Association between Organochlorine Pesticides and Vitamin D in Female Subjects
Abstract
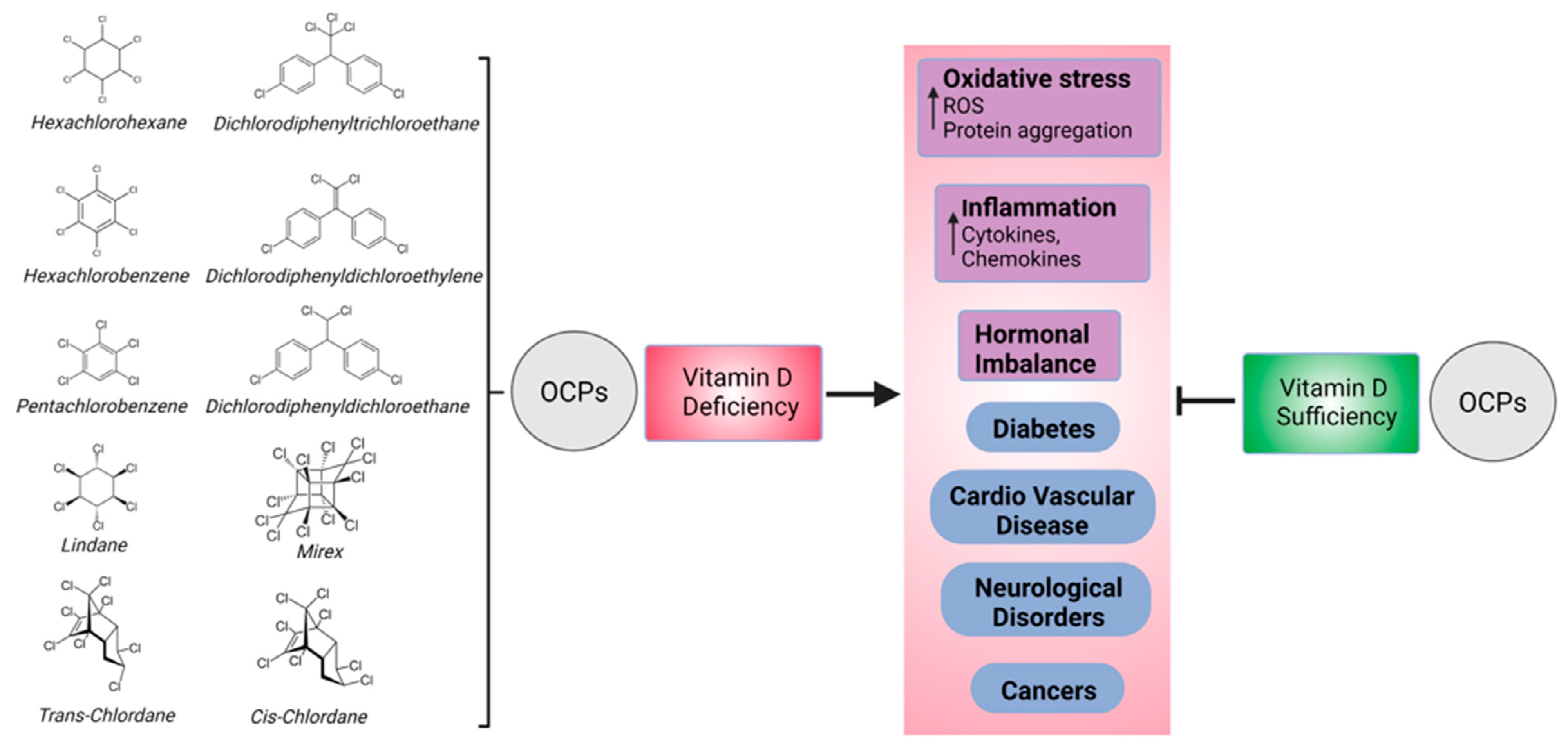
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. OCP Measurement
2.3. Instrumental Analysis
2.4. Quality Assurance/Quality Control
2.5. Vitamin D3 and Biochemical Parameters
2.6. CaM Proteomic Measurement
2.7. Statistics
3. Results
3.1. Whole-Cohort Analysis
3.2. OCP Levels
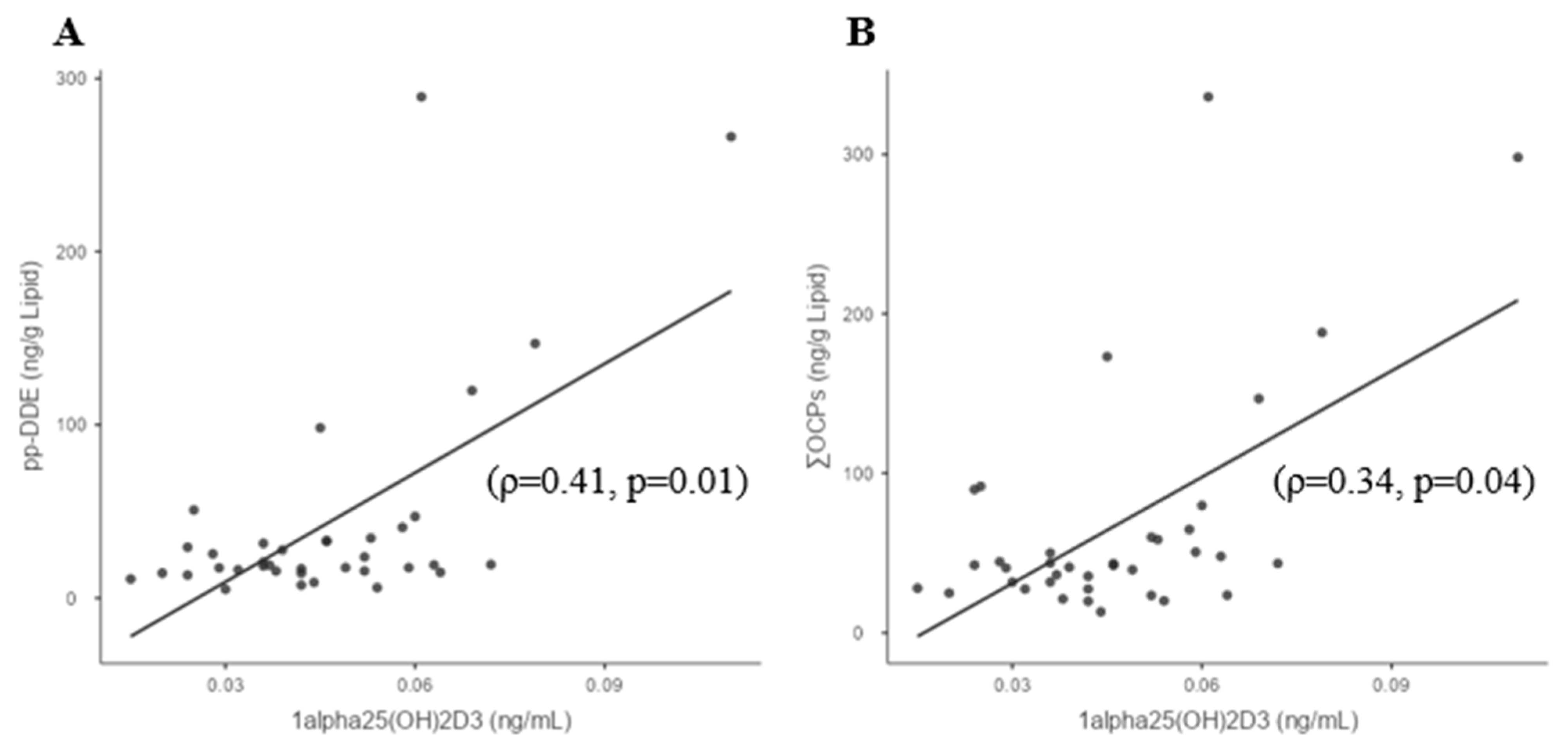
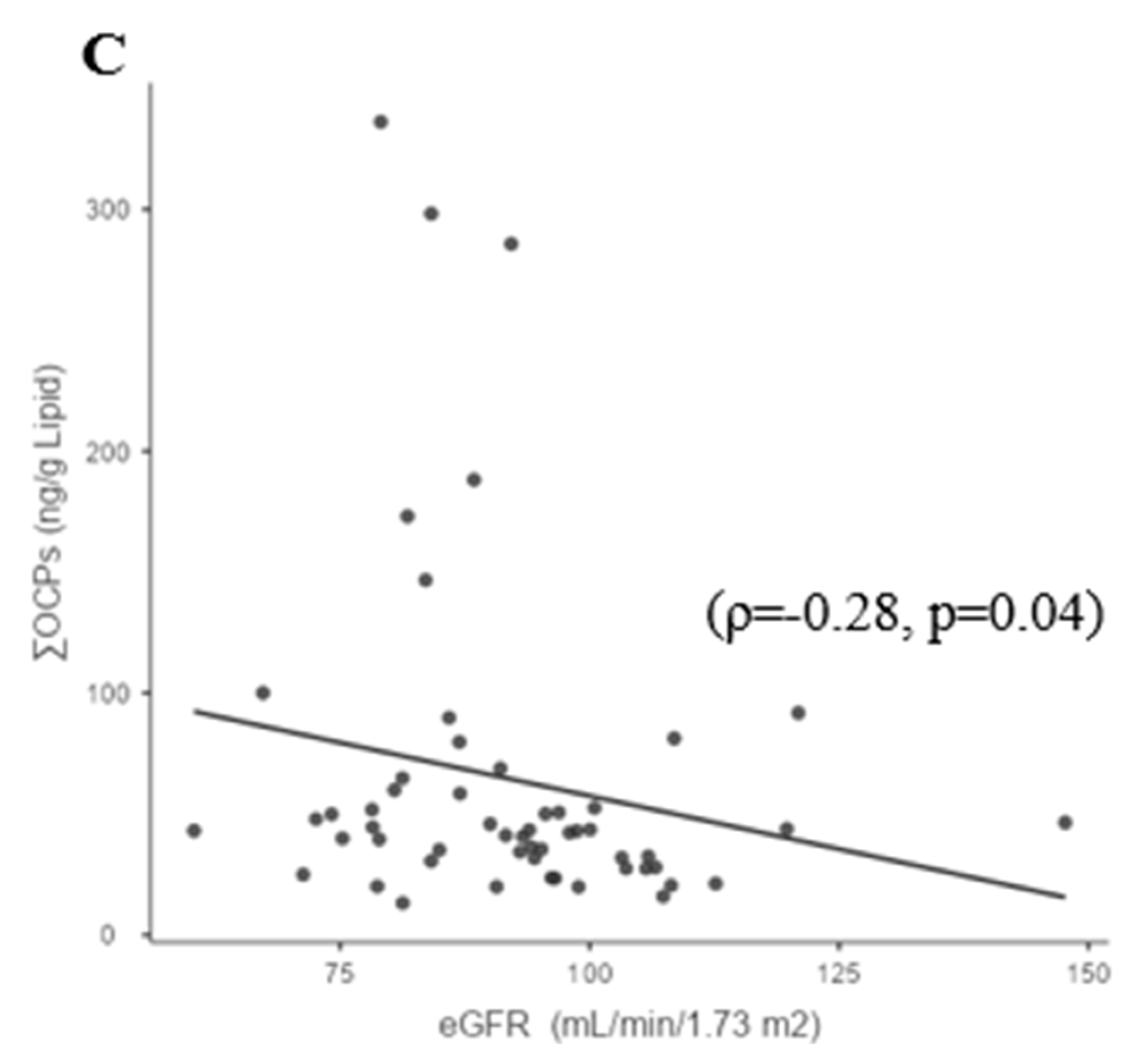
3.3. Whole-Group Correlations
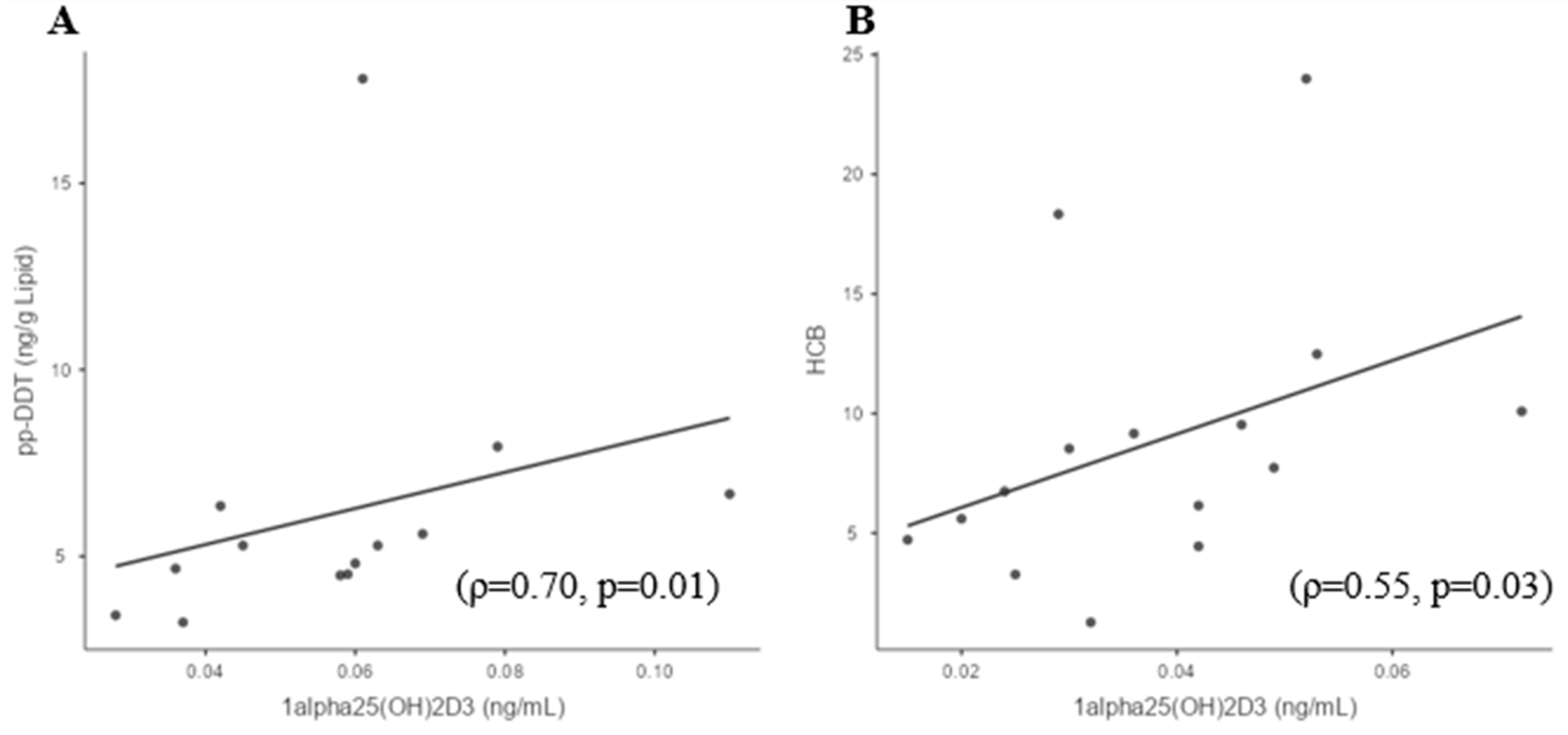
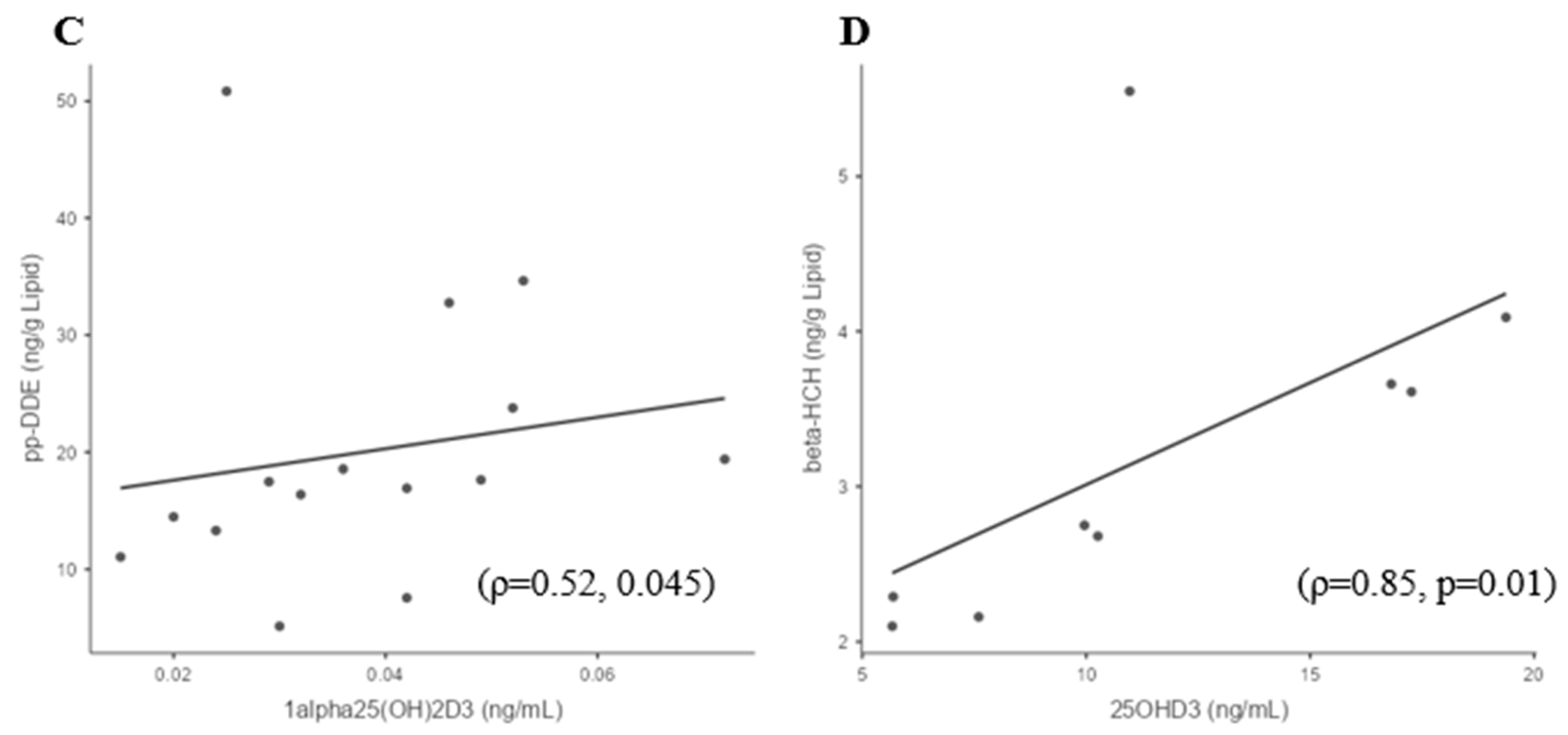
3.4. Sufficient and Deficient 25(OH)D3 Subgroup Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, H.; Aylward, L.L.; Rose, M.; Fernandes, A.; Sedman, P.; Thatcher, N.J.; Atkin, S.L.; Sathyapalan, T. Association of endocrine active environmental compounds with body mass index and weight loss following bariatric surgery. Clin. Endocrinol. 2020, 93, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Buah-Kwofie, A.; Humphries, M.S.; Combrink, X.; Myburgh, J.G. Accumulation of organochlorine pesticides in fat tissue of wild Nile crocodiles (Crocodylus niloticus) from iSimangaliso Wetland Park, South Africa. Chemosphere 2018, 195, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yun, X.; Ruan, Z.; Lu, C.; Shi, Y.; Qin, Q.; Men, Z.; Zou, D.; Du, X.; Xing, B.; et al. Review of hexachlorocyclohexane (HCH) and dichlorodiphenyltrichloroethane (DDT) contamination in Chinese soils. Sci. Total Environ. 2020, 749, 141212. [Google Scholar] [CrossRef]
- Keswani, C.; Dilnashin, H.; Birla, H.; Roy, P.; Tyagi, R.K.; Singh, D.; Rajput, V.D.; Minkina, T.; Singh, S.P. Global footprints of organochlorine pesticides: A pan-global survey. Environ. Geochem. Health 2022, 44, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Rong, M.; Li, M.; He, H.; Zhang, L.; Zhang, S.; Liu, C.; Zhu, Y.; Deng, Y.L.; Chen, P.P.; et al. Serum concentrations of organochlorine pesticides, biomarkers of oxidative stress, and risk of breast cancer. Environ. Pollut. 2021, 286, 117386. [Google Scholar] [CrossRef]
