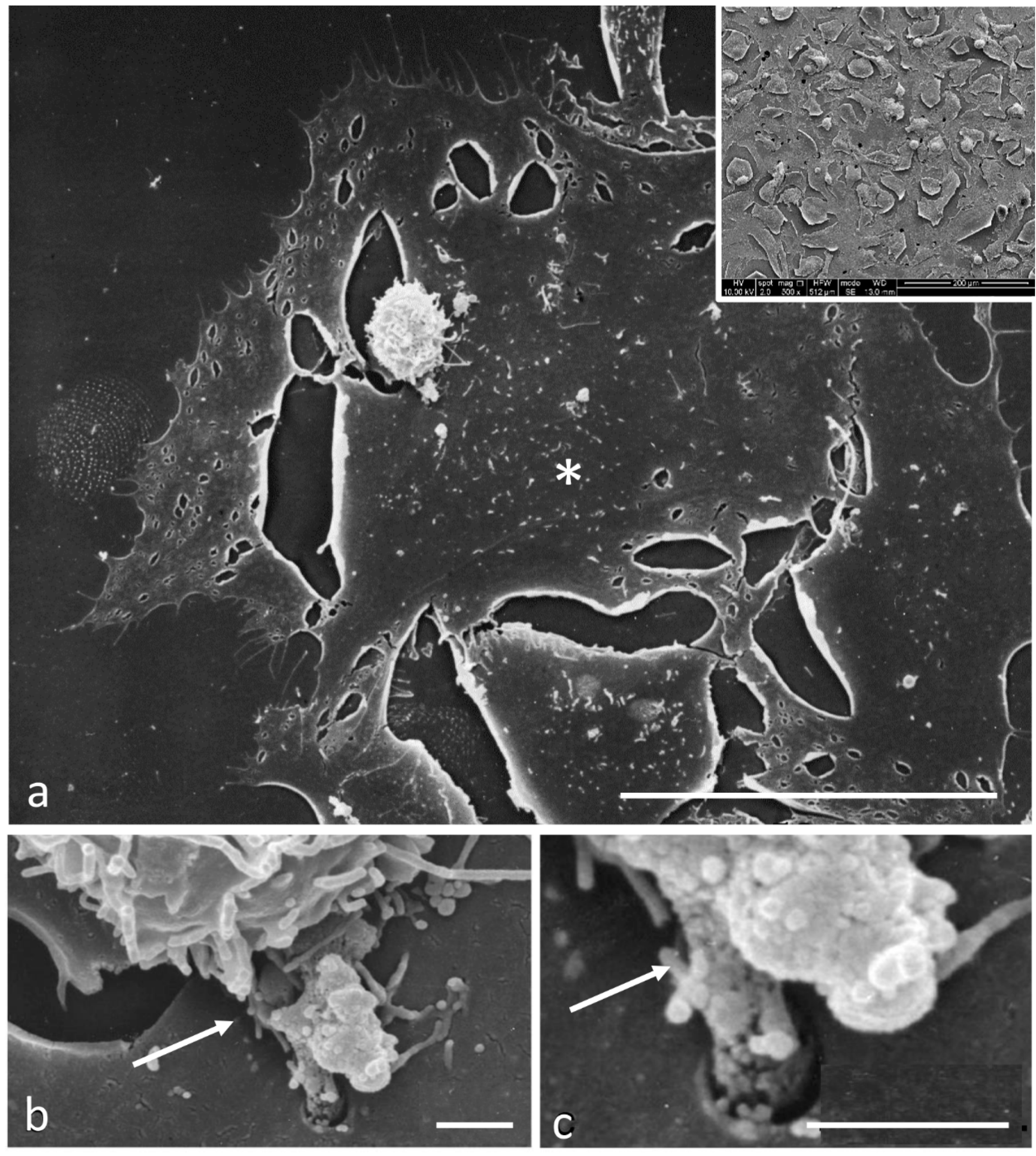
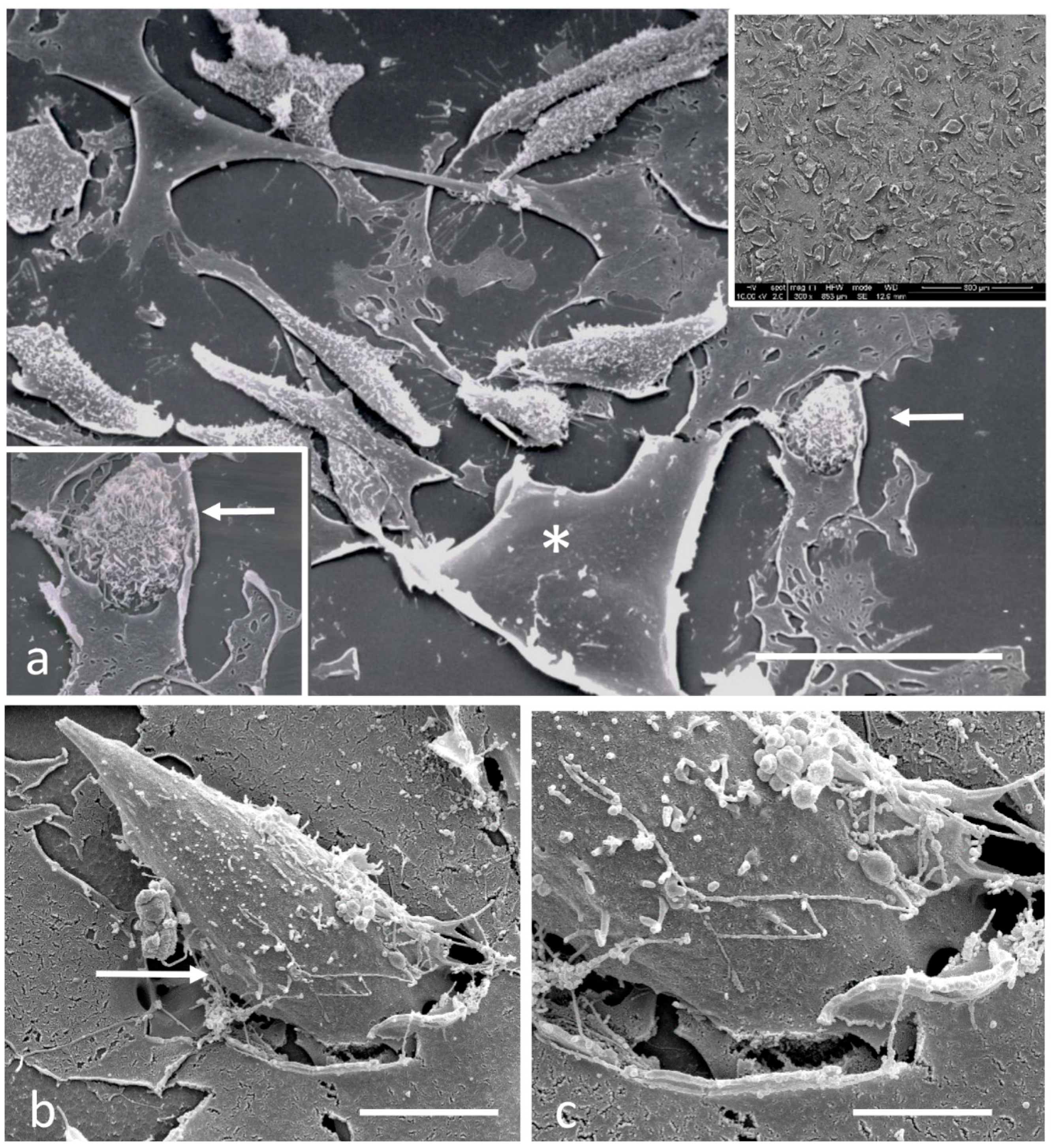
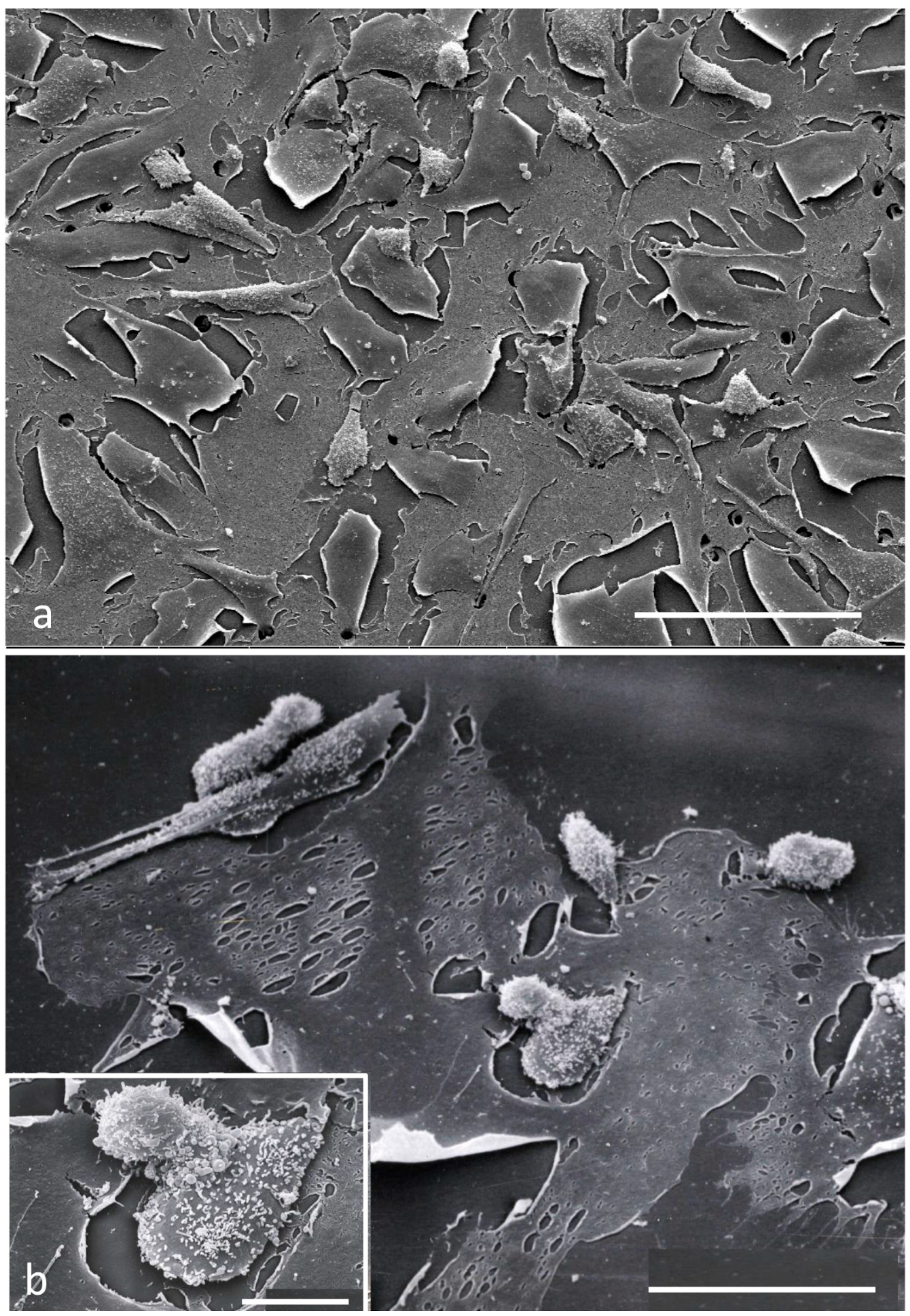
2.4. Scanning Electron Microscopy
2.5. Gelatine Zymography
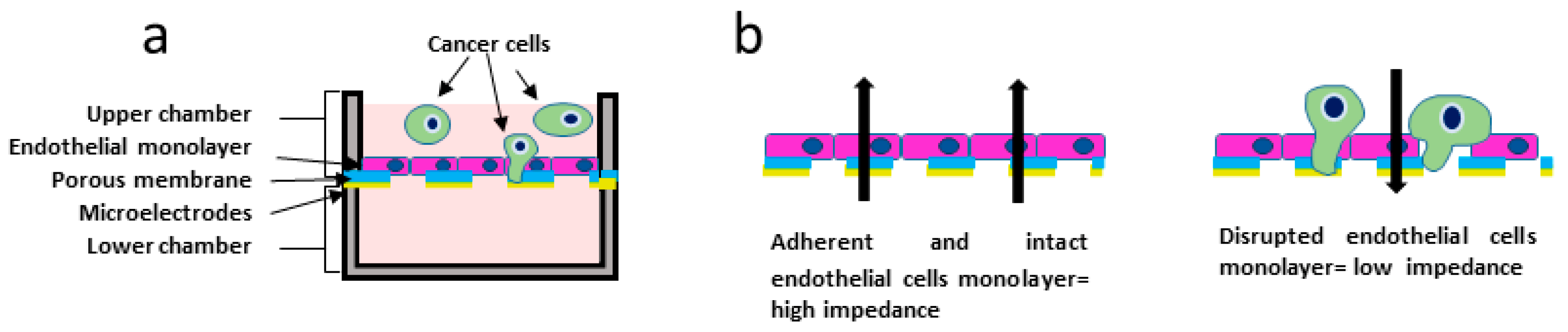
2.6. Electrical Impedance Assay
3. Results
3.1. Analysis of Melanoma Cell–HUVEC Interaction by Laser Scanning Confocal Microscopy
3.2. Electron Microscopy Observations
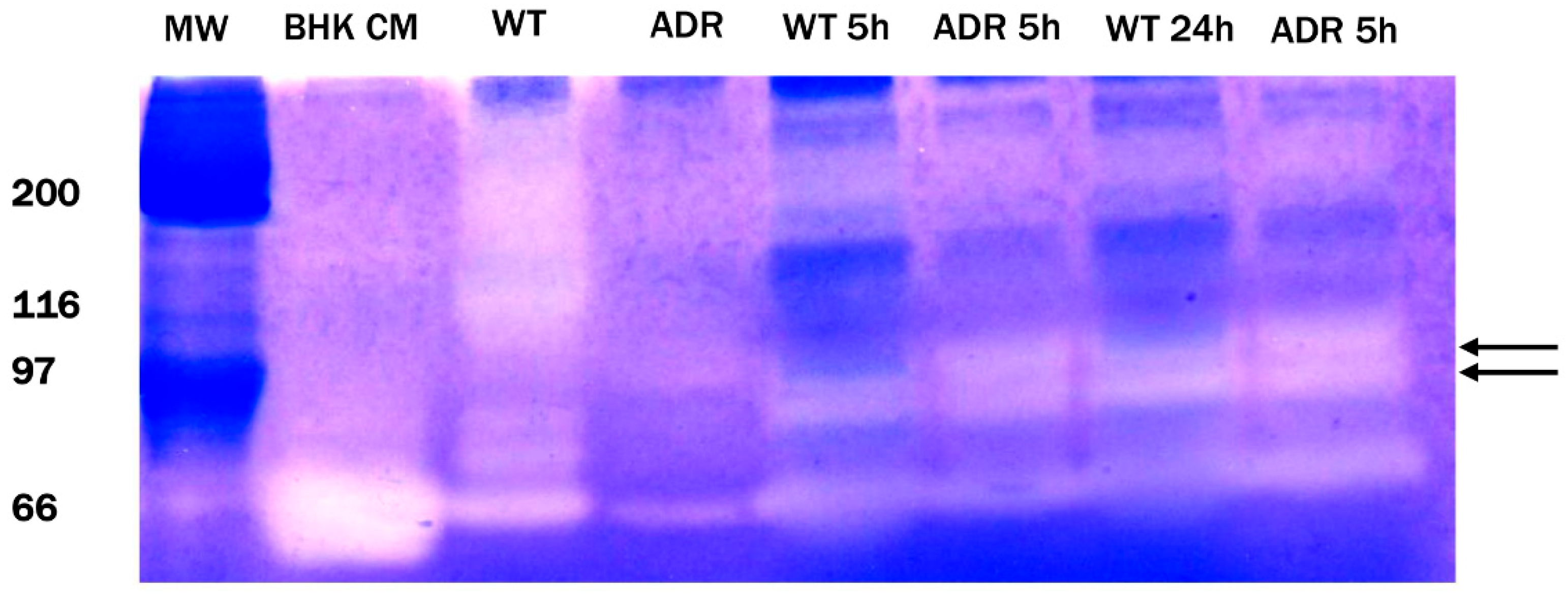
3.3. Gelatine Zymography
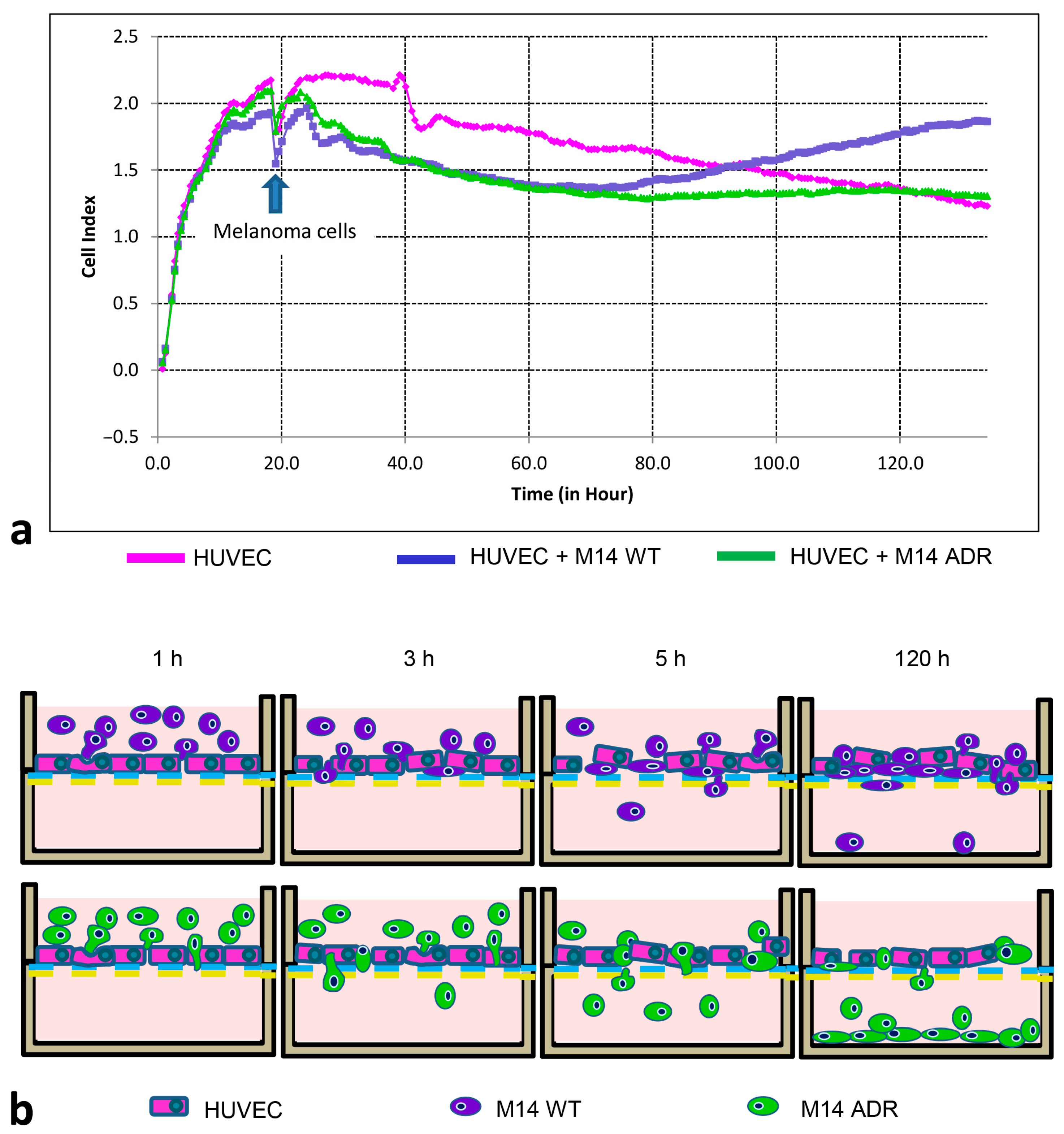
3.4. Electrical Impedance Assay Technique
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liotta, L.A.; Rao, C.N.; Barsky, S.H. Tumor invasion and the extracellular matrix. Lab. Investig. 1983, 49, 636–649. [Google Scholar] [PubMed]
- Orr, F.W.; Wang, H.H.; Lafrenie, R.M.; Scherbarth, S.; Nance, D.M. Interactions between cancer cells and the endothelium in metastasis. J. Pathol. 2000, 190, 310–329. [Google Scholar] [CrossRef]
- Haier, J.; Korb, T.; Hotz, B.; Spiegel, H.-U.; Senninger, N. An intravital model to monitor steps of metastatic tumor cell adhesion within the epatic microcirculation. J. Gastrointest. Surg. 2003, 7, 507–515. [Google Scholar] [CrossRef]
- Salminen, A.T.; Allahyari, Z.; Gholizadeh, S.; McCloskey, M.C.; Ajalik, R.; Cottle, R.N.; Gaborski, T.R.; McGrath, J.L. In vitro Studies of Transendothelial Migration for Biological and Drug Discovery. Front. Med. Technol. 2020, 2, 600616. [Google Scholar] [CrossRef]
- Nicolson, G.L. Metastatic tumor cell interactions with endothelium, basement membrane and tissue. Curr. Opin. Cell Biol. 1989, 1, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Cancer metastasis: Tumor cell and host organ properties important in metastatic to specific secondary sites. Biochim. Biophys. Acta 1988, 948, 175–224. [Google Scholar] [CrossRef]
