Osteopontin in Pulmonary Hypertension
Abstract
1. Introduction
2. Osteopontin Signaling
3. Osteopontin as a Biomarker of Pulmonary Hypertension and Right Ventricular Failure
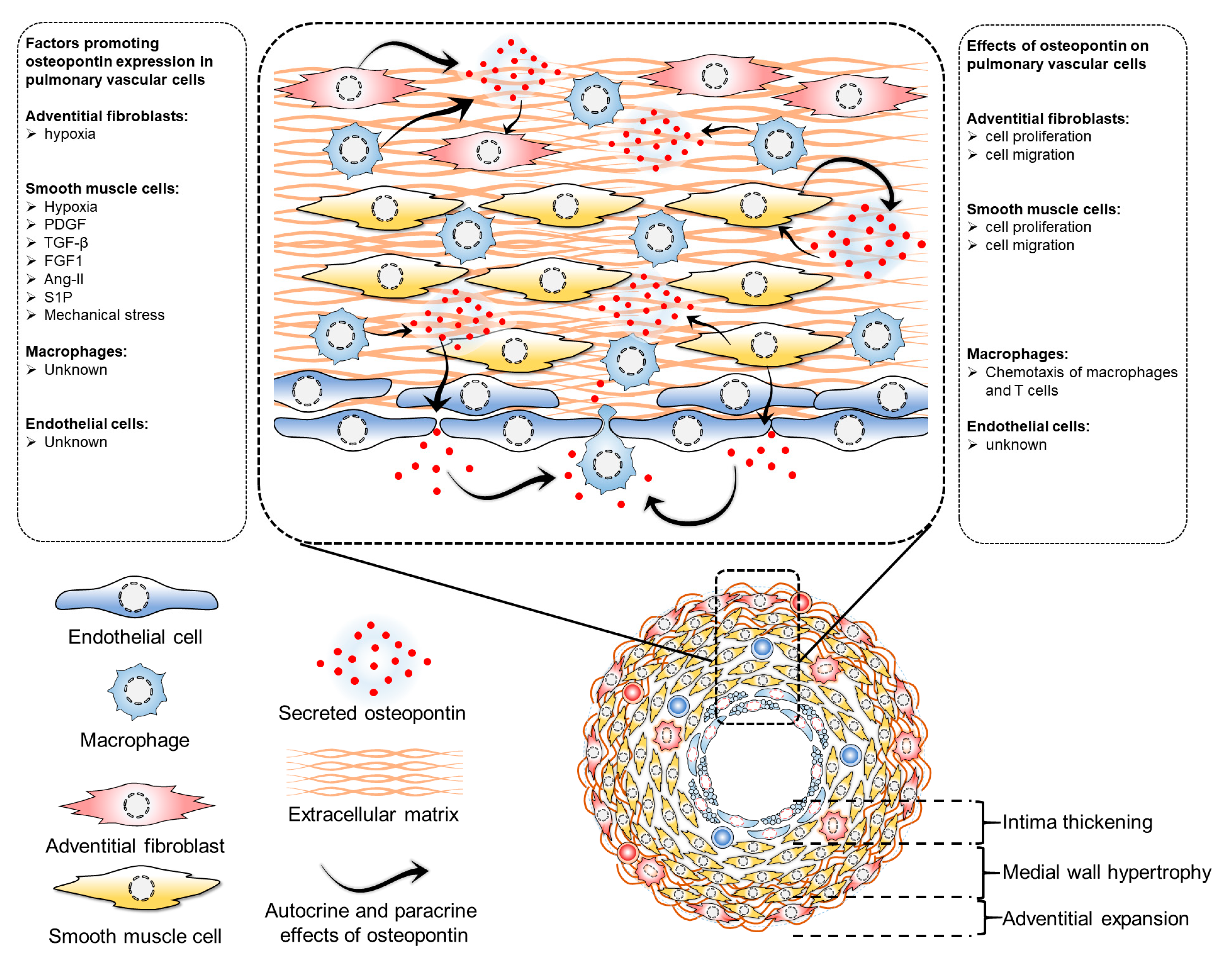
4. Osteopontin in Pulmonary Vascular Cells
4.1. Osteopontin in Pulmonary Artery Endothelial Cells
4.2. Osteopontin in Pulmonary Artery Smooth Muscle Cells
4.3. Osteopontin in Pulmonary Artery Adventitial Fibroblasts
4.4. Osteopontin in Pulmonary Vascular Macrophages
4.5. Osteopontin in Intercellular Communications of Vascular Cells in Pulmonary Vascular Remodeling
5. Osteopontin in Animal Models of Pulmonary Hypertension
6. Osteopontin in Right Ventricular Remodeling
7. Osteopontin as a Treatment Target in Pulmonary Hypertension
8. Future Experimental Perspectives
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmuller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Vara, E.; Ntokou, A.; Dave, J.M.; Jovin, D.G.; Saddouk, F.Z.; Greif, D.M. Vascular pathobiology of pulmonary hypertension. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2022, 42, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Richter, M.J.; Rubin, L.J. The physiological basis of pulmonary arterial hypertension. Eur. Respir. J. 2022, 59, 2102334. [Google Scholar] [CrossRef] [PubMed]
- Cober, N.D.; VandenBroek, M.M.; Ormiston, M.L.; Stewart, D.J. Evolving Concepts in Endothelial Pathobiology of Pulmonary Arterial Hypertension. Hypertension 2022, 79, 1580–1590. [Google Scholar] [CrossRef]
- Morrell, N.W.; Aldred, M.A.; Chung, W.K.; Elliott, C.G.; Nichols, W.C.; Soubrier, F.; Trembath, R.C.; Loyd, J.E. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801899. [Google Scholar] [CrossRef]
- Frump, A.L.; Lahm, T. The Basic Science of Metabolism in Pulmonary Arterial Hypertension. Adv. Pulm. Hypertens. 2018, 17, 95–102. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Wharton, J.; Wilkins, M.R. Metabolomic Insights in Pulmonary Arterial Hypertension. Adv. Pulm. Hypertens. 2018, 17, 103–109. [Google Scholar] [CrossRef]
- Kim, J. Apelin-APJ signaling: A potential therapeutic target for pulmonary arterial hypertension. Mol. Cells 2014, 37, 196. [Google Scholar] [CrossRef]
- Kuhr, F.K.; Smith, K.A.; Song, M.Y.; Levitan, I.; Yuan, J.X. New mechanisms of pulmonary arterial hypertension: Role of Ca2+ signaling. Am. J. Physiol. -Heart Circ. Physiol. 2012, 302, H1546–H1562. [Google Scholar] [CrossRef]
- Ranchoux, B.; Meloche, J.; Paulin, R.; Boucherat, O.; Provencher, S.; Bonnet, S. DNA damage and pulmonary hypertension. Int. J. Mol. Sci. 2016, 17, 990. [Google Scholar] [CrossRef]
- Archer, S.L.; Gomberg-Maitland, M.; Maitland, M.L.; Rich, S.; Garcia, J.G.; Weir, E.K. Mitochondrial metabolism, redox signaling, and fusion: A mitochondria-ROS-HIF-1α-Kv1. 5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am. J. Physiol. -Heart Circ. Physiol. 2008, 294, H570–H578. [Google Scholar] [CrossRef] [PubMed]
- Meloche, J.; Pflieger, A.; Vaillancourt, M.; Graydon, C.; Provencher, S.; Bonnet, S. miRNAs in PAH: Biomarker, therapeutic target or both? Drug. Discov. Today 2014, 19, 1264–1269. [Google Scholar] [CrossRef]
- Gopinath, P.; Natarajan, A.; Sathyanarayanan, A.; Veluswami, S.; Gopisetty, G. The multifaceted role of Matricellular Proteins in health and cancer, as biomarkers and therapeutic targets. Gene 2022, 815, 146137. [Google Scholar] [CrossRef]
- Oldberg, A.; Franzén, A.; Heinegård, D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc. Natl. Acad. Sci. USA 1986, 83, 8819–8823. [Google Scholar] [CrossRef] [PubMed]
- Franzén, A.; Heinegård, D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem. J. 1985, 232, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral. Biol. Med. Off. Publ. Am. Assoc. Oral. Biol. 2000, 11, 279–303. [Google Scholar] [CrossRef]
- Brown, L.F.; Berse, B.; Van de Water, L.; Papadopoulos-Sergiou, A.; Perruzzi, C.A.; Manseau, E.J.; Dvorak, H.F.; Senger, D.R. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell. 1992, 3, 1169–1180. [Google Scholar] [CrossRef]
- Lamort, A.S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef]
- Mackay, C.R.; Terpe, H.J.; Stauder, R.; Marston, W.L.; Stark, H.; Günthert, U. Expression and modulation of CD44 variant isoforms in humans. J. Cell Biol. 1994, 124, 71–82. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol. Pathol. MP 1999, 52, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, Y.U.; Sleeman, J.; Fujii, H.; Herrlich, P.; Hotta, H.; Tanaka, K.; Chikuma, S.; Yagita, H.; Okumura, K.; Murakami, M.; et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999, 59, 219–226. [Google Scholar] [PubMed]
- Rao, G.; Wang, H.; Li, B.; Huang, L.; Xue, D.; Wang, X.; Jin, H.; Wang, J.; Zhu, Y.; Lu, Y.; et al. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Wang, M.J.; Sudhir, P.R.; Chen, G.D.; Chi, C.W.; Chen, J.Y. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN-CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007, 67, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Poggio, P.; Branchetti, E.; Grau, J.B.; Lai, E.K.; Gorman, R.C.; Gorman, J.H., 3rd; Sacks, M.S.; Bavaria, J.E.; Ferrari, G. Osteopontin-CD44v6 interaction mediates calcium deposition via phospho-Akt in valve interstitial cells from patients with noncalcified aortic valve sclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2086–2094. [Google Scholar] [CrossRef]
