Hereditary Breast Cancer in Romania—Molecular Particularities and Genetic Counseling Challenges in an Eastern European Country
Abstract
1. Introduction
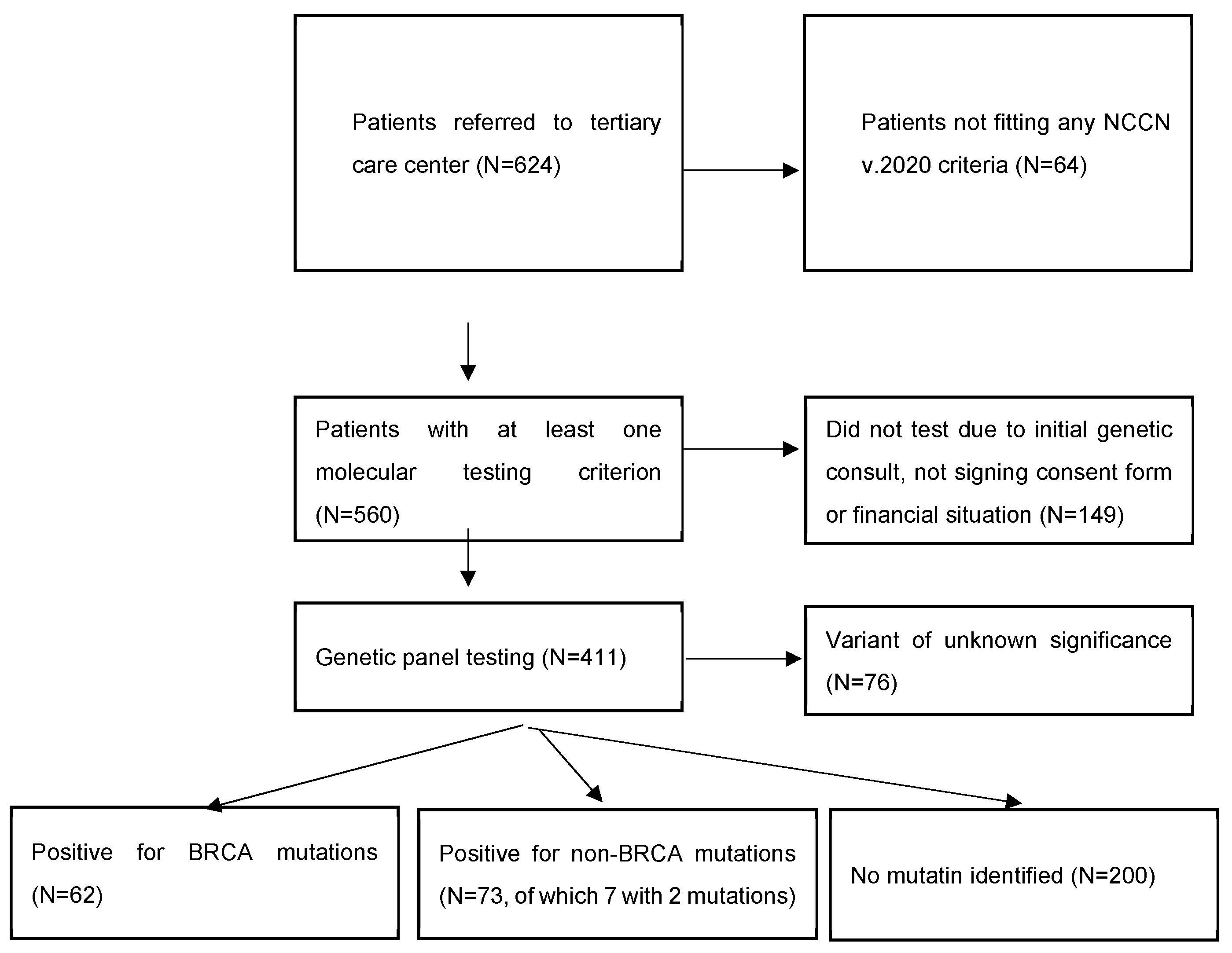
2. Materials and Methods
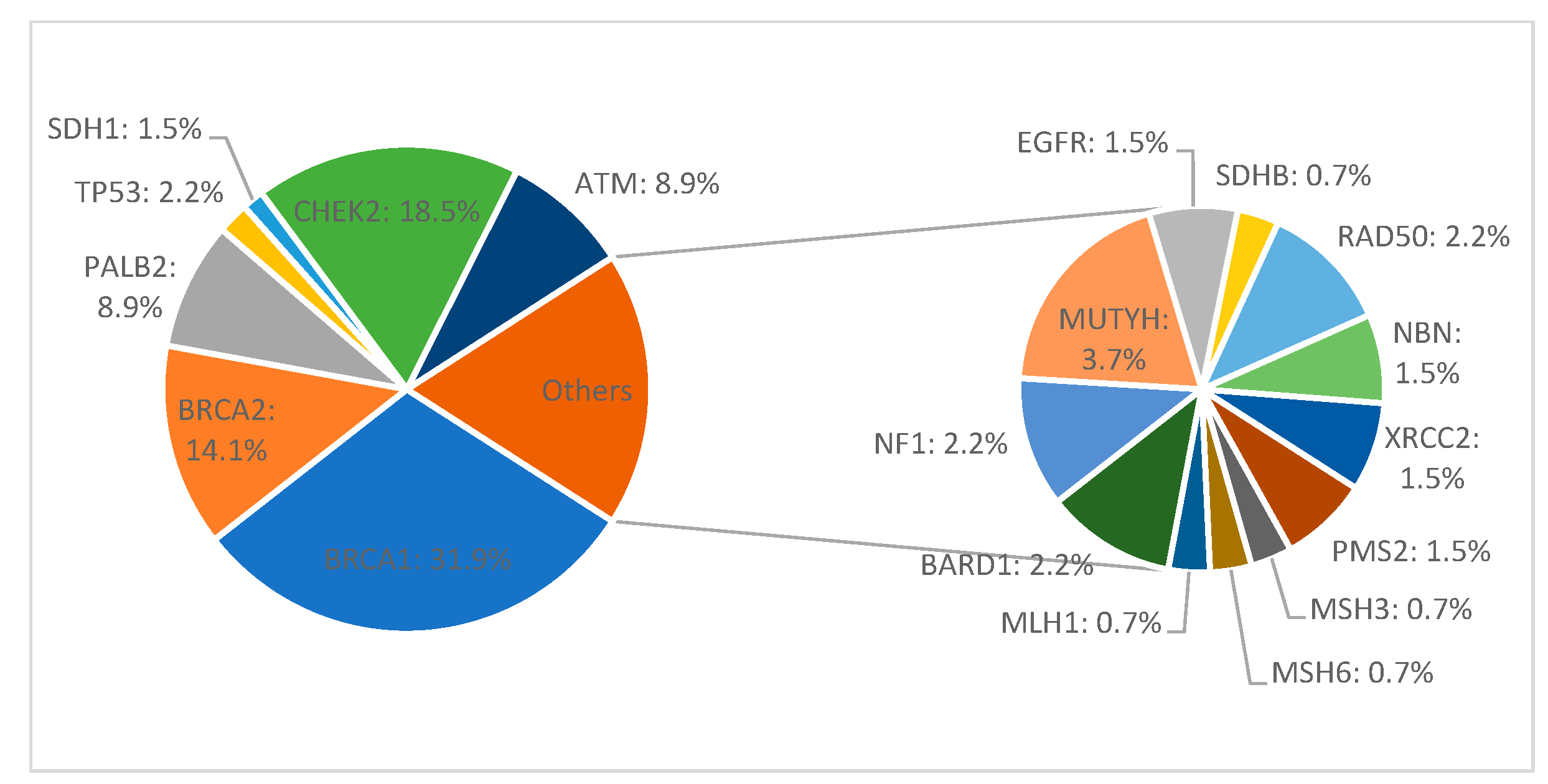
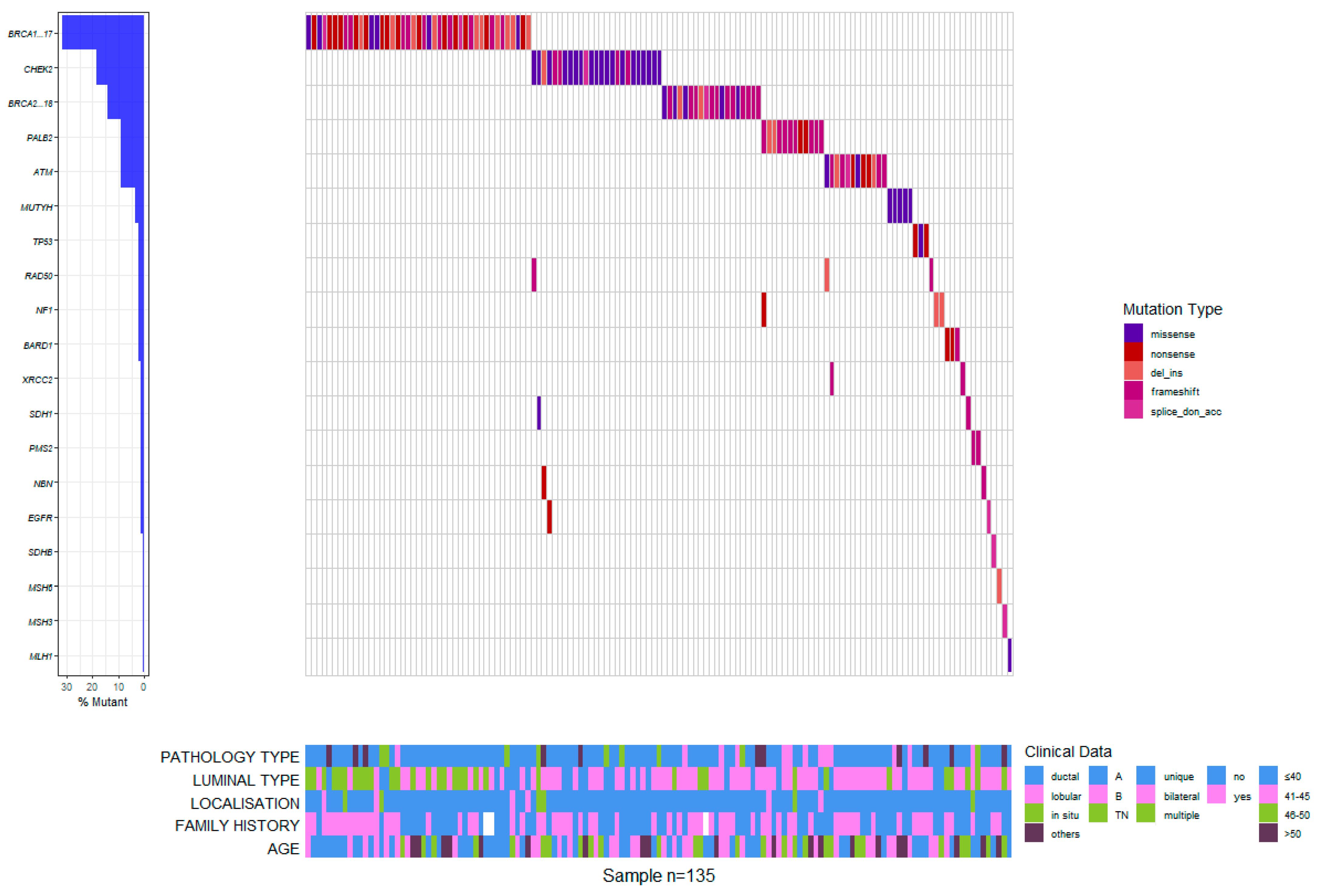
3. Results
4. Discussion
4.1. Mutation Prevalence
4.2. Molecular Subtypes Associations
4.3. Cohort Particularities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furtunescu, F.; Bohiltea, R.E.; Voinea, S.; Georgescu, T.A.; Munteanu, O.; Neacsu, A.; Pop, C.S. Breast cancer mortality gaps in Romanian women compared to the EU after 10 years of accession: Is breast cancer screening a priority for action in Romania? (Review of the Statistics). Exp. Ther. Med. 2021, 21, 268. [Google Scholar] [CrossRef]
- Motoi, G.; Niţă, A.M. The efficiency of public policies and programs for breast cancer prevention. Socio-medical perspectives within a Romania–France comparison. Rom. J. Morphol. Embryol. 2021, 62, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yao, Q.; Xu, Y.; Yu, C.; Zhang, J.; Wang, Q.; Li, J.; Shi, D.; Yu, B.; Zeng, Y.; et al. Characteristics of Germline Non-BRCA Mutation Status of High-Risk Breast Cancer Patients in China and Correlation with High-Risk Factors and Multigene Testing Suggestions. Front. Genet. 2021, 12, 674094. [Google Scholar] [CrossRef]
- Bono, M.; Fanale, D.; Incorvaia, L.; Cancelliere, D.; Fiorino, A.; Calò, V.; Dimino, A.; Filorizzo, C.; Corsini, L.; Brando, C.; et al. Impact of deleterious variants in other genes beyond BRCA1/2 detected in breast/ovarian and pancreatic cancer patients by NGS-based multi-gene panel testing: Looking over the hedge. ESMO Open 2021, 6, 100235. [Google Scholar] [CrossRef]
- LaDuca, H.; Polley, E.C.; Yussuf, A.; Hoang, L.; Gutierrez, S.; Hart, S.N.; Yadav, S.; Hu, C.; Na, J.; Goldgar, D.E.; et al. A clinical guide to hereditary cancer panel testing: Evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet. Med. 2020, 22, 407–415. [Google Scholar] [CrossRef][Green Version]