- Al-Mehdi, A.B.; Tozawa, K.; Fisher, A.B.; Shientag, L.; Lee, A.; Muschel, R.J. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: A new model for metastasis. Nat. Med. 2000, 6, 100–102. [Google Scholar] [CrossRef]
- Friedl, P.; Noble, P.B.; Walton, P.A.; Laird, D.W.; Chauvin, P.J.; Tabah, R.J.; Black, M.; Zänker, K.S. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 1995, 55, 4557–4560. [Google Scholar]
- Heyder, C.; Gloria-Maercker, E.; Entschladen, F.; Hatzmann, W.; Niggemann, B.; Zänker, K.S.; Dittmar, T. Realtime visualization of tumor cell/endothelial cell interactions during transmigration across the endothelial barrier. J. Cancer Res. Clin. Oncol. 2002, 128, 533–538. [Google Scholar] [CrossRef]
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef]
- Roetger, A.; Merscchjann, A.; Dittmar, T.; Jackisch, C.; Barnekow, A.; Brandt, B. Selection of potentially metastatic subpopulations expressing c-erbB-2 from breast cancer tissue by use of an extravasation model. Am. J. Pathol. 1998, 153, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Voura, E.B.; Sandig, M.; Kalnins, V.I.; Siu, C. Cell shape changes and cytoskeleton reorganization during transendothelial migration of human melanoma cells. Cell Tissue Res. 1998, 293, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef]
- Hamidi, H.; Lilja, J.; Ivaska, J. Using xCELLigence RTCA Instrument to Measure Cell Adhesion. Bio. Protoc. 2017, 7, e2646. [Google Scholar] [CrossRef] [PubMed]
- Scrace, S.; O’Neill, E.; Hammond, E.M.; Pires, I.M. Use of the xCELLigence system for real-time analysis of changes in cellular motility and adhesion in physiological conditions. Methods Mol. Biol. 2013, 104, 6295–6306. [Google Scholar] [CrossRef]