- Antignac, J.P.; Figiel, S.; Pinault, M.; Blanchet, P.; Bruyère, F.; Mathieu, R.; Lebdai, S.; Fournier, G.; Rigaud, J.; Mahéo, K.; et al. Persistent organochlorine pesticides in periprostatic adipose tissue from men with prostate cancer: Ethno-geographic variations, association with disease aggressiveness. Environ. Res. 2023, 216, 114809. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhou, T.; Tao, Y.; Feng, Y.; Shen, X.; Mei, S. Exposure to organochlorine pesticides and non-Hodgkin lymphoma: A meta-analysis of observational studies. Sci. Rep. 2016, 6, 25768. [Google Scholar] [CrossRef]
- Rattan, S.; Zhou, C.; Chiang, C.; Mahalingam, S.; Brehm, E.; Flaws, J.A. Exposure to endocrine disruptors during adulthood: Consequences for female fertility. J. Endocrinol. 2017, 233, R109–R129. [Google Scholar] [CrossRef]
- Giulioni, C.; Maurizi, V.; Castellani, D.; Scarcella, S.; Skrami, E.; Balercia, G.; Galosi, A.B. The environmental and occupational influence of pesticides on male fertility: A systematic review of human studies. Andrology 2022, 10, 1250–1271. [Google Scholar] [CrossRef]
- Pathak, R.; Mustafa, M.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K.; Banerjee, B.D. Association between recurrent miscarriages and organochlorine pesticide levels. Clin. Biochem. 2010, 43, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Sun, Y.; Yu, J.; Su, Z.; Tong, M.; Zhang, Y.; Liu, J.; Wang, L.; Li, Z.; Ren, A.; et al. Prenatal exposure to organochlorine pesticides is associated with increased risk for neural tube defects. Sci. Total Environ. 2021, 770, 145284. [Google Scholar] [CrossRef]
- Xu, S.; Yang, X.; Qian, Y.; Luo, Q.; Song, Y.; Xiao, Q. Analysis of serum levels of organochlorine pesticides and related factors in Parkinson’s disease. Neurotoxicology 2022, 88, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Medehouenou, T.C.M.; Ayotte, P.; Carmichael, P.H.; Kröger, E.; Verreault, R.; Lindsay, J.; Dewailly, É.; Tyas, S.L.; Bureau, A.; Laurin, D. Exposure