ROS Production by a Single Neutrophil Cell and Neutrophil Population upon Bacterial Stimulation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umeda, T.; Takahashi, I.; Danjo, K.; Matsuzaka, M.; Nakaji, S. Changes in neutrophil immune functions under different exercise stresses. Nihon Eiseigaku Zasshi. 2011, 66, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Nexar-Qh, J.; Caleiro Seixas, A.E. Adrenergic and cholinergic influence on the production of reactive oxygen species in human neutrophils. Rev. Peru. Med. Exp. Salud Publica 2019, 36, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Van Gemmeren, T.; Schuppner, R.; Grosse, G.M.; Fering, J.; Gabriel, M.M.; Huber, R.; Worthmann, H.; Lichtinghagen, R.; Weissenborn, K. Early Post-Stroke Infections Are Associated with an Impaired Function of Neutrophil Granulocytes. J. Clin. Med. 2020, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.C.; Anderson, M.E.; Edwards, S.W.; Moots, R.J. Neutrophil-derived reactive oxygen species in SSc. Rheumatology 2012, 51, 1166–1169. [Google Scholar] [CrossRef]
- Domerecka, W.; Homa-Mlak, I.; Mlak, R.; Michalak, A.; Wilińska, A.; Kowalska-Kępczyńska, A.; Dreher, P.; Cichoż-Lach, H.; Małecka-Massalska, T. Indicator of Inflammation and NETosis—Low-Density Granulocytes as a Biomarker of Autoimmune Hepatitis. J. Clin. Med. 2022, 11, 2174. [Google Scholar] [CrossRef]
- Christoffersson, G.; Phillipson, M. The neutrophil: One cell on many missions or many cells with different agendas? Cell Tissue Res. 2018, 371, 415–423. [Google Scholar] [CrossRef]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Cerutti, A.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011, 13, 170–180. [Google Scholar] [CrossRef]
- Deniset, J.F.; Surewaard, B.G.; Lee, W.Y.; Kubes, P. Splenic Ly6G high mature and Ly6Gint immature neutrophils contribute to eradication of S. pneumoniae. J. Exp. Med. 2017, 214, 1333–1350. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: «BN1» versus «BN2» TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Abakumova, T.V.; Gening, T.P.; Dolgova, D.R.; Antoneeva, I.I.; Peskov, A.B.; Gening, S.O. Phenotype of circulating neutrophils at different stages of cervical neoplasia. Med. Immunol. 2020, 21, 1127–1138. [Google Scholar] [CrossRef]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 2016, 110, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Christoffersson, G.; Vagesjo, E.; Vandooren, J.; Liden, M.; Massena, S.; Reinert, R.B.; Brissova, M.; Powers, A.C.; Opdenakker, G.; Phillipson, M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood 2012, 120, 4653–4662. [Google Scholar] [CrossRef]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Engblom, C.; Pfirschke, C.; Zilionis, R.; Da Silva Martins, J.; Bos, S.A.; Courties, G.; Faget, J.; Zemmour, D.; Klein, A.; Pittet, M.J.; et al. Osteoblasts remotely supply lung tumors with cancer-promoting Sigle cF high neutrophils. Science 2017, 358, eaal5081. [Google Scholar] [CrossRef] [PubMed]
- Massena, S.; Christoffersson, G.; Vagesjo, E.; Seignez, C.; Gustafsson, K.; Binet, F.; Herrera Hidalgo, C.; Giraud, A.; Lomei, J.; Westrom, S.; et al. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood 2015, 126, 2016–2026. [Google Scholar] [CrossRef]