- Manahan, E.R.; Kuerer, H.M.; Sebastian, M.; Hughes, K.S.; Boughey, J.C.; Euhus, D.M.; Boolbol, S.K.; Taylor, W.A. Consensus Guidelines on Genetic’ Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019, 26, 3025–3031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forbes, C.; Fayter, D.; de Kock, S.; Quek, R.G. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, genetic counseling, and treatment of BRCA-mutated breast cancer. Cancer Manag. Res. 2019, 11, 2321–2337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tung, N.; Garber, J.E. PARP inhibition in breast cancer: Progress made and future hopes. Npj Breast Cancer 2022, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Lilyquist, J.; Ruddy, K.J.; Vachon, C.M.; Couch, F.J. Common Genetic Variation and Breast Cancer Risk-Past, Present, and Future. Cancer Epidemiol. Biomark. Prev. 2018, 27, 380–394. [Google Scholar] [CrossRef][Green Version]
- Hlavac, V.; Kovacova, M.; Elsnerova, K.; Brynychova, V.; Kozevnikovova, R.; Raus, K.; Kopeckova, K.; Mestakova, S.; Vrana, D.; Gatek, J.; et al. Use of Germline Genetic Variability for Prediction of Chemoresistance and Prognosis of Breast Cancer Patients. Cancers 2018, 10, 511. [Google Scholar] [CrossRef][Green Version]
- Vidra, R.; Ciuleanu, T.E.; Nemeș, A.; Pascu, O.; Heroiu, A.M.; Antone, N.; Vidrean, A.I.; Oprean, C.M.; Pop, L.A.; Berindan-Neagoe, I.; et al. Spectrum of BRCA1/2 Mutations in Romanian Breast and Ovarian Cancer Patients. Int. J. Environ. Res. Public Health 2022, 19, 4314. [Google Scholar] [CrossRef]
- Goidescu, I.G.; Caracostea, G.; Eniu, D.T.; Stamatian, F.V. Prevalence of deleterious mutations among patients with breast cancer referred for multigene panel testing in a Romanian population. Med. Pharm. Rep. 2018, 91, 157–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eniu, A.; Pop, L.; Stoian, A.; Dronca, E.; Matei, R.; Ligtenberg, M.; Ouchene, H.; Onisim, A.; Rotaru, O.; Eniu, R.; et al. Understanding BRCA1 and BRCA2 mutated breast cancer cases in Romania: First report on founder mutations in Romanians. Ann. Oncol. 2017, 28, v60–v61. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 27 April 2023).
- RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com (accessed on 27 April 2023).
- Wickham, H. Stringr: Simple, Consistent Wrappers for Common String Operations 2022. Available online: https://CRAN.R-project.org/package=stringr (accessed on 27 April 2023).
- Wickham, H.; Bryan, J. Readxl: Read Excel Files. 2023. Available online: https://CRAN.R-project.org/package=readxl (accessed on 27 April 2023).
