Mesenchymal Stem Cell in Pancreatic Islet Transplantation
Abstract
1. Mesenchymal Stem Cells
2. Stressors Challenging Pancreatic Islet Graft
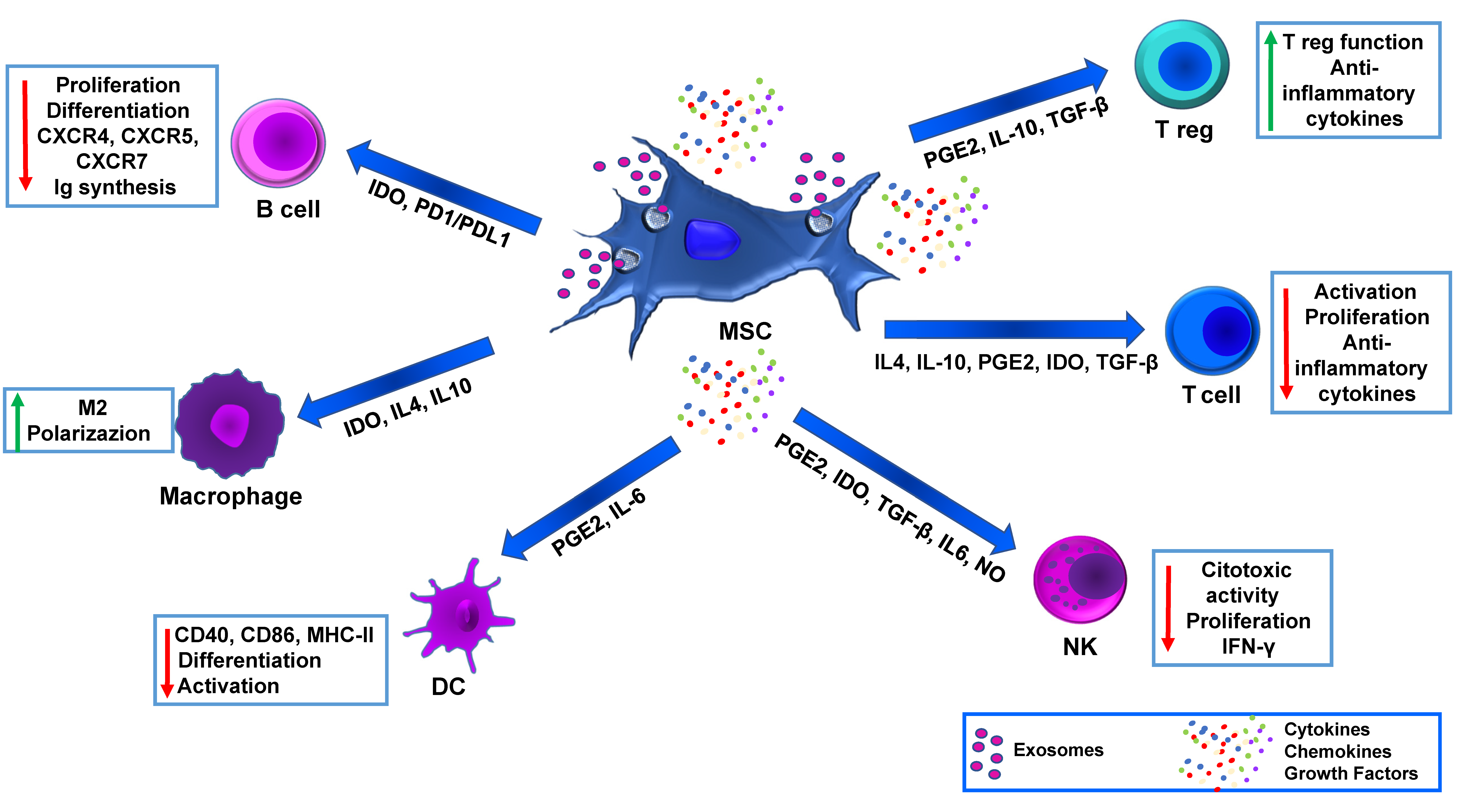
3. MSC Immunomodulation and Advanced Medicinal Therapy
4. Exosomes and Its Potential in MSCs Vascularization
5. Preconditioning of MSC as a Potential Therapeutic Strategy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thanaskody, K.; Jusop, A.S.; Tye, G.J.; Wan Kamarul Zaman, W.S.; Dass, S.A.; Nordin, F. MSCs vs. iPSCs: Potential in therapeutic applications. Front. Cell Dev. Biol. 2022, 10, 1005926. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [CrossRef]
- Davies, L.C.; Alm, J.J.; Heldring, N.; Moll, G.; Gavin, C.; Batsis, I.; Qian, H.; Sigvardsson, M.; Nilsson, B.; Kyllonen, L.E.; et al. Type 1 Diabetes Mellitus Donor Mesenchymal Stromal Cells Exhibit Comparable Potency to Healthy Controls In Vitro. Stem Cells Transl. Med. 2016, 5, 1485–1495. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, O.; Eich, T.; Sundin, A.; Tibell, A.; Tufveson, G.; Andersson, H.; Felldin, M.; Foss, A.; Kyllönen, L.; Langstrom, B.; et al. Positron emission tomography in clinical islet transplantation. Am. J. Transplant. 2009, 9, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef]
- Qi, M.; Kinzer, K.; Danielson, K.K.; Martellotto, J.; Barbaro, B.; Wang, Y.; Bui, J.T.; Gaba, R.C.; Knuttinen, G.; Garcia-Roca, R.; et al. Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: The UIC experience. Acta Diabetol. 2014, 51, 833–843. [Google Scholar] [CrossRef]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef]
- Shrestha, M.; Nguyen, T.T.; Park, J.; Choi, J.U.; Yook, S.; Jeong, J.H. Immunomodulation effect of mesenchymal stem cells in islet transplantation. Biomed. Pharmacother. 2021, 142, 112042. [Google Scholar] [CrossRef]
- Jimenez-Puerta, G.J.; Marchal, J.A.; López-Ruiz, E.; Gálvez-Martín, P. Role of Mesenchymal Stromal Cells as Therapeutic Agents: Potential Mechanisms of Action and Implications in Their Clinical Use. J. Clin. Med. 2020, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Longoni, B.; Szilagyi, E.; Quaranta, P.; Paoli, G.T.; Tripodi, S.; Urbani, S.; Mazzanti, B.; Rossi, B.; Fanci, R.; Demontis, G.C.; et al. Mesenchymal stem cells prevent acute rejection and prolong graft function in pancreatic islet transplantation. Diabetes Technol. Ther. 2010, 12, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Koehler, N.; Buhler, L.; Egger, B.; Gonelle-Gispert, C. Multipotent Mesenchymal Stromal Cells Interact and Support Islet of Langerhans Viability and Function. Front. Endocrinol. 2022, 13, 822191. [Google Scholar] [CrossRef]
- Longoni, B.; Mosca, F. Stem cell-based immunomodulation in type 1 diabetes: Beyond the regenerative approach. Curr. Pharm. Des. 2011, 17, 3229–3242. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.; Kumagai-Braesch, M.; Tibell, A.; Svensson, M.; Flodström-Tullberg, M. Co-transplantation of stromal cells interferes with the rejection of allogeneic islet grafts. Ann. N. Y. Acad. Sci. 2008, 1150, 213–216. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Locatelli, F.; Fibbe, W.E. Mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2009, 1176, 101–117. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann. N. Y. Acad. Sci. 2012, 1266, 107–117. [Google Scholar] [CrossRef]