- Ohta-Ogo, K.; Hao, H.; Ishibashi-Ueda, H.; Hirota, S.; Nakamura, K.; Ohe, T.; Ito, H. CD44 expression in plexiform lesions of idiopathic pulmonary arterial hypertension. Pathol. Int. 2012, 62, 219–225. [Google Scholar] [CrossRef]
- Anwar, A.; Li, M.; Frid, M.G.; Kumar, B.; Gerasimovskaya, E.V.; Riddle, S.R.; McKeon, B.A.; Thukaram, R.; Meyrick, B.O.; Fini, M.A.; et al. Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L1–L11. [Google Scholar] [CrossRef]
- Isobe, S.; Kataoka, M.; Endo, J.; Moriyama, H.; Okazaki, S.; Tsuchihashi, K.; Katsumata, Y.; Yamamoto, T.; Shirakawa, K.; Yoshida, N.; et al. Endothelial-Mesenchymal Transition Drives Expression of CD44 Variant and xCT in Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2019, 61, 367–379. [Google Scholar] [CrossRef]
- Raineri, D.; Dianzani, C.; Cappellano, G.; Maione, F.; Baldanzi, G.; Iacobucci, I.; Clemente, N.; Baldone, G.; Boggio, E.; Gigliotti, C.L.; et al. Osteopontin binds ICOSL promoting tumor metastasis. Commun. Biol. 2020, 3, 615. [Google Scholar] [CrossRef]
- Inoue, M.; Shinohara, M.L. Intracellular osteopontin (iOPN) and immunity. Immunol. Res. 2011, 49, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Petersen, T.E.; Sørensen, E.S. Post-translational modification and proteolytic processing of urinary osteopontin. Biochem. J. 2008, 411, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Iwasaki-Hozumi, H.; Bai, G.; Chagan-Yasutan, H.; Shete, A.; Telan, E.F.; Takahashi, A.; Ashino, Y.; Matsuba, T. Both Full-Length and Protease-Cleaved Products of Osteopontin Are Elevated in Infectious Diseases. Biomedicines 2021, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Kariya, Y. Osteopontin in Cancer: Mechanisms and Therapeutic Targets. Int. J. Transl. Med. 2022, 2, 419–447. [Google Scholar] [CrossRef]
- Harbaum, L.; Rhodes, C.J.; Otero-Núñez, P.; Wharton, J.; Wilkins, M.R. The application of ‘omics’ to pulmonary arterial hypertension. Br. J. Pharm. 2021, 178, 108–120. [Google Scholar] [CrossRef]
- Hojda, S.E.; Chis, I.C.; Clichici, S. Biomarkers in Pulmonary Arterial Hypertension. Diagnostics 2022, 12, 3033. [Google Scholar] [CrossRef]
- Abdalrhim, A.D.; Marroush, T.S.; Austin, E.E.; Gersh, B.J.; Solak, N.; Rizvi, S.A.; Bailey, K.R.; Kullo, I.J. Plasma Osteopontin Levels and Adverse Cardiovascular Outcomes in the PEACE Trial. PLoS ONE 2016, 11, e0156965. [Google Scholar] [CrossRef]
- Schipper, M.E.; Scheenstra, M.R.; van Kuik, J.; van Wichen, D.F.; van der Weide, P.; Dullens, H.F.; Lahpor, J.; de Jonge, N.; De Weger, R.A. Osteopontin: A potential biomarker for heart failure and reverse remodeling after left ventricular assist device support. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2011, 30, 805–810. [Google Scholar] [CrossRef]
- Rosenberg, M.; Zugck, C.; Nelles, M.; Juenger, C.; Frank, D.; Remppis, A.; Giannitsis, E.; Katus, H.A.; Frey, N. Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circulation. Heart Fail. 2008, 1, 43–49. [Google Scholar] [CrossRef]
- Saker, M.; Lipskaia, L.; Marcos, E.; Abid, S.; Parpaleix, A.; Houssaini, A.; Validire, P.; Girard, P.; Noureddine, H.; Boyer, L.; et al. Osteopontin, a Key Mediator Expressed by Senescent Pulmonary Vascular Cells in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1879–1890. [Google Scholar] [CrossRef]
- Mura, M.; Cecchini, M.J.; Joseph, M.; Granton, J.T. Osteopontin lung gene expression is a marker of disease severity in pulmonary arterial hypertension. Respirology 2019, 24, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, X.; Teng, X.; Gu, H.; Yuan, W.; Meng, J.; Li, J.; Zheng, Z.; Wei, Y.; Hu, S. Osteopontin plays important roles in pulmonary arterial hypertension induced by systemic-to-pulmonary shunt. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 7236–7251. [Google Scholar] [CrossRef]
- Bellan, M.; Piccinino, C.; Tonello, S.; Minisini, R.; Giubertoni, A.; Sola, D.; Pedrazzoli, R.; Gagliardi, I.; Zecca, E.; Calzaducca, E.; et al. Role of Osteopontin as a Potential Biomarker of Pulmonary Arterial Hypertension in Patients with Systemic Sclerosis and Other Connective Tissue Diseases (CTDs). Pharmaceuticals 2021, 14, 394. [Google Scholar] [CrossRef]
- Hetman, O.; Krakhmalova, O. Osteopontin as a marker of pulmonary hypertension in patients with coronary heart disease combined with chronic obstructive pulmonary disease. Cardiovasc. Res. 2016, 111 (Suppl. 1), S113–S114. [Google Scholar]
- Yao, X.; Jing, T.; Wang, T.; Gu, C.; Chen, X.; Chen, F.; Feng, H.; Zhao, H.; Chen, D.; Ma, W. Molecular Characterization and Elucidation of Pathways to Identify Novel Therapeutic Targets in Pulmonary Arterial Hypertension. Front. Physiol. 2021, 12, 694702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Niu, Y.; Luo, L.; Lu, Z.; Chen, Q.; Zhang, S.; Guo, Q.; Li, L.; Gou, D. Decoding ceRNA regulatory network in the pulmonary artery of hypoxia-induced pulmonary hypertension (HPH) rat model. Cell. Biosci. 2022, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Keranov, S.; Dörr, O.; Jafari, L.; Liebetrau, C.; Keller, T.; Troidl, C.; Riehm, J.; Rutsatz, W.; Bauer, P.; Kriechbaum, S.; et al. Osteopontin and galectin-3 as biomarkers of maladaptive right ventricular remodeling in pulmonary hypertension. Biomark. Med. 2021, 15, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Kölmel, S.; Hobohm, L.; Käberich, A.; Krieg, V.J.; Bochenek, M.L.; Wenzel, P.; Wiedenroth, C.B.; Liebetrau, C.; Hasenfuß, G.; Mayer, E.; et al. Potential Involvement of Osteopontin in Inflammatory and Fibrotic Processes in Pulmonary Embolism and Chronic Thromboembolic Pulmonary Hypertension. Thromb. Haemost. 2019, 119, 1332–1346. [Google Scholar] [CrossRef]