to polychlorinated biphenyls and organochlorine pesticides and risk of dementia, Alzheimer’s disease and cognitive decline in an older population: A prospective analysis from the Canadian Study of Health and Aging. Environ. Health 2019, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J.; Gabure, S.; Maise, J.; Snipes, S.; Peete, M.; Whalen, M.M. The organochlorine pesticides pentachlorophenol and dichlorodiphenyltrichloroethane increase secretion and production of interleukin 6 by human immune cells. Environ. Toxicol. Pharmacol. 2019, 72, 103263. [Google Scholar] [CrossRef]
- Koner, B.C.; Banerjee, B.D.; Ray, A. Organochlorine pesticide-induced oxidative stress and immune suppression in rats. Indian J. Exp. Biol. 1998, 36, 395–398. [Google Scholar]
- Jeddy, Z.; Kordas, K.; Allen, K.; Taylor, E.V.; Northstone, K.; Dana Flanders, W.; Namulanda, G.; Sjodin, A.; Hartman, T.J. Prenatal exposure to organochlorine pesticides and early childhood communication development in British girls. Neurotoxicology 2018, 69, 121–129. [Google Scholar] [CrossRef]
- Evangelou, E.; Ntritsos, G.; Chondrogiorgi, M.; Kavvoura, F.K.; Hernández, A.F.; Ntzani, E.E.; Tzoulaki, I. Exposure to pesticides and diabetes: A systematic review and meta-analysis. Environ. Int. 2016, 91, 60–68. [Google Scholar] [CrossRef]
- Zago, A.M.; Faria, N.M.X.; Fávero, J.L.; Meucci, R.D.; Woskie, S.; Fassa, A.G. Pesticide exposure and risk of cardiovascular disease: A systematic review. Glob. Public Health 2022, 17, 3944–3966. [Google Scholar] [CrossRef]
- Costa, L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 2015, 131, 135–148. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Wetterslev, J.; Simonetti, R.G.; Bjelakovic, M.; Gluud, C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst. Rev. 2014, Cd007470. [Google Scholar] [CrossRef] [PubMed]