- Takahashi, T.; Tibell, A.; Ljung, K.; Saito, Y.; Gronlund, A.; Osterholm, C.; Holgersson, J.; Lundgren, T.; Ericzon, B.G.; Corbascio, M.; et al. Multipotent mesenchymal stromal cells synergize with costimulation blockade in the inhibition of immune responses and the induction of Foxp3+ regulatory T cells. Stem Cells Transl. Med. 2014, 3, 1484–1494. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, F.; Fang, H.; Huang, H. Effect of mesenchymal stem cells on renal injury in rats with severe acute pancreatitis. Exp. Biol. Med. 2013, 238, 687–695. [Google Scholar] [CrossRef]
- Tu, X.H.; Song, J.X.; Xue, X.J.; Guo, X.W.; Ma, Y.X.; Chen, Z.Y.; Zou, Z.D.; Wang, L. Role of bone marrow-derived mesenchymal stem cells in a rat model of severe acute pancreatitis. World J. Gastroenterol. 2012, 18, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.F.; Bai, Z.L. Protective effects of transplanted and mobilized bone marrow stem cells on mice with severe acute pancreatitis. World J. Gastroenterol. 2003, 9, 2274–2277. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Song, S.U.; Yi, T.; Jeon, M.S.; Hong, S.W.; Zheng, H.M.; Lee, H.S.; Choi, M.J.; Lee, D.H.; Hong, S.S. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology 2011, 140, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Bai, B.; Liu, C.X.; Wang, S.Q.; Jiang, X.; Zhu, C.L.; Zhao, Q.C. Effect of umbilical cord mesenchymal stem cells on treatment of severe acute pancreatitis in rats. Cytotherapy 2013, 15, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Hu, G.; Wan, R.; Yu, G.; Cang, X.; Ni, J.; Xiong, J.; Hu, Y.; Xing, M.; Fan, Y.; et al. Role of bone marrow mesenchymal stem cells in L-arg-induced acute pancreatitis: Effects and possible mechanisms. Int. J. Clin. Exp. Pathol. 2015, 8, 4457–4468. [Google Scholar]
- Shahjalal, H.M.; Abdal Dayem, A.; Lim, K.M.; Jeon, T.I.; Cho, S.G. Generation of pancreatic β cells for treatment of diabetes: Advances and challenges. Stem Cell Res. Ther. 2018, 9, 355. [Google Scholar] [CrossRef]
- Barachini, S.; Montali, M.; Panvini, F.M.; Carnicelli, V.; Gatti, G.L.; Piolanti, N.; Bonicoli, E.; Scaglione, M.; Buda, G.; Parchi, P.D. Mesangiogenic Progenitor Cells Are Tissue Specific and Cannot Be Isolated From Adipose Tissue or Umbilical Cord Blood. Front. Cell Dev. Biol. 2021, 9, 669381. [Google Scholar] [CrossRef]
- Barachini, S.; Pacini, S.; Montali, M.; Panvini, F.M.; Carnicelli, V.; Piolanti, N.; Bonicoli, E.; Scaglione, M.; Parchi, P.D. Mesangiogenic Progenitor Cells and musculoskeletal tissue regeneration: Differences between adipose-derived and bone marrow-derived cells? J. Biol. Regul. Homeost. Agents 2020, 34, 33–38. [Google Scholar]
- Montali, M.; Barachini, S.; Pacini, S.; Panvini, F.M.; Petrini, M. Isolating Mesangiogenic Progenitor Cells (MPCs) from Human Bone Marrow. J. Vis. Exp. 2016, 15, e54225. [Google Scholar] [CrossRef]
- Montali, M.; Panvini, F.M.; Barachini, S.; Ronca, F.; Carnicelli, V.; Mazzoni, S.; Petrini, I.; Pacini, S. Human adult mesangiogenic progenitor cells reveal an early angiogenic potential, which is lost after mesengenic differentiation. Stem Cell Res. Ther. 2017, 8, 106. [Google Scholar] [CrossRef]