- Kumar, S.; Lu, B.; Davra, V.; Hornbeck, P.; Machida, K.; Birge, R.B. Tyrosine Phosphorylation Regulates PDGF-BB-inducible Src Activation and Breast Tumorigenicity and Metastasis. Mol. Cancer Res. 2018, 16, 173–183. [Google Scholar] [CrossRef]
- Mudduluru, G.; Large, N.; Park, T. Impedance-based Real-time Measurement of Cancer Cell Migration and Invasion. J. Vis. Exp. 2020, 158, e60997. [Google Scholar] [CrossRef]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Salvatore, G.; Mondello, F.; Arancia, G.; Molinari, A. Terpinen-4-ol, the main component of Melaleuca alternifolia (tea tree) oil inhibits the in vitro growth of human melanoma cells. J. Investig. Dermatol. 2004, 122, 349–360. [Google Scholar] [CrossRef]
- Molinari, A.; Stringaro, A.; Gentile, M.; Colone, M.; Toccacieli, L.; Arancia, G. Invasive properties of multidrug resistant human melanoma cells. Ital. J. Anat. Embryol. 2005, 110, 135–141. [Google Scholar]
- Colone, M.; Calcabrini, A.; Toccacieli, L.; Bozzuto, G.; Stringaro, A.; Gentile, M.; Cianfriglia, M.; Ciervo, A.; Caraglia, M.; Budillon, A.; et al. The multidrug transporter P-Glycoprotein: A mediator of melanoma invasion? J. Investig. Dermatol. 2008, 128, 957–971. [Google Scholar] [CrossRef]
- Bozzuto, G.; Condello, M.; Molinari, A. Migratory behaviour of tumour cells: A scanning electron microscopy study. Ann. Ist. Super Sanità 2015, 51, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.J. Live Cell Imaging and Analysis of Cancer-Cell Transmigration Through Endothelial Monolayers. Methods Mol. Biol. 2022, 2441, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Casali, B.C.; Gozzer, L.T.; Baptista, M.P.; Altei, W.F.; Selistre-de-Araújo, H.S. The Effects of αvβ3 Integrin Blockage in Breast Tumor and Endothelial Cells under Hypoxia In Vitro. Int. J. Mol. Sci. 2022, 23, 1745. [Google Scholar] [CrossRef] [PubMed]