- Osorio Landa, H.K.; Perez Diaz, I.; Laguna Barcenas, S.D.C.; Lopez Navarro, J.M.; Abella Roa, M.F.; Corral Orozco, M.; Mancilla Ortega, J.P.; Martinez Duarte, D.A.; Morales Montalvo, S.I.; Muzquiz Aguirre, S.; et al. Association of serum vitamin D levels with chronic disease and mortality. Nutr. Hosp. 2020, 37, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yokoyama, K.; Yokoo, T.; Urashima, M. Role of vitamin D in diabetes mellitus and chronic kidney disease. World J. Diabetes 2016, 7, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Husemoen, L.L.; Thuesen, B.H.; Fenger, M.; Jorgensen, T.; Glumer, C.; Svensson, J.; Ovesen, L.; Witte, D.R.; Linneberg, A. Serum 25(OH)D and type 2 diabetes association in a general population: A prospective study. Diabetes Care 2012, 35, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C. Vitamin D and diabetes: Where do we stand? Diabetes Res. Clin. Pract. 2015, 108, 201–209. [Google Scholar] [CrossRef]
- Butler, A.E.; Dargham, S.R.; Latif, A.; Mokhtar, H.R.; Robay, A.; Chidiac, O.M.; Jayyousi, A.; Al Suwaidi, J.; Crystal, R.G.; Abi Khalil, C.; et al. Association of vitamin D3 and its metabolites in subjects with and without Type 2 diabetes and their relationship to diabetes complications. Ther. Adv. Chronic Dis. 2020, 11, 2040622320924159. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 22–27. [Google Scholar] [CrossRef]
- Han, X.; Zhang, F.; Meng, L.; Xu, Y.; Li, Y.; Li, A.; Turyk, M.E.; Yang, R.; Wang, P.; Zhang, J.; et al. Exposure to organochlorine pesticides and the risk of type 2 diabetes in the population of East China. Ecotoxicol. Environ. Saf. 2020, 190, 110125. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, Y.M.; Bae, S.G.; Jacobs, D.R., Jr.; Lee, D.H. Associations between organochlorine pesticides and vitamin D deficiency in the U.S. population. PLoS ONE 2012, 7, e30093. [Google Scholar] [CrossRef] [PubMed]