- Skidmore, Z.L.; Wagner, A.H.; Lesurf, R.; Campbell, K.M.; Kunisaki, J.; Griffith, O.L.; Griffith, M. GenVisR: Genomic Visualizations in R. Bioinformatics 2016, 32, 3012–3014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janavičius, R. Founder BRCA1/2 mutations in the Europe: Implications for hereditary breast-ovarian cancer prevention and control. EPMA J. 2010, 1, 397–412. [Google Scholar] [CrossRef][Green Version]
- Górski, B.; Byrski, T.; Huzarski, T.; Jakubowska, A.; Menkiszak, J.; Gronwald, J.; Płużańska, A.; Bębenek, M.; Fischer-Maliszewska, Ł.; Grzybowska, E.; et al. Founder Mutations in the BRCA1 Gene in Polish Families with Breast-Ovarian Cancer. Am. J. Hum. Genet. 2000, 66, 1963–1968. [Google Scholar] [CrossRef][Green Version]
- Kaufman, B.; Laitman, Y.; Gronwald, J.; Lubinski, J.; Friedman, E. Haplotype of the C61G BRCA1 Mutation in Polish and Jewish Individuals. Genet. Test. Mol. Biomark. 2009, 13, 465–469. [Google Scholar] [CrossRef]
- Bogdanova, N.; Antonenkova, N.; Rogov, Y.; Karstens, J.; Hillemanns, P.; Dörk, T. High frequency and allele-specific differences of BRCA1 founder mutations in breast cancer and ovarian cancer patients from Belarus. Clin. Genet. 2010, 78, 364–372. [Google Scholar] [CrossRef]
- Negură, L.; Duşa, C.P.; Balmuş, M.I.; Azoicăi, D.; Negură, A.M.; Marinca, M.V.; Miron, L. BRCA1 5382insC founder mutation has not a significative recurrent presence in Northeastern Romanian cancer patients. Rom. J. Morphol. Embryol. 2015, 56, 379–385. [Google Scholar]
- Janatova, M.; Kleibl, Z.; Stribrna, J.; Panczak, A.; Vesela, K.; Zimovjanova, M.; Kleiblova, P.; Dundr, P.; Soukupova, J.; Pohlreich, P. The PALB2 gene is a strong candidate for clinical testing in BRCA1- and BRCA2-negative hereditary breast cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2323–2332. [Google Scholar] [CrossRef][Green Version]
- Wojcik, P.; Jasiowka, M.; Strycharz, E.; Sobol, M.; Hodorowicz-Zaniewska, D.; Skotnicki, P.; Byrski, T.; Blecharz, P.; Marczyk, E.; Cedrych, I.; et al. Recurrent mutations of BRCA1, BRCA2 and PALB2 in the population of breast and ovarian cancer patients in Southern Poland. Hered. Cancer Clin. Pract. 2016, 14, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsaousis, G.N.; Papadopoulou, E.; Apessos, A.; Agiannitopoulos, K.; Pepe, G.; Kampouri, S.; Diamantopoulos, N.; Floros, T.; Iosifidou, R.; Katopodi, O.; et al. Analysis of hereditary cancer syndromes by using a panel of genes: Novel and multiple pathogenic mutations. BMC Cancer 2019, 19, 535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hilz, P.; Heinrihsone, R.; Pätzold, L.A.; Qi, Q.; Trofimovics, G.; Gailite, L.; Irmejs, A.; Gardovskis, J.; Miklasevics, E.; Daneberga, Z. Allelic variants of breast cancer susceptibility genes PALB2 and RECQL in the Latvian population. Hered. Cancer Clin. Pract. 