- Pietrovito, L.; Leo, A.; Gori, V.; Lulli, M.; Parri, M.; Becherucci, V.; Piccini, L.; Bambi, F.; Taddei, M.L.; Chiarugi, P. Bone marrow--derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition. Mol. Oncol. 2018, 12, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, K.; Kwiecień, E.; Sośniak, J.; Zimoląg, E.; Guzik, E.; Sroka, J.; Madeja, Z.; Czyż, J. Fenofibrate Interferes with the Diapedesis of Lung Adenocarcinoma Cells through the Interference with Cx43/EGF-Dependent Intercellular Signaling. Cancers 2018, 10, 363. [Google Scholar] [CrossRef]
- Arvanitis, C.; Khuon, S.; Spann, R.; Ridge, K.M.; Chew, T.-M. Structure and biomechanics of the endothelial transcellular circumferential invasion array in tumor invasion. PLoS ONE 2014, 9, e89758. [Google Scholar] [CrossRef]
- Peyri, N.; Berard, M.; Fauvel-Lafeve, F.; Trochon, V.; Arbeille, B.; Lu, H.; Legrand, C.; Crepin, M. Breast tumor cells transendothelial migration induces endothelial cell anoikis through extracellular matrix degradation. Anticancer Res. 2009, 29, 2347–2355. [Google Scholar]
- Mierke, C.T.; Zitterbart, D.P.; Kollmannsberger, P.; Raupach, C.; Schlötzer-Schrehardt, U.; Goecke, T.W.; Behrens, J.; Fabry, B. Breakdown of the endothelial barrier function in tumor cell transmigration. Biophys. J. 2008, 94, 2832–2846. [Google Scholar] [CrossRef]
- Leroy-Dudal, J.; Demeilliers, C.; Gallet, O.; Pauthe, E.; Dutoit, S.; Agniel, R.; Gauduchon, P.; Carreiras, F. Transmigration of human ovarian adenocarcinoma cells through endothelial extracellular matrix involves alpha V integrins and the participation of MMP2. Int. J. Cancer 2005, 114, 531–543. [Google Scholar] [CrossRef]
- Greco, C.; Zupi, G. Biological features and in vitro chemosensitivity of a new model of human melanoma. Anticancer Res. 1987, 7, 839–844. [Google Scholar]