- Schæbel, L.K.; Bonefeld-Jørgensen, E.C.; Vestergaard, H.; Andersen, S. The influence of persistent organic pollutants in the traditional Inuit diet on markers of inflammation. PLoS ONE 2017, 12, e0177781. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.E.; Courtney, K.D.; Donaldson, W.E. Impairment of calcium homeostasis by hexachlorobenzene (HCB) exposure in Fischer 344 rats. J. Toxicol. Environ. Health 1988, 23, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; Kumar, N.; Drage, D.S.; Cunningham, T.K.; Sathyapalan, T.; Mueller, J.F.; Atkin, S.L. A case-control study of polychlorinated biphenyl association with metabolic and hormonal outcomes in polycystic ovary syndrome. J. Environ. Sci. Health C Toxicol. Carcinog. 2022, 40, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Papageorgiou, M.; Deshmukh, H.; Kilpatrick, E.S.; Mann, V.; Corless, L.; Abouda, G.; Rigby, A.S.; Atkin, S.L.; Sathyapalan, T. A Randomized, Controlled Trial of Vitamin D Supplementation on Cardiovascular Risk Factors, Hormones, and Liver Markers in Women with Polycystic Ovary Syndrome. Nutrients 2019, 11, 188. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Sathyapalan, T.; Butler, A.E.; Atkin, S.L. Vitamin D association with coagulation factors in polycystic ovary syndrome is dependent upon body mass index. J. Transl. Med. 2021, 19, 239. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Sathyapalan, T.; Butler, A.E.; Atkin, S.L. Coagulation factor dysregulation in polycystic ovary syndrome is an epiphenomenon of obesity. Clin. Endocrinol. 2023, 98, 796–802. [Google Scholar] [CrossRef]
- Birkett, M.A.; Day, S.J. Internal pilot studies for estimating sample size. Stat. Med. 1994, 13, 2455–2463. [Google Scholar] [CrossRef]
- Siddharth, M.; Datta, S.K.; Bansal, S.; Mustafa, M.; Banerjee, B.D.; Kalra, O.P.; Tripathi, A.K. Study on organochlorine pesticide levels in chronic kidney disease patients: Association with estimated glomerular filtration rate and oxidative stress. J. Biochem. Mol. Toxicol. 2012, 26, 241–247. [Google Scholar] [CrossRef]