- Rubis, P.; Toton-Zuranska, J.; Wisniowska-Smialek, S.; Kolton-Wroz, M.; Wolkow, P.; Wypasek, E.; Rudnicka-Sosin, L.; Pawlak, A.; Kozanecki, K.; Tomkiewicz-Pajak, L.; et al. Right ventricular morphology and function is not related with microRNAs and fibrosis markers in dilated cardiomyopathy. Cardiol. J. 2017, 25, 722–731. [Google Scholar] [CrossRef]
- Kazimli, A.V.; Ryzhkov, A.V.; Goncharova, N.S.; Naymushin, A.V.; Moiseeva, O.M. Myeloperoxidase, osteopontin and asymmetrical dimethylarginine as biomarkers of pulmonary hypertension severity. Eur. Heart J. 2013, 34 (Suppl. S1). [Google Scholar] [CrossRef]
- Lorenzen, J.M.; Nickel, N.; Kramer, R.; Golpon, H.; Westerkamp, V.; Olsson, K.M.; Haller, H.; Hoeper, M.M. Osteopontin in patients with idiopathic pulmonary hypertension. Chest 2011, 139, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Meyer, F.J.; Gruenig, E.; Schuster, T.; Lutz, M.; Lossnitzer, D.; Wipplinger, R.; Katus, H.A.; Frey, N. Osteopontin (OPN) improves risk stratification in pulmonary hypertension (PH). Int. J. Cardiol. 2012, 155, 504–505. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.; Wharton, J.; Howard, L.; Gibbs, J.; Wilkins, M. S96 Novel biomarkers in idiopathic pulmonary arterial hypertension. Thorax 2010, 65 (Suppl. S4), A44. [Google Scholar] [CrossRef]
- Rosenberg, M.; Meyer, F.J.; Gruenig, E.; Lutz, M.; Lossnitzer, D.; Wipplinger, R.; Katus, H.A.; Frey, N. Osteopontin predicts adverse right ventricular remodelling and dysfunction in pulmonary hypertension. Eur. J. Clin. Investig. 2012, 42, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Shingai, M.; Aso, N.; Hazuku, T.; Nasu, M. Osteopontin is released from the heart into the coronary circulation in patients with a previous anterior wall myocardial infarction. Circ. J. Off. J. Jpn. Circ. Soc. 2003, 67, 742–744. [Google Scholar] [CrossRef]
- Ayoub, C.; Nozza, A.; Denault, A.; Deschamps, A.; Dupuis, J. Pulmonary production of osteopontin in humans: Effects of left ventricular systolic dysfunction and cardiopulmonary bypass. J. Card. Fail. 2013, 19, 816–820. [Google Scholar] [CrossRef]
- Schäfer, S.; Ellinghaus, P.; Janssen, W.; Kramer, F.; Lustig, K.; Milting, H.; Kast, R.; Klein, M. Chronic inhibition of phosphodiesterase 5 does not prevent pressure-overload-induced right-ventricular remodelling. Cardiovasc. Res. 2009, 82, 30–39. [Google Scholar] [CrossRef]
- Sugiyama, T.; Yoshimoto, T.; Hirono, Y.; Suzuki, N.; Sakurada, M.; Tsuchiya, K.; Minami, I.; Iwashima, F.; Sakai, H.; Tateno, T.; et al. Aldosterone increases osteopontin gene expression in rat endothelial cells. Biochem. Biophys. Res. Commun. 2005, 336, 163–167. [Google Scholar] [CrossRef]
- Senger, D.R.; Ledbetter, S.R.; Claffey, K.P.; Papadopoulos-Sergiou, A.; Peruzzi, C.A.; Detmar, M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am. J. Pathol. 1996, 149, 293–305. [Google Scholar]
- Sadaghianloo, N.; Contenti, J.; Dufies, M.; Parola, J.; Rouleau, M.; Lee, S.; Peyron, J.F.; Fabbri, L.; Hassen-Khodja, R.; Pouysségur, J.; et al. Co-culture of human fibroblasts, smooth muscle and endothelial cells promotes osteopontin induction in hypoxia. J. Cell Mol. Med. 2020, 24, 2931–2941. [Google Scholar] [CrossRef]
- Hamada, Y.; Nokihara, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Matsuo, M.; Umakoshi, Y.; Takahashi, J.; Matsuura, N. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochem. Biophys. Res. Commun. 2003, 310, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Wing, T.T.; Erikson, D.W.; Burghardt, R.C.; Bazer, F.W.; Bayless, K.J.; Johnson, G.A. OPN binds alpha V integrin to promote endothelial progenitor cell incorporation into vasculature. Reproduction 2020, 159, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, E.E.; Liew, A.; Mashayekhi, K.; Dockery, P.; McDermott, J.; Kealy, B.; Flynn, A.; Duffy, A.; Coleman, C.; O’Regan, A.; et al. Pretreatment of endothelial progenitor cells with osteopontin enhances cell therapy for peripheral vascular disease. Cell. Transplant. 2012, 21, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.N.; Guo, Q.; Zhang, Q.; Ren, L.; Ren, X.Y.; Nie, M.L.; Xu, L.; Long, Q.M.; Guo, Y.F.; Zhao, W.; et al. Proangiogenic functions of osteopontin-derived synthetic peptide RSKSKKFRR in endothelial cells and postischemic brain. Neuroreport 2021, 32, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Johnson, J.N.; Singh, K.; Singh, M. Impairment of myocardial angiogenic response in the absence of osteopontin. Microcirculation 2007, 14, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, G.; Zhu, Y.; Peng, X.; Li, T.; Liu, L. Relationship of Cx43 regulation of vascular permeability to osteopontin-tight junction protein pathway after sepsis in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R1–R11. [Google Scholar] [CrossRef]
- Chang, C.J.; Lai, Y.J.; Tung, Y.C.; Wu, L.S.; Hsu, L.A.; Tseng, C.N.; Chang, G.J.; Yang, K.C.; Yeh, Y.H. Osteopontin mediation of disturbed flow-induced endothelial mesenchymal transition through CD44 is a novel mechanism of neointimal hyperplasia in arteriovenous fistulae for hemodialysis access. Kidney Int. 2023, 103, 702–718. [Google Scholar] [CrossRef]