- Battaglia, M.F.; Balducci, L.; Finocchiaro, M. Use of Hoechst 33258 fluorochrome for detection of mycoplasma contamination in cell cultures: Development of a technique based on simultaneous fixation and staining. Boll. Ist. Sieroter. Milan. 1980, 59, 155–158. [Google Scholar] [PubMed]
- Overall, C.M.; King, A.E.; Sam, D.K.; Ong, A.D.; Lau, T.T.; Wallon, U.M.; DeClerck, Y.A.; Atherstone, J. Identification of the tissue inhibitor of metalloproteinases-2 (TIMP-2) binding site on the hemopexin carboxyl domain of human gelatinase A by site-directed mutagenesis: The hierarchical role in binding TIMP-2 of the unique cationic clusters of hemopexin modules III and IV. J. Biol. Chem. 1999, 274, 4421–4429. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.; Kirstein, S. Real-time, label-free monitoring of cellular invasion and migration with the xCELLigence system. Nat. Methods 2009, 6, v–vi. [Google Scholar] [CrossRef]
- Zink, D.; Fischer, A.; Nickerson, J. Nuclear structure in cancer cells. Nat. Rev. Cancer 2004, 4, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Gerlitz, G. The Emerging Roles of Heterochromatin in Cell Migration. Front. Cell Dev. Biol. 2020, 8, 394. [Google Scholar] [CrossRef]
- Rahim, S.; Üren, A. A real-time electrical impedance-based technique to measure invasion of endothelial cell monolayer by cancer cells. J. Vis. Exp. 2011, 50, 2792. [Google Scholar] [CrossRef]
- Wyckoff, J.B.; Jones, J.G.; Condeelis, J.S.; Segall, J.E. A critical step in metastasis:in vivo analysis of intravasation at the primary tumour. Cancer Res. 2000, 60, 2504–2511. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, K. Visualizing cancer extravasation: From mechanistic studies to drug development. Cancer Metastatis Rev. 2021, 40, 71–88. [Google Scholar] [CrossRef]