- Lv, J.; Guo, L.; Gu, Y.; Xu, Y.; Xue, Q.; Yang, X.; Wang, Q.N.; Meng, X.M.; Xu, D.X.; Pan, X.F.; et al. National temporal trend for organophosphate pesticide DDT exposure and associations with chronic kidney disease using age-adapted eGFR model. Environ. Int. 2022, 169, 107499. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, N.; Daniel, P.; Šrámek, J.; Jelínek, M.; Šrámková, V.; Němcová, V.; Balušíková, K.; Halada, P.; Kovář, J. Upregulation of vitamin D-binding protein is associated with changes in insulin production in pancreatic beta-cells exposed to p,p’-DDT and p,p’-DDE. Sci. Rep. 2019, 9, 18026. [Google Scholar] [CrossRef]
- Ellison, T.I.; Dowd, D.R.; MacDonald, P.N. Calmodulin-dependent kinase IV stimulates vitamin D receptor-mediated transcription. Mol. Endocrinol. 2005, 19, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, H.G.; Myrtle, J.F.; Norman, A.W. Effects of organochlorine insecticides on metabolism of cholecalciferol (vitamin D 3) in Rachitic cockerel. J. Agric. Food Chem. 1972, 20, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Siddiqee, M.H.; Bhattacharjee, B.; Siddiqi, U.R.; MeshbahurRahman, M. High prevalence of vitamin D deficiency among the South Asian adults: A systematic review and meta-analysis. BMC Public Health 2021, 21, 1823. [Google Scholar] [CrossRef]
- Shah, H.K.; Sharma, T.; Banerjee, B.D. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere 2020, 246, 125691. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Zhong, K.; Wang, C.; Xu, X. The associations between endocrine disrupting chemicals and markers of inflammation and immune responses: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2022, 234, 113382. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Querfeld, U. Vitamin D and inflammation. Pediatr. Nephrol. 2013, 28, 605–610. [Google Scholar] [CrossRef]