- Khan, S.A.; Lopez-Chua, C.A.; Zhang, J.; Fisher, L.W.; Sørensen, E.S.; Denhardt, D.T. Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J. Cell. Biochem. 2002, 85, 728–736. [Google Scholar] [CrossRef]
- Evans, C.E.; Cober, N.D.; Dai, Z.; Stewart, D.J.; Zhao, Y.Y. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur. Respir. J. 2021, 58, 2003957. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, S.E.; Jang, M.A.; Kim, C.D. Osteopontin plays a key role in vascular smooth muscle cell proliferation via EGFR-mediated activation of AP-1 and C/EBPβ pathways. Pharmacol. Res. 2016, 108, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.S.; Schnapp, L.M.; Lin, X.; Taubman, M.B. Osteopontin deficiency in rat vascular smooth muscle cells is associated with an inability to adhere to collagen and increased apoptosis. Lab. Investig. A J. Tech. Methods Pathol. 2000, 80, 1603–1615. [Google Scholar] [CrossRef] [PubMed]
- Chaulet, H.; Desgranges, C.; Renault, M.A.; Dupuch, F.; Ezan, G.; Peiretti, F.; Loirand, G.; Pacaud, P.; Gadeau, A.P. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ. Res. 2001, 89, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Han, M.; Wen, J.K.; Li, A.Y. Osteopontin stimulates vascular smooth muscle cell migration by inducing FAK phosphorylation and ILK dephosphorylation. Biochem. Biophys. Res. Commun. 2007, 356, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Corjay, M.H.; Diamond, S.M.; Schlingmann, K.L.; Gibbs, S.K.; Stoltenborg, J.K.; Racanelli, A.L. alphavbeta3, alphavbeta5, and osteopontin are coordinately upregulated at early time points in a rabbit model of neointima formation. J. Cell. Biochem. 1999, 75, 492–504. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Phadke, S.A.; Batlle, D.; Sahai, A. Hypoxia stimulates osteopontin expression and proliferation of cultured vascular smooth muscle cells: Potentiation by high glucose. Diabetes 2001, 50, 1482–1490. [Google Scholar] [CrossRef]
- Wang, X.; Louden, C.; Ohlstein, E.H.; Stadel, J.M.; Gu, J.L.; Yue, T.L. Osteopontin expression in platelet-derived growth factor-stimulated vascular smooth muscle cells and carotid artery after balloon angioplasty. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1365–1372. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Dai, Z.; Sun, Y. Intermittent high glucose enhances proliferation of vascular smooth muscle cells by upregulating osteopontin. Mol. Cell. Endocrinol. 2009, 313, 64–69. [Google Scholar] [CrossRef]
- Seo, K.W.; Lee, S.J.; Ye, B.H.; Kim, Y.W.; Bae, S.S.; Kim, C.D. Mechanical stretch enhances the expression and activity of osteopontin and MMP-2 via the Akt1/AP-1 pathways in VSMC. J. Mol. Cell. Cardiol. 2015, 85, 13–24. [Google Scholar] [CrossRef]
- Fu, G.X.; Xu, C.C.; Zhong, Y.; Zhu, D.L.; Gao, P.J. Aldosterone-induced osteopontin expression in vascular smooth muscle cells involves MR, ERK, and p38 MAPK. Endocrine 2012, 42, 676–683. [Google Scholar] [CrossRef]
- Lyle, A.N.; Joseph, G.; Fan, A.E.; Weiss, D.; Landázuri, N.; Taylor, W.R. Reactive oxygen species regulate osteopontin expression in a murine model of postischemic neovascularization. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Gadeau, A.P.; Campan, M.; Millet, D.; Candresse, T.; Desgranges, C. Osteopontin overexpression is associated with arterial smooth muscle cell proliferation in vitro. Arterioscler. Thromb. 1993, 13, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Jalvy, S.; Renault, M.A.; Leen, L.L.; Belloc, I.; Bonnet, J.; Gadeau, A.P.; Desgranges, C. Autocrine expression of osteopontin contributes to PDGF-mediated arterial smooth muscle cell migration. Cardiovasc. Res. 2007, 75, 738–747. [Google Scholar] [CrossRef]
- Weintraub, A.S.; Giachelli, C.M.; Krauss, R.S.; Almeida, M.; Taubman, M.B. Autocrine secretion of osteopontin by vascular smooth muscle cells regulates their adhesion to collagen gels. Am. J. Pathol. 1996, 149, 259–272. [Google Scholar] [PubMed]
- Bendeck, M.P.; Irvin, C.; Reidy, M.; Smith, L.; Mulholland, D.; Horton, M.; Giachelli, C.M. Smooth muscle cell matrix metalloproteinase production is stimulated via alpha(v)beta(3) integrin. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Zhang, H.; Tian, C.; Sun, X.; Qian, X.; Meng, Y.; Guo, X.; Chang, Q. Proliferative Vascular Smooth Muscle Cells Stimulate Extracellular Matrix Production via Osteopontin/p38 MAPK Signaling Pathway. Cardiology 2021, 146, 646–655. [Google Scholar] [CrossRef]
- Dey, N.B.; Boerth, N.J.; Murphy-Ullrich, J.E.; Chang, P.L.; Prince, C.W.; Lincoln, T.M. Cyclic GMP-dependent protein kinase inhibits osteopontin and thrombospondin production in rat aortic smooth muscle cells. Circ. Res. 1998, 82, 139–146. [Google Scholar] [CrossRef]
- Li, P.; Oparil, S.; Feng, W.; Chen, Y.F. Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J. Appl. Physiol. 2004, 97, 1550–1558. [Google Scholar] [CrossRef]
- Li, P.; Oparil, S.; Novak, L.; Cao, X.; Shi, W.; Lucas, J.; Chen, Y.F. ANP signaling inhibits TGF-beta-induced Smad2 and Smad3 nuclear translocation and extracellular matrix expression in rat pulmonary arterial smooth muscle cells. J. Appl. Physiol. 2007, 102, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Aikeremu, N.; Shi, W.Y.; Tang, X.C.; Gao, R.J.; Kong, L.J.; Zhang, J.R.; Qin, W.J.; Zhang, A.M.; Ma, K.T.; et al. Inhibition of KIR2.1 decreases pulmonary artery smooth muscle cell proliferation and migration. Int. J. Mol. Med. 2022, 50, 119. [Google Scholar] [CrossRef]