- Montali, M.; Barachini, S.; Panvini, F.M.; Carnicelli, V.; Fulceri, F.; Petrini, I.; Pacini, S. Growth Factor Content in Human Sera Affects the Isolation of Mesangiogenic Progenitor Cells (MPCs) from Human Bone Marrow. Front. Cell Dev. Biol. 2016, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Pacini, S.; Barachini, S.; Montali, M.; Carnicelli, V.; Fazzi, R.; Parchi, P.; Petrini, M. Mesangiogenic Progenitor Cells Derived from One Novel CD64(bright)CD31(bright)CD14(neg) Population in Human Adult Bone Marrow. Stem Cells Dev. 2016, 25, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Panvini, F.M.; Pacini, S.; Montali, M.; Barachini, S.; Mazzoni, S.; Morganti, R.; Ciancia, E.M.; Carnicelli, V.; Petrini, M. High NESTIN Expression Marks the Endosteal Capillary Network in Human Bone Marrow. Front. Cell Dev. Biol. 2020, 8, 596452. [Google Scholar] [CrossRef] [PubMed]
- Lablanche, S.; Vantyghem, M.C.; Kessler, L.; Wojtusciszyn, A.; Borot, S.; Thivolet, C.; Girerd, S.; Bosco, D.; Bosson, J.L.; Colin, C.; et al. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wassmer, C.H.; Perrier, Q.; Combescure, C.; Pernin, N.; Parnaud, G.; Cottet-Dumoulin, D.; Brioudes, E.; Bellofatto, K.; Lebreton, F.; Berishvili, E.; et al. Impact of ischemia time on islet isolation success and posttransplantation outcomes: A retrospective study of 452 pancreas isolations. Am. J. Transplant. 2021, 21, 1493–1502. [Google Scholar] [CrossRef]
- Gurgul-Convey, E.; Mehmeti, I.; Plötz, T.; Jörns, A.; Lenzen, S. Sensitivity profile of the human EndoC-βH1 beta cell line to proinflammatory cytokines. Diabetologia 2016, 59, 2125–2133. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Ricordi, C.; Sakuma, Y.; Yamamoto, T.; Misawa, R.; Mita, A.; Molano, R.D.; Vaziri, N.D.; Pileggi, A.; Ichii, H. Divergent antioxidant capacity of human islet cell subsets: A potential cause of beta-cell vulnerability in diabetes and islet transplantation. PLoS ONE 2018, 13, e0196570. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, H.; Theisinger, B.; Guenther, B.; Johnson, P.R.; Brandhorst, D. Pancreatic L-Glutamine Administration Protects Pig Islets From Cold Ischemic Injury and Increases Resistance Toward Inflammatory Mediators. Cell Transplant. 2016, 25, 531–538. [Google Scholar] [CrossRef]
- Nzuza, S.; Ndwandwe, D.E.; Owira, P.M.O. Naringin protects against HIV-1 protease inhibitors-induced pancreatic β-cell dysfunction and apoptosis. Mol. Cell Endocrinol. 2016, 437, 1–10. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Tan, K.N.; Gotteland, M.; Borges, K. Sulforaphane Protects against High Cholesterol-Induced Mitochondrial Bioenergetics Impairments, Inflammation, and Oxidative Stress and Preserves Pancreatic. Oxid. Med. Cell. Longev. 2017, 2017, 3839756. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Kaviani, M.; Jabbarpour, Z.; Geramizadeh, B.; Motevaseli, E.; Nikeghbalian, S.; Shamsaeefar, A.; Motazedian, N.; Al-Abdullah, I.H.; Ghahremani, M.H.; et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci. Rep. 2019, 9, 11701. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Park, S.H.; Jetha, A.; Aikin, R.; Tremblay, M.; Paraskevas, S. Evidence of endoplasmic reticulum stress mediating cell death in transplanted human islets. Cell Transplant. 2012, 21, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L.; MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Ding, Y.; Liang, X.; Zhang, Y.; Yi, L.; Shum, H.C.; Chen, Q.; Chan, B.P.; Fan, H.; Liu, Z.; Tergaonkar, V.; et al. Rap1 deficiency-provoked paracrine dysfunction impairs immunosuppressive potency of mesenchymal stem cells in allograft rejection of heart transplantation. Cell Death Dis. 2018, 9, 386. [Google Scholar] [CrossRef]