2019, 17, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cybulski, C.; Kluźniak, W.; Huzarski, T.; Wokołorczyk, D.; Kashyap, A.; Jakubowska, A.; Szwiec, M.; Byrski, T.; Dębniak, T.; Górski, B.; et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: A prospective cohort analysis. Lancet Oncol. 2015, 16, 638–644. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef][Green Version]
- Suspitsin, E.; Sokolenko, A.; Bizin, I.; Tumakova, A.; Guseva, M.; Sokolova, N.; Vakhlyarskaya, S.; Kondratenko, I.; Imyanitov, E. ATM mutation spectrum in Russian children with ataxia-telangiectasia. Eur. J. Med. Genet. 2020, 63, 103630. [Google Scholar] [CrossRef] [PubMed]
- Kluska, A.; Balabas, A.; Piatkowska, M.; Czarny, K.; Paczkowska, K.; Nowakowska, D.; Mikula, M.; Ostrowski, J. PALB2 mutations in BRCA1/2-mutation negative breast and ovarian cancer patients from Poland. BMC Med. Genomics 2017, 10, 14. [Google Scholar] [CrossRef][Green Version]
- Neben, C.L.; Zimmer, A.D.; Stedden, W.; van den Akker, J.; O’Connor, R.; Chan, R.C.; Chen, E.; Tan, Z.; Leon, A.; Ji, J.; et al. Multi-Gene Panel Testing of 23,179 Individuals for Hereditary Cancer Risk Identifies Pathogenic Variant Carriers Missed by Current Genetic Testing Guidelines. J. Mol. Diagn. 2019, 21, 646–657. [Google Scholar] [CrossRef][Green Version]
- Boonen, R.A.; Wiegant, W.W.; Celosse, N.; Vroling, B.; Heijl, S.; Kote-Jarai, Z.; Mijuskovic, M.; Cristea, S.; Solleveld-Westerink, N.; van Wezel, T.; et al. Functional Analysis Identifies Damaging CHEK2 Missense Variants Associated with Increased Cancer Risk. Cancer Res. 2022, 82, 615–631. [Google Scholar] [CrossRef]
- Bychkovsky, B.L.; Agaoglu, N.B.; Horton, C.; Zhou, J.; Yussuf, A.; Hemyari, P.; Richardson, M.E.; Young, C.; LaDuca, H.; McGuinness, D.L.; et al. Differences in Cancer Phenotypes Among Frequent CHEK2 Variants and Implications for Clinical Care—Checking CHEK2. JAMA Oncol. 2022, 8, 1598–1606. [Google Scholar] [CrossRef]
- Ivanov, M.; Sharova, M.; Olsen, A.; Lebedeva, A.; Ignatova, E.; Mouse, G.; Mileyko, V. Letter to the Editor: CHEK2 I157T-Pluto Among Numerous Low-Risk Genetic Factors Requiring Discharge From a Range of Pathogenic Variants? J. Natl. Compr. Cancer Netw. 2022, 20. [Google Scholar] [CrossRef]