- Reymond, N.; d’Agua, B.B.; Ridley, A.J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 2013, 13, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Strilic, B.; Offermanns, S. Intravascular survival and extravasation of tumour cells. Cancer Cell 2017, 32, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef]
- Kim, S.; Wan, Z.; Jeon, J.S.; Kamm, R.D. Microfluidic vascular models of tumor cell extravasation. Front. Oncol. 2022, 12, 1052192. [Google Scholar] [CrossRef]
- Simoneau, B.; Houle, F.; Huot, J. Regulation of endothelial permeability and transendothelial migration of cancer cells by tropomyosin-1 phosphorylation. Vasc. Cell 2012, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Endothelial cell’s biomechanical properties are regulated by invasive cancer cells. Mol. Biosyst. 2012, 8, 1639–1649. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- García-Román, J.; Zentella-Dehesa, A. Vascular permeability changes involved in tumour metastasis. Cancer Lett. 2013, 335, 259–269. [Google Scholar] [CrossRef]
- De Bruyn, P.P.; Cho, Y. Vascular endothelial invasion via transcellular passage by malignant cells in the primary stage of metastases formation. J. Ultrastruct. Res. 1982, 81, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Khuon, S.; Liang, L.; Dettman, R.W.; Sporn, P.H.S.; Wysolmerski, R.B.; Chew, T.-L. Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: A three-dimensional FRET study. J. Cell Sci. 2010, 123, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Buccione, R.; Caldieri, G.; Ayala, I. Invadopodia: Specialized tumour cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009, 28, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.L.; Auger, F.A.; Huot, J. Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP. Oncogene 2006, 25, 6563–6573. [Google Scholar] [CrossRef] [PubMed]