- Teuben, M.; Heeres, M.; Blokhuis, T.; Hollman, A.; Vrisekoop, N.; Tan, E.; Pfeifer, R.; Pape, H.C.; Koenderman, L.; Leenen, L.P.N. Instant intra-operative neutropenia despite the emergence of banded (CD16 dim/CD62L bright) neutrophils in peripheral blood—An observational study during extensive trauma-surgery in pigs. Injury 2020, 52, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Saprykin, V.P.; Kuznetsov, S.L. Morphological variants of neutrophilic granulocytes in the blood of practically normal humans. Morphologiia 2001, 120, 37–41. [Google Scholar]
- Pleskova, S.N.; Balalaeva, I.V.; Gushchina, I.I.; Seregeeva, E.A.; Zdobnova, T.A.; Deev, S.M.; Turchin, I.V. Differences in the functional activity of human neutrophilic granulocytes in their interactions with semiconductor quantum dots. Morphologiia 2009, 135, 47–49. [Google Scholar]
- Tsuda, Y.; Takahashi, H.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 2004, 21, 215–226. [Google Scholar] [CrossRef]
- Gerasimov, I.G.; Ignatov, D. Functional heterogenicity of human blood neutrophils: Generation of oxygen active species. Tsitologiia 2001, 43, 432–436. [Google Scholar]
- Valenta, H.; Erard, M.; Dupré-Crochet, S.; Nüβe, O. The NADPH Oxidase and the Phagosome. Adv. Exp. Med. Biol. 2020, 1246, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of soft matter nanomechanics by atomic force microscopy and optical tweezers: A comprehensive review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, H.F.; Cartagena-Rivera, A.X.; Raman, A.; Ávila, A. Evaluation of the elastic Young’s modulus and cytotoxicity variations in fibroblasts exposed to car-bon-based nanomaterials. J. Nanobiotechnol. 2019, 17, 32. [Google Scholar] [CrossRef]
- Lachgar, A.; Sojic, N.; Arbault, S.; Bruce, D.; Sarasin, A.; Amatore, C.; Bizzini, B.; Zagury, D.; Vuillaume, M. Amplification of the inflammatory cellular redox state by human immunodeficiency virus type 1-immunosuppressive tat and gp160 proteins. J. Virol. 1999, 73, 1447–1452. [Google Scholar] [CrossRef]
- Arbault, S.; Pantano, P.; Sojic, N.; Amatore, C.; Best-Belpomme, M.; Sarasin, A.; Vuillaume, M. Activation of the NADPH oxidase in human fibroblasts by mechanical intrusion of a single cell with an ultramicroelectrode. Carcinogenesis 1997, 18, 569–574. [Google Scholar] [CrossRef]
- Arbault, S.; Sojic, N.; Bruce, D.; Amatore, C.; Sarasin, A.; Vuillaume, M. Oxidative stress in cancer prone xeroderma pigmentosumfibroblasts. Real-time and single cell monitoring of superoxide and nitric oxide production with microelectrodes. Carcinogenesis 2004, 25, 509–515. [Google Scholar] [CrossRef]
- Amatore, C.; Arbault, S.; Bouton, C.; Drapier, J.-C.; Ghandour, H.; Koh, A.C.W. Real-time amperometric analysis of reactive oxygen and nitrogen species released by single immunostimulated macrophages. Chembiochem 2008, 9, 1472–1480. [Google Scholar] [CrossRef]
- Hu, K.; Li, Y.; Rotenberg, S.A.; Amatore, C.; Mirkin, M.V. Electrochemical measurements of reactive oxygen and nitrogen species inside single phagolysosomes of living macrophages. J. Am. Chem. Soc. 2019, 141, 4564–4568. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Oleinick, A.; Jiang, H.; Liao, Q.-L.; Qiu, Q.-F.; Svir, I.; Liu, Y.-L.; Amatore, C.; Huang, W.-H. lectrochemical monitoring of ROS/RNS homeostasis within individual phagolysosomes inside single macrophages. Angew. Chem. Int. Ed. Engl. 2019, 58, 7753–7756. [Google Scholar] [CrossRef]