- Xing, Y.; Zheng, X.; Li, G.; Liao, L.; Cao, W.; Xing, H.; Shen, T.; Sun, L.; Yang, B.; Zhu, D. MicroRNA-30c contributes to the development of hypoxia pulmonary hypertension by inhibiting platelet-derived growth factor receptor beta expression. Int. J. Biochem. Cell Biol. 2015, 64, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, T.; Liu, X.; Yu, H.; Hao, Z.; Chen, Y.; Zhang, C.; Liu, Y.; Li, Q.; Mao, M.; et al. Modulation of Pulmonary Vascular Remodeling in Hypoxia: Role of 15-LOX-2/15-HETE-MAPKs Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 35, 2079–2097. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, J.; Zhu, Y.; Feng, W.; Zhai, C.; Liu, L.; Shi, W.; Wang, Q.; Zhang, Q.; Chai, L.; et al. S1P induces pulmonary artery smooth muscle cell proliferation by activating calcineurin/NFAT/OPN signaling pathway. Biochem. Biophys. Res. Commun. 2019, 516, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Gotlieb, A.I. Recent Developments in Vascular Adventitial Pathobiology: The Dynamic Adventitia as a Complex Regulator of Vascular Disease. Am. J. Pathol. 2020, 190, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Nozik-Grayck, E.; Gerasimovskaya, E.; Anwar, A.; Li, M.; Riddle, S.; Frid, M. The adventitia: Essential role in pulmonary vascular remodeling. Compr. Physiol. 2011, 1, 141–161. [Google Scholar] [CrossRef]
- Li, G.; Oparil, S.; Kelpke, S.S.; Chen, Y.F.; Thompson, J.A. Fibroblast growth factor receptor-1 signaling induces osteopontin expression and vascular smooth muscle cell-dependent adventitial fibroblast migration in vitro. Circulation 2002, 106, 854–859. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.F.; Kelpke, S.S.; Oparil, S.; Thompson, J.A. Estrogen attenuates integrin-beta(3)-dependent adventitial fibroblast migration after inhibition of osteopontin production in vascular smooth muscle cells. Circulation 2000, 101, 2949–2955. [Google Scholar] [CrossRef]
- Gao, X.; Jia, G.; Guttman, A.; DePianto, D.J.; Morshead, K.B.; Sun, K.H.; Ramamoorthi, N.; Vander Heiden, J.A.; Modrusan, Z.; Wolters, P.J.; et al. Osteopontin Links Myeloid Activation and Disease Progression in Systemic Sclerosis. Cell Rep. Med. 2020, 1, 100140. [Google Scholar] [CrossRef]
- Scatena, M.; Liaw, L.; Giachelli, C.M. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2302–2309. [Google Scholar] [CrossRef]
- Bruemmer, D.; Collins, A.R.; Noh, G.; Wang, W.; Territo, M.; Arias-Magallona, S.; Fishbein, M.C.; Blaschke, F.; Kintscher, U.; Graf, K.; et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J. Clin. Investig. 2003, 112, 1318–1331. [Google Scholar] [CrossRef]
- Nyström, T.; Dunér, P.; Hultgårdh-Nilsson, A. A constitutive endogenous osteopontin production is important for macrophage function and differentiation. Exp. Cell Res. 2007, 313, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.A.; Wilson, C.L.; Raines, E.W.; Tang, J.; Giachelli, C.M.; Scatena, M. Osteopontin mediates macrophage chemotaxis via α4 and α9 integrins and survival via the α4 integrin. J. Cell. Biochem. 2013, 114, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.F.; Seshadri, V.; Huang, K.; Shao, J.S.; Cai, J.; Vattikuti, R.; Schumacher, A.; Loewy, A.P.; Denhardt, D.T.; Rittling, S.R.; et al. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ. Res. 2006, 98, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.L.; Frid, M.G.; Kunrath, C.L.; Karoor, V.; Anwar, A.; Wagner, B.D.; Strassheim, D.; Stenmark, K.R. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L238–L250. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Agrawal, V.; Lawrie, A.; Bonnet, S. The Latest in Animal Models of Pulmonary Hypertension and Right Ventricular Failure. Circ. Res. 2022, 130, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Mamazhakypov, A.; Sommer, N.; Assmus, B.; Tello, K.; Schermuly, R.T.; Kosanovic, D.; Sarybaev, A.S.; Weissmann, N.; Pak, O. Novel Therapeutic Targets for the Treatment of Right Ventricular Remodeling: Insights from the Pulmonary Artery Banding Model. Int. J. Environ. Res. Public. Health 2021, 18, 8297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Feng, J.A.; Li, P.; Xing, D.; Ambalavanan, N.; Oparil, S. Atrial natriuretic peptide-dependent modulation of hypoxia-induced pulmonary vascular remodeling. Life Sci. 2006, 79, 1357–1365. [Google Scholar] [CrossRef]
- Chen, Y.F.; Feng, J.A.; Li, P.; Xing, D.; Zhang, Y.; Serra, R.; Ambalavanan, N.; Majid-Hassan, E.; Oparil, S. Dominant negative mutation of the TGF-beta receptor blocks hypoxia-induced pulmonary vascular remodeling. J. Appl. Physiol. 2006, 100, 564–571. [Google Scholar] [CrossRef]
- Behringer, A.; Trappiel, M.; Berghausen, E.M.; Ten Freyhaus, H.; Wellnhofer, E.; Odenthal, M.; Blaschke, F.; Er, F.; Gassanov, N.; Rosenkranz, S.; et al. Pioglitazone alleviates cardiac and vascular remodelling and improves survival in monocrotaline induced pulmonary arterial hypertension. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 369–379. [Google Scholar] [CrossRef]
- Wang, Y.; Han, D.D.; Wang, H.M.; Liu, M.; Zhang, X.H.; Wang, H.L. Downregulation of osteopontin is associated with fluoxetine amelioration of monocrotaline-induced pulmonary inflammation and vascular remodelling. Clin. Exp. Pharmacol. Physiol. 2011, 38, 365–372. [Google Scholar] [CrossRef]