- He, J.G.; Xie, Q.L.; Li, B.B.; Zhou, L.; Yan, D. Exosomes Derived from IDO1-Overexpressing Rat Bone Marrow Mesenchymal Stem Cells Promote Immunotolerance of Cardiac Allografts. Cell Transplant. 2018, 27, 1657–1683. [Google Scholar] [CrossRef]
- Zhang, Y.; Chiu, S.; Liang, X.; Gao, F.; Zhang, Z.; Liao, S.; Liang, Y.; Chai, Y.H.; Low, D.J.; Tse, H.F.; et al. Rap1-mediated nuclear factor-kappaB (NF-κB) activity regulates the paracrine capacity of mesenchymal stem cells in heart repair following infarction. Cell Death Discov. 2015, 1, 15007. [Google Scholar] [CrossRef] [PubMed]
- Eldor, R.; Abel, R.; Sever, D.; Sadoun, G.; Peled, A.; Sionov, R.; Melloul, D. Inhibition of nuclear factor-κB activation in pancreatic β-cells has a protective effect on allogeneic pancreatic islet graft survival. PLoS ONE 2013, 8, e56924. [Google Scholar] [CrossRef] [PubMed]
- Hematti, P.; Kim, J.; Stein, A.P.; Kaufman, D. Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant. Rev. 2013, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fan, X.; Liu, Y.; Jie, P.; Mazhar, M.; Dechsupa, N.; Wang, L. Immunomodulatory Mechanisms and Therapeutic Potential of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2023, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Sousa, M.R.R.; Alencar-Silva, T.; Carvalho, J.L.; Saldanha-Araujo, F. Mesenchymal stem cells immunomodulation: The road to IFN-γ licensing and the path ahead. Cytokine Growth Factor Rev. 2019, 47, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.; Thangavelu, L.; Aravindhan, S.; Zekiy, A.O.; Jarahian, M.; Chartrand, M.S.; Pathak, Y.; Marofi, F.; Shamlou, S.; Hassanzadeh, A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res. Ther. 2021, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Raffaghello, L.; Bianchi, G.; Meloni, F.; Salis, A.; Millo, E.; Ferrone, S.; Barnaba, V.; Pistoia, V. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells 2008, 26, 1275–1287. [Google Scholar] [CrossRef]
- Rostami, Z.; Khorashadizadeh, M.; Naseri, M. Immunoregulatory properties of mesenchymal stem cells: Micro-RNAs. Immunol. Lett. 2020, 219, 34–45. [Google Scholar] [CrossRef]
- Petrini, I.; Sollini, M.; Bartoli, F.; Barachini, S.; Montali, M.; Pardini, E.; Burzi, I.S.; Erba, P.A. ED-B-Containing Isoform of Fibronectin in Tumor Microenvironment of Thymomas: A Target for a Theragnostic Approach. Cancers 2022, 14, 2592. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Mannucci, S.; Calderan, L.; Quaranta, P.; Antonini, S.; Mosca, F.; Longoni, B.; Marzola, P.; Boschi, F. Quantum dots labelling allows detection of the homing of mesenchymal stem cells administered as immunomodulatory therapy in an experimental model of pancreatic islets transplantation. J. Anat. 2017, 230, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, O.; Quaranta, P.; Raffa, V.; Funel, N.; Campani, D.; Pelliccioni, S.; Longoni, B.; Mosca, F.; Pietrabissa, A.; Cuschieri, A. Magnetic carbon nanotubes: A new tool for shepherding mesenchymal stem cells by magnetic fields. Nanomedicine 2011, 6, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, M.; Vedel, S.; Skafte-Pedersen, P.; Sabourin, D.; Collas, P.; Bruus, H.; Dufva, M. The role of paracrine and autocrine signaling in the early phase of adipogenic differentiation of adipose-derived stem cells. PLoS ONE 2013, 8, e63638. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Longoni, B.; Fasciani, I.; Kolachalam, S.; Pietrantoni, I.; Marampon, F.; Petragnano, F.; Aloisi, G.; Coppolino, M.F.; Rossi, M.; Scarselli, M.; et al. Neurotoxic and Neuroprotective Role of Exosomes in Parkinson’s Disease. Curr. Pharm. Des. 2019, 25, 4510–4522. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Zhang, K.; Cao, Q.; Liu, T.; Li, J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res. Ther. 2021, 12, 561. [Google Scholar] [CrossRef]
- Canning, P.; Alwan, A.; Khalil, F.; Zhang, Y.; Opara, E.C. Perspectives and Challenges on the Potential Use of Exosomes in Bioartificial Pancreas Engineering. Ann. Biomed. Eng. 2022, 50, 1177–1186. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Ranghino, A.; Cantaluppi, V.; Grange, C.; Vitillo, L.; Fop, F.; Biancone, L.; Deregibus, M.C.; Tetta, C.; Segoloni, G.P.; Camussi, G. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int. J. Immunopathol. Pharmacol. 2012, 25, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ. Res. 2011, 109, 724–728. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-derived exosomes: A novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Biancone, L.; Figliolini, F.; Beltramo, S.; Medica, D.; Deregibus, M.C.; Galimi, F.; Romagnoli, R.; Salizzoni, M.; Tetta, C.; et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012, 21, 1305–1320. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Liu, Y.M.; Niu, X.; Yin, J.Y.; Hu, B.; Guo, S.C.; Fan, Y.; Wang, Y.; Wang, N.S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef]
- Newton, W.C.; Kim, J.W.; Luo, J.Z.Q.; Luo, L. Stem cell-derived exosomes: A novel vector for tissue repair and diabetic therapy. J. Mol. Endocrinol. 2017, 59, R155–R165. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Liu, D.; Weizmann, Y.; Mahato, R.I. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J. Control. Release 2016, 238, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Gomari, H.; Forouzandeh Moghadam, M.; Soleimani, M.; Ghavami, M.; Khodashenas, S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int. J. Nanomed. 2019, 14, 5679–5690. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Weng, J.; Guo, L.; Chen, X.; Du, X. Novel insights into MSC-EVs therapy for immune diseases. Biomark Res. 2019, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Ding, J.; Zheng, Z.H.; Wu, Z.B.; Zhu, P. Long-term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Mol. Med. Rep. 2012, 6, 1183–1189. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Greijer, A.E.; van der Wall, E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 2004, 57, 1009–1014. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Tarnawski, A.S. Critical role of hypoxia sensor—HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 2012, 19, 90–97. [Google Scholar] [CrossRef]