- Park, B.; Hopper, J.L.; Win, A.K.; Dowty, J.G.; Sung, H.K.; Ahn, C.; Kim, S.-W.; Lee, M.H.; Lee, J.; Lee, J.W.; et al. Reproductive factors as risk modifiers of breast cancer in BRCA mutation carriers and high-risk non-carriers. Oncotarget 2017, 8, 102110–102118. [Google Scholar] [CrossRef][Green Version]
- Cybulski, C.; Wokołorczyk, D.; Jakubowska, A.; Huzarski, T.; Byrski, T.; Gronwald, J.; Masojć, B.; Dębniak, T.; Górski, B.; Blecharz, P.; et al. Risk of Breast Cancer in Women with a CHEK2 Mutation with and Without a Family History of Breast Cancer. J. Clin. Oncol. 2011, 29, 3747–3752. [Google Scholar] [CrossRef]
- Gallagher, S.; Hughes, E.; Kurian, A.W.; Domchek, S.M.; Garber, J.; Probst, B.; Morris, B.; Tshiaba, P.; Meek, S.; Rosenthal, E.; et al. Comprehensive Breast Cancer Risk Assessment for CHEK2 and ATM Pathogenic Variant Carriers Incorporating a Polygenic Risk Score and the Tyrer-Cuzick Model. JCO Precis. Oncol. 2021, 5, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Win, A.K.; Reece, J.C.; Dowty, J.G.; Buchanan, D.D.; Clendenning, M.; Rosty, C.; Southey, M.C.; Young, J.P.; Cleary, S.P.; Kim, H.; et al. Risk of extracolonic cancers for people with biallelic and monoallelic mutations in MUTYH: Extracolonic cancer risks for people with biallelic and monoallelic MUTYH mutations. Int. J. Cancer 2016, 139, 1557–1563. [Google Scholar] [CrossRef][Green Version]
- Wasielewski, M.; Out, A.A.; Vermeulen, J.; Nielsen, M.; Ouweland, A.V.D.; Tops, C.M.J.; Wijnen, J.T.; Vasen, H.; Weiss, M.M.; Klijn, J.G.M.; et al. Increased MUTYH mutation frequency among Dutch families with breast cancer and colorectal cancer. Breast Cancer Res. Treat. 2010, 124, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Rennert, G.; Lejbkowicz, F.; Cohen, I.; Pinchev, M.; Rennert, H.S.; Barnett-Griness, O. MutYH mutation carriers have increased breast cancer risk. Cancer 2012, 118, 1989–1993. [Google Scholar] [CrossRef]
- VCV000140877.35—ClinVar—NCBI. Available online: https://www.ncbi.nlm.nih.gov/clinvar/variation/140877/?new_evidence=true (accessed on 18 February 2023).