- Peng, X.; Li, H.X.; Shao, H.J.; Li, G.W.; Sun, J.; Xi, Y.H.; Li, H.Z.; Wang, X.Y.; Wang, L.N.; Bai, S.Z.; et al. Involvement of calcium-sensing receptors in hypoxia-induced vascular remodeling and pulmonary hypertension by promoting phenotypic modulation of small pulmonary arteries. Mol. Cell. Biochem. 2014, 396, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Mamazhakypov, A.; Sartmyrzaeva, M.; Sarybaev, A.S.; Schermuly, R.; Sydykov, A. Clinical and Molecular Implications of Osteopontin in Heart Failure. Curr. Issues Mol. Biol. 2022, 44, 3573–3597. [Google Scholar] [CrossRef] [PubMed]
- Nadadur, R.D.; Umar, S.; Wong, G.; Eghbali, M.; Iorga, A.; Matori, H.; Partow-Navid, R.; Eghbali, M. Reverse right ventricular structural and extracellular matrix remodeling by estrogen in severe pulmonary hypertension. J. Appl. Physiol. 2012, 113, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Klusonova, P.; Rehakova, L.; Borchert, G.; Vagnerova, K.; Neckar, J.; Ergang, P.; Miksik, I.; Kolar, F.; Pacha, J. Chronic intermittent hypoxia induces 11beta-hydroxysteroid dehydrogenase in rat heart. Endocrinology 2009, 150, 4270–4277. [Google Scholar] [CrossRef]
- Park, J.F.; Clark, V.R.; Banerjee, S.; Hong, J.; Razee, A.; Williams, T.; Fishbein, G.; Saddic, L.; Umar, S. Transcriptomic Analysis of Right Ventricular Remodeling in Two Rat Models of Pulmonary Hypertension: Identification and Validation of Epithelial-to-Mesenchymal Transition in Human Right Ventricular Failure. Circulation. Heart Fail. 2021, 14, e007058. [Google Scholar] [CrossRef]
- Bandopadhyay, M.; Bulbule, A.; Butti, R.; Chakraborty, G.; Ghorpade, P.; Ghosh, P.; Gorain, M.; Kale, S.; Kumar, D.; Kumar, S.; et al. Osteopontin as a therapeutic target for cancer. Expert. Opin. Ther. Targets 2014, 18, 883–895. [Google Scholar] [CrossRef]
- Hunter, C.; Bond, J.; Kuo, P.C.; Selim, M.A.; Levinson, H. The role of osteopontin and osteopontin aptamer (OPN-R3) in fibroblast activity. J. Surg. Res. 2012, 176, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yousefi, K.; Ding, W.; Singh, J.; Shehadeh, L.A. Osteopontin RNA aptamer can prevent and reverse pressure overload-induced heart failure. Cardiovasc. Res. 2017, 113, 633–643. [Google Scholar] [CrossRef]
- Dai, J.; Matsui, T.; Abel, E.D.; Dedhar, S.; Gerszten, R.E.; Seidman, C.E.; Seidman, J.G.; Rosenzweig, A. Deep sequence analysis of gene expression identifies osteopontin as a downstream effector of integrin-linked kinase (ILK) in cardiac-specific ILK knockout mice. Circulation. Heart Fail. 2014, 7, 184–193. [Google Scholar] [CrossRef]
- Dahiya, S.; Givvimani, S.; Bhatnagar, S.; Qipshidze, N.; Tyagi, S.C.; Kumar, A. Osteopontin-stimulated expression of matrix metalloproteinase-9 causes cardiomyopathy in the mdx model of Duchenne muscular dystrophy. J. Immunol. 2011, 187, 2723–2731. [Google Scholar] [CrossRef]

 —indicates that animal models with the corresponding osteopontin manipulations are available; NA—studies with the corresponding animal models and osteopontin manipulations are not available. (B) No experimental studies have evaluated effects of recombinant osteopontin application or osteopontin-neutralizing antibodies. In order to elucidate the cell specific roles of osteopontin, the Cre/LoxP system can be utilized using cell-specific promoter systems. Further, to better characterize the role of osteopontin in such models it is recommended to use invasive catheterization (C) to measure right atrial (RA) pressure, right ventricular (RV) systolic pressure (RVSP), RV diastolic pressure (RVDP), aortic pressure, left ventricular (LV) systolic pressure (LVSP) and LV diastolic pressure (LVDP). Echocardiographic imaging (D) of the heart is also warranted to inform additional characteristics of the RV including both systolic and diastolic functions, including the following parameters: the ratio of pulmonary artery acceleration time to pulmonary artery ejection time (PAAT/PAET), tricuspid annulus systolic excursion (TAPSE), RV annulus systolic velocity (RV-S´), RV internal diameter (RVID), RV wall thickness (RVWT), stroke volume (SV), cardiac output (CO), LV eccentricity index (LVEI), tricuspid valve inflow velocities (TV E/A), and tricuspid annulus lateral velocities (TV E´/A´). Following the terminal catheterization and echocardiography assessments, lung and heart tissues (E) can be evaluated ex vivo for pulmonary artery (PA) wall thickness, muscularization and inflammation, as well as lung capillary density. RV tissue can be assessed for RV fibrosis, cardiomyocyte hypertrophy, inflammation, and angiogenesis. Furthermore, lung and RV tissues can be studied for the expression of genes and proteins involved in various pathological processes including inflammation, extracellular matrix (ECM) synthesis and endothelial-to-mesenchymal transition (EndMT). Finally, the exact cellular roles of osteopontin can be studied in vitro (F) using cell culture techniques under both osteopontin loss- and gain-of-function conditions to assess cell proliferation, migration, and apoptosis. Employing such strategies in rodent pulmonary hypertension models, and ex vivo tissue and in vitro cell culture experiments may be necessary to fully characterize the role of osteopontin in pulmonary hypertension.