- Voura, E.B.; Ramjeesingh, R.A.; Montgomery, A.M.; Siu, C.H. Involvement of integrin alpha(v)beta(3) and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol. Biol. Cell 2001, 12, 2699–2710. [Google Scholar] [CrossRef]
- Clucas, J.; Valderrama, F. ERM proteins in cancer progression. J. Cell Sci. 2014, 127, 267–275. [Google Scholar] [CrossRef]
- Luciani, F.; Molinari, A.; Lozupone, F.; Calcabrini, A.; Lugini, L.; Stringaro, A.; Puddu, P.; Arancia, G.; Cianfriglia, M.; Fais, S. P-glycoprotein-actin association through ERM family proteins: A role in P-glycoprotein function in human cells of lymphoid origin. Blood 2002, 99, 641–648. [Google Scholar] [CrossRef]
- Bozzuto, G.; Colone, M.; Toccacieli, L.; Stringaro, A.; Molinari, A. Tea tree oil might combat melanoma. Planta Med. 2011, 77, 54–56. [Google Scholar] [CrossRef]
- Sandig, M.; Voura, E.B.; Kalnins, V.I.; Siu, C.H. Role of cadherins in the transendothelial migration of melanoma cells in culture. Cell Motil. Cytoskelet. 1997, 38, 351–364. [Google Scholar] [CrossRef]
- Carman, C.V.; Springer, T.A. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 2004, 167, 377–388. [Google Scholar] [CrossRef]
- Martinelli, R.; Kamei, M.; Sage, P.T.; Massol, R.; Varghese, L.; Sciuto, T.; Toporsian, M.; Dvorak, A.M.; Kirchhausen, T.; Springer, T.A.; et al. Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier microwounds. J. Cell Biol. 2013, 201, 449–465. [Google Scholar] [CrossRef]
- van Buul, J.D.; Allingham, M.J.; Samson, T.; Meller, J.; Boulter, E.; García-Mata, R.; Burridge, K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J. Cell Biol. 2007, 178, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Herman, H.; Fazakas, C.; Haskó, J.; Molnár, K.; Mészáros, Á.; Nyúl-Tóth, Á.; Szabó, G.; Erdélyi, F.; Ardelean, A.; Hermenean, A.; et