- Wei, X.; Li, X.; Du, J.; Ge, X.; Sun, Y.; Li, X.; Xun, Z.; Liu, W.; Wang, Z.Y.; Li, Y.C. Vitamin D Deficiency Exacerbates Colonic Inflammation Due to Activation of the Local Renin-Angiotensin System in the Colon. Dig. Dis. Sci. 2021, 66, 3813–3821. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Geng, X.P.; Wang, M.W.; Wang, H.Q.; Zhang, C.; He, X.; Liang, S.M.; Xu, D.X.; Chen, X. Vitamin D deficiency exacerbates hepatic oxidative stress and inflammation during acetaminophen-induced acute liver injury in mice. Int. Immunopharmacol. 2021, 97, 107716. [Google Scholar] [CrossRef] [PubMed]
- Glinski, A.; Liebel, S.; Pelletier, È.; Voigt, C.L.; Randi, M.A.; Campos, S.X.; Oliveira Ribeiro, C.A.; Filipak Neto, F. Toxicological interactions of silver nanoparticles and organochlorine pesticides in mouse peritoneal macrophages. Toxicol. Mech. Methods 2016, 26, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Massawe, R.; Drabo, L.; Whalen, M. Effects of pentachlorophenol and dichlorodiphenyltrichloroethane on secretion of interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) from human immune cells. Toxicol. Mech. Methods 2017, 27, 223–235. [Google Scholar] [CrossRef] [PubMed]
- McMullin, J.L.; Codner, J.; Patel, S.G.; Sharma, J.; Hu, X.; Jones, D.P.; Weber, C.J.; Saunders, N.D. Environmental Chemicals and their Association with Hyperparathyroidism. World J. Surg. 2023, 47, 296–303. [Google Scholar] [CrossRef] [PubMed]
| Female Subjects (n = 58) | ||
|---|---|---|
| Mean | SD | |
| Age (years) | 31.9 | 4.6 |
| BMI (kg/m2) | 25.7 | 3.7 |
| CRP (mg/L) | 2.6 | 2.5 |
| TSH (mU/L) | 2.4 | 2.2 |
| Free-T3 (pmol/L) | 4.8 | 0.7 |
| Free-T4 (pmol/L) | 11.3 | 1.8 |
| 25(OH)D3 (ng/mL) | 23 | 11.2 |
| 1,25(OH)2D3 (ng/mL) | 0.05 | 0.02 |
| Calcium (mmol/L) | 2.3 | 0.07 |
| Urea (nmol/L) | 4.66 | 5.21 |
| Creatinine (nmol/L) | 66 | 8.86 |
| eGFR (mL/min/1.73 m2) | 92.3 | 14.8 |
| PDE1A (RFU) | 789 | 1060 |
| CAMK2A (RFU) | 373 | 453 |
| CaMK2B (RFU) | 859 | 1423 |
| CaMK1D (RFU) | 1695 | 580 |
| CaMK2D (RFU) | 2379 | 3462 |
| CaMK1 (RFU) | 4681 | 1371 |
| CaMKK α (RFU) | 343 | 463 |
| PeCB (ng/g Lipid) | 9.12 | 9.1 |
| α-HCH (ng/g Lipid) | <LOR | |
| β-HCH (ng/g Lipid) | 4.63 | 4.92 |
| HCB (ng/g Lipid) | 9.81 | 6.5 |
| Lindane (ng/g Lipid) | 1.35 | 0.28 |