—indicates that animal models with the corresponding osteopontin manipulations are available; NA—studies with the corresponding animal models and osteopontin manipulations are not available. (B) No experimental studies have evaluated effects of recombinant osteopontin application or osteopontin-neutralizing antibodies. In order to elucidate the cell specific roles of osteopontin, the Cre/LoxP system can be utilized using cell-specific promoter systems. Further, to better characterize the role of osteopontin in such models it is recommended to use invasive catheterization (C) to measure right atrial (RA) pressure, right ventricular (RV) systolic pressure (RVSP), RV diastolic pressure (RVDP), aortic pressure, left ventricular (LV) systolic pressure (LVSP) and LV diastolic pressure (LVDP). Echocardiographic imaging (D) of the heart is also warranted to inform additional characteristics of the RV including both systolic and diastolic functions, including the following parameters: the ratio of pulmonary artery acceleration time to pulmonary artery ejection time (PAAT/PAET), tricuspid annulus systolic excursion (TAPSE), RV annulus systolic velocity (RV-S´), RV internal diameter (RVID), RV wall thickness (RVWT), stroke volume (SV), cardiac output (CO), LV eccentricity index (LVEI), tricuspid valve inflow velocities (TV E/A), and tricuspid annulus lateral velocities (TV E´/A´). Following the terminal catheterization and echocardiography assessments, lung and heart tissues (E) can be evaluated ex vivo for pulmonary artery (PA) wall thickness, muscularization and inflammation, as well as lung capillary density. RV tissue can be assessed for RV fibrosis, cardiomyocyte hypertrophy, inflammation, and angiogenesis. Furthermore, lung and RV tissues can be studied for the expression of genes and proteins involved in various pathological processes including inflammation, extracellular matrix (ECM) synthesis and endothelial-to-mesenchymal transition (EndMT). Finally, the exact cellular roles of osteopontin can be studied in vitro (F) using cell culture techniques under both osteopontin loss- and gain-of-function conditions to assess cell proliferation, migration, and apoptosis. Employing such strategies in rodent pulmonary hypertension models, and ex vivo tissue and in vitro cell culture experiments may be necessary to fully characterize the role of osteopontin in pulmonary hypertension.
 —indicates that animal models with the corresponding osteopontin manipulations are available; NA—studies with the corresponding animal models and osteopontin manipulations are not available. (B) No experimental studies have evaluated effects of recombinant osteopontin application or osteopontin-neutralizing antibodies. In order to elucidate the cell specific roles of osteopontin, the Cre/LoxP system can be utilized using cell-specific promoter systems. Further, to better characterize the role of osteopontin in such models it is recommended to use invasive catheterization (C) to measure right atrial (RA) pressure, right ventricular (RV) systolic pressure (RVSP), RV diastolic pressure (RVDP), aortic pressure, left ventricular (LV) systolic pressure (LVSP) and LV diastolic pressure (LVDP). Echocardiographic imaging (D) of the heart is also warranted to inform additional characteristics of the RV including both systolic and diastolic functions, including the following parameters: the ratio of pulmonary artery acceleration time to pulmonary artery ejection time (PAAT/PAET), tricuspid annulus systolic excursion (TAPSE), RV annulus systolic velocity (RV-S´), RV internal diameter (RVID), RV wall thickness (RVWT), stroke volume (SV), cardiac output (CO), LV eccentricity index (LVEI), tricuspid valve inflow velocities (TV E/A), and tricuspid annulus lateral velocities (TV E´/A´). Following the terminal catheterization and echocardiography assessments, lung and heart tissues (E) can be evaluated ex vivo for pulmonary artery (PA) wall thickness, muscularization and inflammation, as well as lung capillary density. RV tissue can be assessed for RV fibrosis, cardiomyocyte hypertrophy, inflammation, and angiogenesis. Furthermore, lung and RV tissues can be studied for the expression of genes and proteins involved in various pathological processes including inflammation, extracellular matrix (ECM) synthesis and endothelial-to-mesenchymal transition (EndMT). Finally, the exact cellular roles of osteopontin can be studied in vitro (F) using cell culture techniques under both osteopontin loss- and gain-of-function conditions to assess cell proliferation, migration, and apoptosis. Employing such strategies in rodent pulmonary hypertension models, and ex vivo tissue and in vitro cell culture experiments may be necessary to fully characterize the role of osteopontin in pulmonary hypertension.