- Stokes, R.A.; Cheng, K.; Deters, N.; Lau, S.M.; Hawthorne, W.J.; O’Connell, P.J.; Stolp, J.; Grey, S.; Loudovaris, T.; Kay, T.W.; et al. Hypoxia-inducible factor-1α (HIF-1α) potentiates β-cell survival after islet transplantation of human and mouse islets. Cell Transplant. 2013, 22, 253–266. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, M.; Wang, Y.; Pasha, Z.; Li, T.; Ashraf, M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J. Mol. Cell. Cardiol. 2007, 42, 1036–1044. [Google Scholar] [CrossRef]
- Hendrawan, S.; Kusnadi, Y.; Lagonda, C.A.; Fauza, D.; Lheman, J.; Budi, E.; Manurung, B.S.; Baer, H.U.; Tansil Tan, S. Wound healing potential of human umbilical cord mesenchymal stem cell conditioned medium: An. Vet. World 2021, 14, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Touani, F.K.; Borie, M.; Azzi, F.; Trudel, D.; Noiseux, N.; Der Sarkissian, S.; Lerouge, S. Pharmacological Preconditioning Improves the Viability and Proangiogenic Paracrine Function of Hydrogel-Encapsulated Mesenchymal Stromal Cells. Stem Cells Int. 2021, 2021, 6663467. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, A.A.; Abbaszadeh, H.A.; Roozbahany, N.A.; Pourbakht, A.; Khoshsirat, S.; Niri, H.H.; Peyvandi, H.; Niknazar, S. Deferoxamine promotes mesenchymal stem cell homing in noise-induced injured cochlea through PI3K/AKT pathway. Cell Prolif 2018, 51, e12434. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; Wang, H.S.; Lin, G.J.; Chou, S.C.; Chu, T.H.; Chuan, W.T.; Lu, Y.J.; Weng, Z.C.; Su, C.H.; Hsieh, P.S.; et al. Undifferentiated Wharton’s Jelly Mesenchymal Stem Cell Transplantation Induces Insulin-Producing Cell Differentiation and Suppression of T-Cell-Mediated Autoimmunity in Nonobese Diabetic Mice. Cell Transplant. 2015, 24, 1555–1570. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Shaw, G.; Duffy, N.; Islam, M.N.; O’Shea, P.M.; O’Toole, D.; Howard, L.; Murphy, J.M. Survival/Adaptation of Bone Marrow-Derived Mesenchymal Stem Cells After Long-Term Starvation Through Selective Processes. Stem Cells 2019, 37, 813–827. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Shaw, G.; Coleman, C.M.; Chen, X.Z.; Connolly, D.; Palamá, E.M.F.; Gentili, C.; Contessotto, P.; Murphy, M.J. Regenerative and Anti-Inflammatory Potential of Regularly Fed, Starved Cells and Extracellular Vesicles In Vivo. Cells 2022, 11, 2696. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barachini, S.; Biso, L.; Kolachalam, S.; Petrini, I.; Maggio, R.; Scarselli, M.; Longoni, B. Mesenchymal Stem Cell in Pancreatic Islet Transplantation. Biomedicines 2023, 11, 1426. https://doi.org/10.3390/biomedicines11051426
Barachini S, Biso L, Kolachalam S, Petrini I, Maggio R, Scarselli M, Longoni B. Mesenchymal Stem Cell in Pancreatic Islet Transplantation. Biomedicines. 2023; 11(5):1426. https://doi.org/10.3390/biomedicines11051426
Chicago/Turabian StyleBarachini, Serena, Letizia Biso, Shivakumar Kolachalam, Iacopo Petrini, Roberto Maggio, Marco Scarselli, and Biancamaria Longoni. 2023. "Mesenchymal Stem Cell in Pancreatic Islet Transplantation" Biomedicines 11, no. 5: 1426. https://doi.org/10.3390/biomedicines11051426
APA StyleBarachini, S., Biso, L., Kolachalam, S., Petrini, I., Maggio, R., Scarselli, M., & Longoni, B. (2023). Mesenchymal Stem Cell in Pancreatic Islet Transplantation. Biomedicines, 11(5), 1426. https://doi.org/10.3390/biomedicines11051426