- Honrado, E.; Benítez, J.; Palacios, J. The Pathology of Hereditary Breast Cancer. Hered. Cancer Clin. Pract. 2004, 2, 131–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stoppa-Lyonnet, D. The biological effects and clinical implications of BRCA mutations: Where do we go from here? Eur. J. Hum. Genet. 2016, 24 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef][Green Version]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krammer, J.; Pinker-Domenig, K.; Robson, M.E.; Gönen, M.; Bernard-Davila, B.; Morris, E.A.; Mangino, D.A.; Jochelson, M.S. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2017, 163, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.L.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Pathology of Breast and Ovarian Cancers among BRCA1 and BRCA2 Mutation Carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Biomark. Prev. 2012, 21, 134–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. JNCI J. Natl. Cancer Inst. 2020, 113, 808–819. [Google Scholar] [CrossRef]
- Lakhani, S.R.; Van De Vijver, M.J.; Jacquemier, J.; Anderson, T.J.; Osin, P.P.; McGuffog, L.; Easton, D.F. The Pathology of Familial Breast Cancer: Predictive Value of Immunohistochemical Markers Estrogen Receptor, Progesterone Receptor, HER-2, and p53 in Patients with Mutations in BRCA1 and BRCA2. J. Clin. Oncol. 2002, 20, 2310–2318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hacking, S.M.; Wang, Y. Practical Issues of Ki-67 Evaluation in Breast Cancer Clinical Practice. J. Clin. Transl. Pathol. 2022. [Google Scholar] [CrossRef]
- Mrouj, K.; Andrés-Sánchez, N.; Dubra, G.; Singh, P.; Sobecki, M.; Chahar, D.; Al Ghoul, E.; Aznar, A.B.; Prieto, S.; Pirot, N.; et al. Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2026507118. [Google Scholar] [CrossRef]
- Cocoş, R.; Schipor, S.; Hervella, M.; Cianga, P.; Popescu, R.; Bănescu, C.; Constantinescu, M.; Martinescu, A.; Raicu, F. Genetic affinities among the historical provinces of Romania and Central Europe as revealed by an mtDNA analysis. BMC Genet. 2017, 18, 20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katz, S.J.; Ward, K.C.; Hamilton, A.S.; McLeod, M.C.; Wallner, L.P.; Morrow, M.; Jagsi, R.; Hawley, S.T.; Kurian, A.W. Gaps in Receipt of Clinically Indicated Genetic Counseling After Diagnosis of Breast Cancer. J. Clin. Oncol. 2018, 36, 1218–1224. [Google Scholar] [CrossRef]
| Gene | Mutation | Number of Patients |
|---|---|---|
| BRCA1 | c.3607 C>T (p.Arg1203Ter) | 12 |
| c.181T>G (p.Cys61Gly) | 8 | |
| c.5266dupC (p.Gln1756Profs) | 6 | |
| c.68_69delAG (p.GluValfs) | 3 | |
| c.1687C˃T (p.Glu563Ter) | 2 | |
| c.843_846del (p.Ser282fs) | 1 | |