| Trans-Chlordane (ng/g Lipid) | 1.4 | 0.97 |
| cis-Chlordane (ng/g Lipid) | 1.59 | 0.58 |
| p,p′-DDE (ng/g Lipid) | 40.7 | 60.4 |
| o,p′-DDE (ng/g Lipid) | 2.23 | 0.32 |
| o,p′-DDD (ng/g Lipid) | <LOR | |
| p,p′-DDD+o,p′-DDT (ng/g Lipid) | <LOR | |
| p,p′-DDT (ng/g Lipid) | 4.99 | 2.3 |
| Mirex (ng/g Lipid) | 3.27 | 0.69 |
| ƩOCPs (ng/g Lipid) | 63.3 | 66.8 |
| PeCB | β-HCH | HCB | Lindane | trans-Chlordane | cis-Chlordane | o,p′-DDE | p,p′-DDE | p,p′-DDT | Mirex | ƩOCPs | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D3 | 0.289 | 0.174 | 0.101 | 0.393 | −0.14 | 0.325 | 0.374 | 0.272 | 0.096 | −0.204 | 0.284 |
| (0.192) | (0.339) | (0.502) | (0.40) | (0.648) | (0.091) | (0.258) | (0.061) | (0.595) | (0.361) | (0.051) | |
| 1,25(OH)2D3 | −0.108 | 0.315 | 0.333 | −0.2 | −0.205 | 0.278 | 0.06 | 0.408 | 0.364 | −0.03 | 0.34 |
| (0.66) | (0.14) | (0.05) | (0.92) | (0.74) | 0.263 | (0.89) | (0.01) | (0.09) | (0.92) | (0.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brennan, E.; Butler, A.E.; Nandakumar, M.; Drage, D.S.; Sathyapalan, T.; Atkin, S.L. Association between Organochlorine Pesticides and Vitamin D in Female Subjects. Biomedicines 2023, 11, 1451. https://doi.org/10.3390/biomedicines11051451
Brennan E, Butler AE, Nandakumar M, Drage DS, Sathyapalan T, Atkin SL. Association between Organochlorine Pesticides and Vitamin D in Female Subjects. Biomedicines. 2023; 11(5):1451. https://doi.org/10.3390/biomedicines11051451
Chicago/Turabian StyleBrennan, Edwina, Alexandra E. Butler, Manjula Nandakumar, Daniel S. Drage, Thozhukat Sathyapalan, and Stephen L. Atkin. 2023. "Association between Organochlorine Pesticides and Vitamin D in Female Subjects" Biomedicines 11, no. 5: 1451. https://doi.org/10.3390/biomedicines11051451
APA StyleBrennan, E., Butler, A. E., Nandakumar, M., Drage, D. S., Sathyapalan, T., & Atkin, S. L. (2023). Association between Organochlorine Pesticides and Vitamin D in Female Subjects. Biomedicines, 11(5), 1451. https://doi.org/10.3390/biomedicines11051451