al. Paracellular and transcellular migration of metastatic cells through the cerebral endothelium. J. Cell Mol. Med. 2019, 23, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; D’Avenio, G.; Condello, M.; Sennato, S.; Battaglione, E.; Familiari, G.; Molinari, A.; Grigioni, M. Label-free cell based impedance measurements of ZnO nanoparticles-human lung cell interaction: A comparison with MTT, NR, Trypan blue and cloning efficiency assays. J. Nanobiotechnol. 2021, 19, 306. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzuto, G.; Colone, M.; Toccacieli, L.; Molinari, A.; Calcabrini, A.; Stringaro, A. Interaction of Drug-Sensitive and -Resistant Human Melanoma Cells with HUVEC Cells: A Label-Free Cell-Based Impedance Study. Biomedicines 2023, 11, 1544. https://doi.org/10.3390/biomedicines11061544
Bozzuto G, Colone M, Toccacieli L, Molinari A, Calcabrini A, Stringaro A. Interaction of Drug-Sensitive and -Resistant Human Melanoma Cells with HUVEC Cells: A Label-Free Cell-Based Impedance Study. Biomedicines. 2023; 11(6):1544. https://doi.org/10.3390/biomedicines11061544
Chicago/Turabian StyleBozzuto, Giuseppina, Marisa Colone, Laura Toccacieli, Agnese Molinari, Annarica Calcabrini, and Annarita Stringaro. 2023. "Interaction of Drug-Sensitive and -Resistant Human Melanoma Cells with HUVEC Cells: A Label-Free Cell-Based Impedance Study" Biomedicines 11, no. 6: 1544. https://doi.org/10.3390/biomedicines11061544
APA StyleBozzuto, G., Colone, M., Toccacieli, L., Molinari, A., Calcabrini, A., & Stringaro, A. (2023). Interaction of Drug-Sensitive and -Resistant Human Melanoma Cells with HUVEC Cells: A Label-Free Cell-Based Impedance Study. Biomedicines, 11(6), 1544. https://doi.org/10.3390/biomedicines11061544