| c.212+1G>T | 1 | |
| c.4327C>T (p.Arg1443Ter) | 1 | |
| c.5030_5033delCTAA (p.Thr1677Ilefs) | 1 | |
| c.1018C>T (p.Arg340Ter) | 1 | |
| c.4065_4068del (p.Asn1355fs) | 1 | |
| c.3700_3704del (p.Val1234fs) | 1 | |
| c.737del (p.Asp245_Leu246isTer) | 1 | |
| c.5251C>T (p.Arg1751Ter) | 1 | |
| c.213-12A>G | 1 | |
| c.1636_1654del (p.Met546fs) | 1 | |
| c.211A>G (p.Arg71Gly) | 1 | |
| BRCA2 | c.9371A>T (p.Asn3124Ile) | 3 |
| c.2808_2811del (p.Ala938Profs) | 2 | |
| c.5796_5797del (p.His1932fs) | 2 | |
| c.7878G>C (p.Trp2626Cys) | 2 | |
| c.2944A>C (p.Ile982Leu) | 1 | |
| c.7230delT (p.Phe2410Leufs) | 1 | |
| c.9253delA (p.Thr3085Glnfs) | 1 | |
| c.3545_3546delT (p.Phe1182Terfs) | 1 | |
| c.793+1G>A | 1 | |
| c.5576_5579del (p.Ile1859fs) | 1 | |
| c.3680_3681del (p.Leu1227fs) | 1 | |
| c.8680C>T (p.Gln2894Ter) | 1 | |
| c.729_732del (p.Asn243fs) | 1 | |
| c.5946del (p.Ser1982fs) | 1 | |
| PALB2 | c.172_175delTTGT (p.Gln60Agfs) | 4 |
| c.2257C>T (p.Arg753Ter) | 3 | |
| c.93dupA (p.Leu32Thrfs) | 1 | |
| c.757_758del (p.Leu253fs) | 1 | |
| c.1037_1041del (p.Lys346fs) | 1 | |
| c.93dup (p.Leu32fs) | 1 | |
| c.1002C>A (p.Tyr334Ter) | 1 | |
| TP53 | C.586C>T (P.Arg196ter) | 1 |
| c.1025G>C (p.Arg342Pro) | 1 | |
| c.916C>T (p.Arg306Ter) | 1 | |
| SDH1 | c.1531C>T (p.Gln511Ter) | 2 |
| ATM | c.1564_1565delGA (p.Glu522Ilefs) | 5 |
| c.935dup (p.Leu312Phef*s6) | 1 | |
| c.6095G>A (p.Arg2032Lys) | 1 | |
| c.8585-2A>C | 1 | |
| c.5980A>T (p.Lys1994Ter) | 1 | |
| c.4768C>T (p.Leu1590Phe) | 1 | |
| c.5644C>T (p.Arg1882Ter) | 1 | |
| c.5932G>T (p.Glu1978Ter) | 1 | |
| CHEK2 | c.470T>C (p.Ile157Thr) | 18 |
| c.902delT, p.(Leu301Trpfs*3) | 3 | |
| c.917G>C (p.Gly306Ala) | 1 | |
| c.1312G>T (p.Asp438Tyr) | 1 | |
| c.444+1G>A | 1 | |
| c.1100del (p.Thr367fs) | 1 | |
| BARD1 | c.1690C>T (p.Gln564Ter) | 1 |
| c.632T>A (p.Leu211Ter) | 1 | |
| c.176_177del (p.Glu59fs) | 1 | |
| RAD50 | c.326_329del (p.Thr109fs) | 3 |
| PMS2 | c.1239dup (p.Asp414fs) | 1 |
| c.1076dupT(p.Leu359Phefs) | 1 | |
| MSH3 | c.2436-1G>A | 1 |
| MSH6 | ex.1-6del | 1 |
| MLH1 | c.544A>G (p.Arg182Gly) | 1 |
| MUTYH | c.650G>A (p.Arg217His) | 3 |
| c.1187G>A (p.Gly396Asp) | 1 | |
| c.536A>G (p.Tyr179Cys) | 1 | |
| NF1 | ex.5 CNV | 1 |
| ex.7del | 1 | |
| c.2410-1G>A | 1 | |
| NBN | c.657_661del (p.Lys219fs) | 2 |
| SDHB | c.423+1G>A | 1 |
| XRCC2 | c.190C>T (p.Arg64Ter) | 2 |
| EGFR | c.2061+2T>C | 2 |