—indicates that animal models with the corresponding osteopontin manipulations are available; NA—studies with the corresponding animal models and osteopontin manipulations are not available. (B) No experimental studies have evaluated effects of recombinant osteopontin application or osteopontin-neutralizing antibodies. In order to elucidate the cell specific roles of osteopontin, the Cre/LoxP system can be utilized using cell-specific promoter systems. Further, to better characterize the role of osteopontin in such models it is recommended to use invasive catheterization (C) to measure right atrial (RA) pressure, right ventricular (RV) systolic pressure (RVSP), RV diastolic pressure (RVDP), aortic pressure, left ventricular (LV) systolic pressure (LVSP) and LV diastolic pressure (LVDP). Echocardiographic imaging (D) of the heart is also warranted to inform additional characteristics of the RV including both systolic and diastolic functions, including the following parameters: the ratio of pulmonary artery acceleration time to pulmonary artery ejection time (PAAT/PAET), tricuspid annulus systolic excursion (TAPSE), RV annulus systolic velocity (RV-S´), RV internal diameter (RVID), RV wall thickness (RVWT), stroke volume (SV), cardiac output (CO), LV eccentricity index (LVEI), tricuspid valve inflow velocities (TV E/A), and tricuspid annulus lateral velocities (TV E´/A´). Following the terminal catheterization and echocardiography assessments, lung and heart tissues (E) can be evaluated ex vivo for pulmonary artery (PA) wall thickness, muscularization and inflammation, as well as lung capillary density. RV tissue can be assessed for RV fibrosis, cardiomyocyte hypertrophy, inflammation, and angiogenesis. Furthermore, lung and RV tissues can be studied for the expression of genes and proteins involved in various pathological processes including inflammation, extracellular matrix (ECM) synthesis and endothelial-to-mesenchymal transition (EndMT). Finally, the exact cellular roles of osteopontin can be studied in vitro (F) using cell culture techniques under both osteopontin loss- and gain-of-function conditions to assess cell proliferation, migration, and apoptosis. Employing such strategies in rodent pulmonary hypertension models, and ex vivo tissue and in vitro cell culture experiments may be necessary to fully characterize the role of osteopontin in pulmonary hypertension.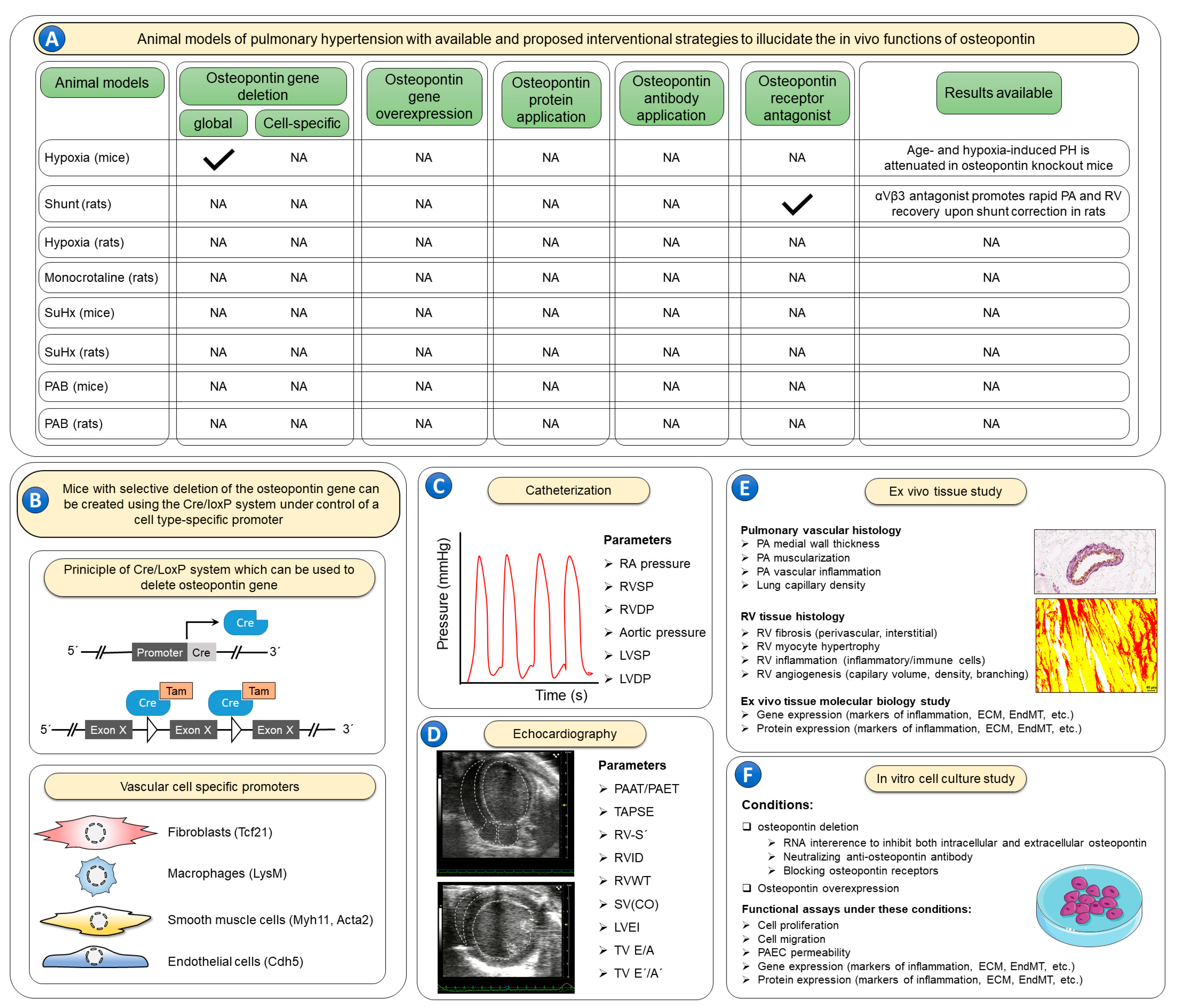
| Subjects | Main Findings | Studies |
|---|---|---|
| IPAH (n = 35) | Circulating osteopontin levels correlated with WHO-FC and 6MWD. A cut-off of osteopontin (53.4 ng/mL) predicted significant differences in survival at 4.0 ± 2.2-year follow-up. | [53] |
| IPAH (n = 95) (retrospective cohort (n = 70), prospective cohort (n = 25), control (n = 40) | In both retrospective and prospective cohorts, circulating osteopontin levels correlated with mPAP and NT-BNP. In the retrospective cohort, osteopontin levels also correlated with age, 6MWD, and NYHA class. Multivariate Cox analysis demonstrated that baseline osteopontin levels were independent predictors of mortality. | [51] |
| PH (n = 71), control (n = 40) | Patients with advanced right heart failure revealed higher levels of circulating osteopontin compared to less symptomatic ones (NYHA III-IV vs. NYHA I-II). Osteopontin was a strong independent predictor of all-cause mortality within 24 months of follow-up. | [52] |
| PH (PAH + CTEPH) (n = 71) | Circulating osteopontin levels correlated with RVEDD, TAPSE, and RV-S´. Patients with RV dysfunction had higher levels of osteopontin compared to those without RV dysfunction (956 ng/mL vs. 628 ng/mL). ROC analysis revealed that an osteopontin concentration of 694.2 ng⁄mL detects RV dilatation. | [54] |
| PH (PAH + CTEPH) (n = 62), control (n = 12) | Circulating osteopontin levels in PH patients were elevated compared to those in healthy control subjects. Circulating osteopontin levels predicted decreased 6MWD. Osteopontin levels were associated with NT-proBNP, RVEDD (echo), RVD (MRI), and PA distensibility index. | [50] |
| CAD-COPD (n = 131) | Circulating osteopontin levels correlated with mPAP and 6MWD. Osteopontin levels > 43 ng/mL were a statistically significant predictor of PH in patients with CAD-COPD. | [44] |
| CHD (n = 22), CHD-PAH (n = 25), control (n = 24) | Circulating osteopontin levels increase with the development of PAH and Eisenmenger syndrome. Circulating osteopontin levels correlated with mPAP, CI, and TPVR. | [42] |
| DCM (n = 70) (DCM without RVD (n = 15) and with RVD (n = 55)) | Circulating osteopontin levels in DCM patients correlated with RVD1, RVD2, and sPAP. | [49] |
| PH (n = 62), DCM (n = 34), LVH (LVH; n = 47), control (n = 38) | Circulating osteopontin levels were higher in PH, DCM, and LVH patients compared to those in the controls. Osteopontin concentrations in PH patients with maladaptive RV were significantly higher than in those with adaptive RV of CTEPH origin. In PH patients, osteopontin levels were correlated with TAPSE/sPAP, RVEDD, mPAP, PVR, NT-pro-BNP, NYHA-FC. | [47] |
| CTD (n = 113) | CTD-PAH patients showed significantly higher circulating osteopontin levels than patients with CTD alone. Osteopontin levels were independently associated with PAH diagnosis. | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamazhakypov, A.; Maripov, A.; Sarybaev, A.S.; Schermuly, R.T.; Sydykov, A. Osteopontin in Pulmonary Hypertension. Biomedicines 2023, 11, 1385. https://doi.org/10.3390/biomedicines11051385
Mamazhakypov A, Maripov A, Sarybaev AS, Schermuly RT, Sydykov A. Osteopontin in Pulmonary Hypertension. Biomedicines. 2023; 11(5):1385. https://doi.org/10.3390/biomedicines11051385
Chicago/Turabian StyleMamazhakypov, Argen, Abdirashit Maripov, Akpay S. Sarybaev, Ralph Theo Schermuly, and Akylbek Sydykov. 2023. "Osteopontin in Pulmonary Hypertension" Biomedicines 11, no. 5: 1385. https://doi.org/10.3390/biomedicines11051385
APA StyleMamazhakypov, A., Maripov, A., Sarybaev, A. S., Schermuly, R. T., & Sydykov, A. (2023). Osteopontin in Pulmonary Hypertension. Biomedicines, 11(5), 1385. https://doi.org/10.3390/biomedicines11051385