| Variable | BRCA Mutation (N = 62) | Non-BRCA Mutation (N = 73) | VUS (N = 76) | No Mutation (N = 200) |
|---|---|---|---|---|
| Diagnosis age | 41.387 ± 8.084 | 44.466 ± 7.02 | 44.7 | 45.62 ± 7.367 |
| Positive family history of cancer | 32 (54.2%) † | 33 (45.2%) | 32 (42.1%) | 49 (24.5%) |
| Histology | ||||
| Ductal (312–75.9%) | 50 (80.6%) | 52 (71.2%) | 48 (63.1%) | 162 (81%) |
| In situ 30 (30–7.3%) | 4 (6.5%) | 5 (6.8%) | 11 (14.4%) | 10 (5%) |
| Lobular (53–12.9%) | 4 (6.5%) | 9 (12.3%) | 14 (18.4%) | 26 (13%) |
| Others (16–3.9%) | 4 (6.5%) | 7 (9.6%) | 3 (4.1%) | 2 (1%) |
| Molecular subtype | ||||
| Luminal A (72–17.5%) | 10 (16.1%) | 15 (20.5%) | 28 (36.8%) | 19 (9.5%) |
| Luminal B (285–69.3%) | 28 (45.2%) | 49 (67.1%) | 42 (55.2%) | 166 (83%) |
| TN (54–13.1%) | 24 (38.7%) | 9 (12.3%) | 6 (7.9%) | 15 (7.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cătană, A.; Trifa, A.P.; Achimas-Cadariu, P.A.; Bolba-Morar, G.; Lisencu, C.; Kutasi, E.; Chelaru, V.F.; Muntean, M.; Martin, D.L.; Antone, N.Z.; et al. Hereditary Breast Cancer in Romania—Molecular Particularities and Genetic Counseling Challenges in an Eastern European Country. Biomedicines 2023, 11, 1386. https://doi.org/10.3390/biomedicines11051386
Cătană A, Trifa AP, Achimas-Cadariu PA, Bolba-Morar G, Lisencu C, Kutasi E, Chelaru VF, Muntean M, Martin DL, Antone NZ, et al. Hereditary Breast Cancer in Romania—Molecular Particularities and Genetic Counseling Challenges in an Eastern European Country. Biomedicines. 2023; 11(5):1386. https://doi.org/10.3390/biomedicines11051386
Chicago/Turabian StyleCătană, Andreea, Adrian P. Trifa, Patriciu A. Achimas-Cadariu, Gabriela Bolba-Morar, Carmen Lisencu, Eniko Kutasi, Vlad F. Chelaru, Maximilian Muntean, Daniela L. Martin, Nicoleta Z. Antone, and et al. 2023. "Hereditary Breast Cancer in Romania—Molecular Particularities and Genetic Counseling Challenges in an Eastern European Country" Biomedicines 11, no. 5: 1386. https://doi.org/10.3390/biomedicines11051386
APA StyleCătană, A., Trifa, A. P., Achimas-Cadariu, P. A., Bolba-Morar, G., Lisencu, C., Kutasi, E., Chelaru, V. F., Muntean, M., Martin, D. L., Antone, N. Z., Fetica, B., Pop, F., & Militaru, M. S. (2023). Hereditary Breast Cancer in Romania—Molecular Particularities and Genetic Counseling Challenges in an Eastern European Country. Biomedicines, 11(5), 1386. https://doi.org/10.3390/biomedicines11051386