- Vaneev, A.; Gorelkin, P.; Garanina, A.S.; Lopatukhina, H.V.; Vodopyanov, S.S.; Alova, A.V.; Raybaya, O.; Akasov, R.A.; Zhang, Y.; Erofeev, A.S.; et al. In vitro and in vivo electrochemical measurement of reactive oxygen species after treatment with anticancer drugs. Anal. Chem. 2020, 92, 8010–8014. [Google Scholar] [CrossRef] [PubMed]
- Obraztsov, I.V.; Godkov, M.A.; Polimova, A.M.; Demin, E.M.; Proskurnina, E.V.; Vladimirov, Y.A. Evaluation of the func-tional activity of whole blood neutrophils by a two stage stimulation: A new approach to the chemiluminescent analysis. Russ. J. Immunol. 2015, 9, 418–425. [Google Scholar]
- Pleskova, S.N.; Kriukov, R.N.; Razumkova, E.V.; Zubkov, S.Y.; Abarbanel, N.A. Features of phagocytosis of opsonized and nonopsonized bacteria Staphylococcus aureus and Escherichia coli by human neutrophil granulocytes, studied by atomic force microscopy. Cell Tissue Biol. 2018, 60, 623–631. [Google Scholar] [CrossRef]
- Dahlgren, C.; Karlsson, A.; Bylund, J. Intracellular neutrophil oxidants: From laboratory curiosity to clinical reality. J. Immunol. 2019, 202, 3127–3134. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox. Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Dahlgren, C.; Gabl, M.; Holdfeldt, A.; Winther, M.; Forsman, H. Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria. Biochem. Pharmacol. 2016, 114, 22–39. [Google Scholar] [CrossRef]
- Bylund, J.; Brown, K.L.; Movitz, C.; Dahlgren, C.; Karlsson, A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: How, where, and what for? Free Radic. Biol. Med. 2010, 49, 1834–1845. [Google Scholar] [CrossRef]
- Karlsson, A.; Nixon, J.B.; McPhail, L.C. Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: Dependent or independent of phosphatidylinositol 3-kinase. J. Leukoc. Biol. 2000, 67, 396–404. [Google Scholar] [CrossRef]
- Lundqvist, H.; Foilin, P.; Khalfan, L.; Dahlgren, C. Phorbol myristate acetate-induced NADPH oxidase activity in human neutrophils: Only half the story has been told. J. Leukoc. Biol. 1996, 59, 270–279. [Google Scholar] [CrossRef]
- Gupta, E.; Kumar, S.; Srivastava, V.K.; Saxena, J.; Siddiqui, A.J.; Mehta, S.; Kaushik, S.; Jyoti, A. Unravelling the differential Host immuno-inflammatory responses to Staphylococcus aureus and Escherichia coli infections in sepsis. Vaccines 2022, 10, 1648. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, J.S.; Lee, Y.Y.; Kim, D.Y.; Kang, J.L.; Kim, H.S. Anti-inflammatory and anti-oxidant mechanisms of an MMP-8 inhibitor in lipoteichoic acid-stimulated rat primary astrocytes: Involvement of NF-κB, Nrf2, and PPAR-γ signaling pathways. J. Neuroinflamm. 2018, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Hadley, J.S.; Wang, J.E.; Foster, S.J.; Thiemermann, C.; Hinds, C.J. Peptidoglycan of Staphylococcus aureus upregulates monocyte expression of CD14, toll-like receptor 2 (TLR2), and TLR4 in human blood: Possible implications for riming of lipopolysac-charide signaling. Infect. Immun. 2005, 73, 7613–7619. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Joo, H.-S.; Chatterjee, S.S.; Otto, M. Phenol-soluble modulins—Critical determinants of staphylococcal viru-lence. FEMS Microbiol. Rev. 2014, 38, 698–719. [Google Scholar] [CrossRef] [PubMed]
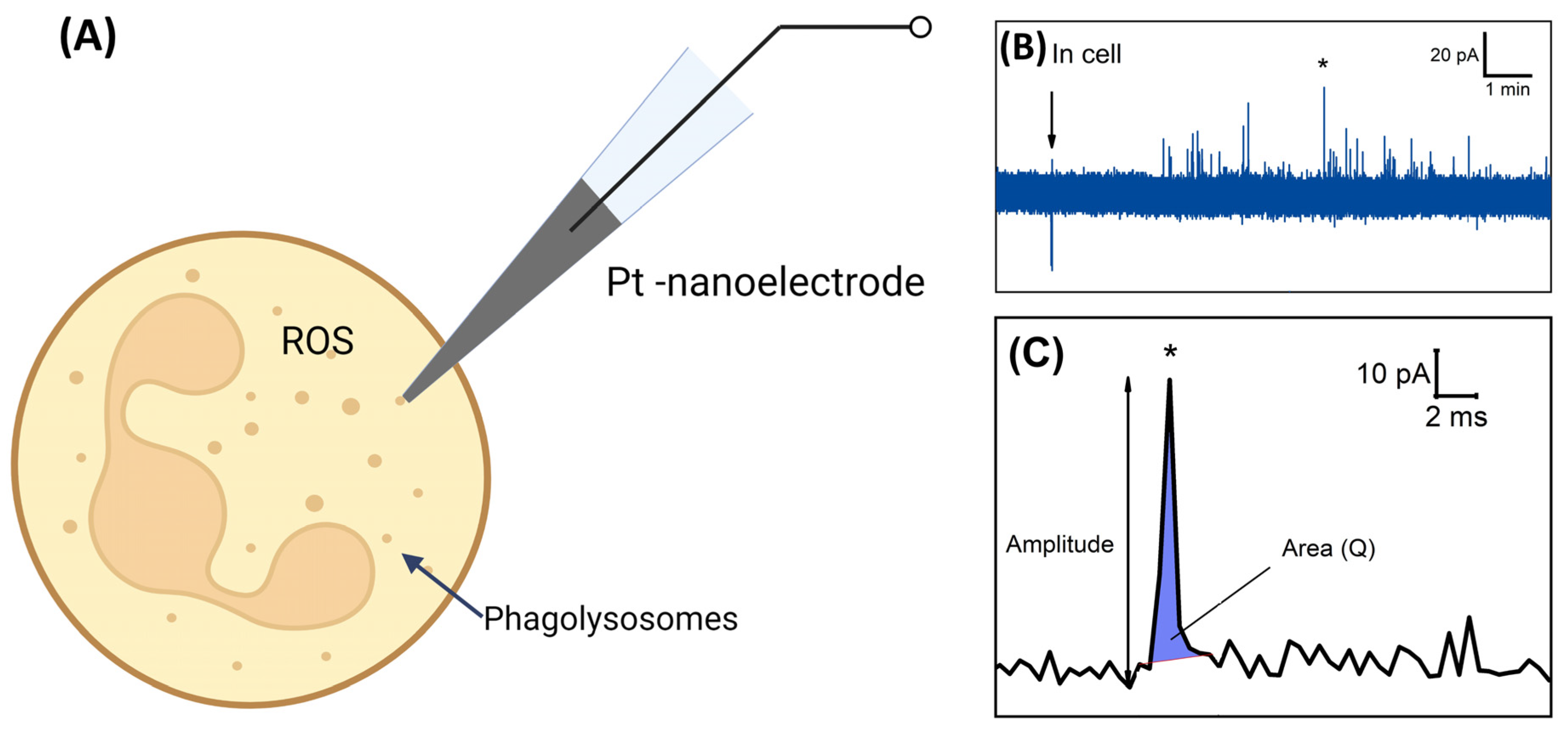
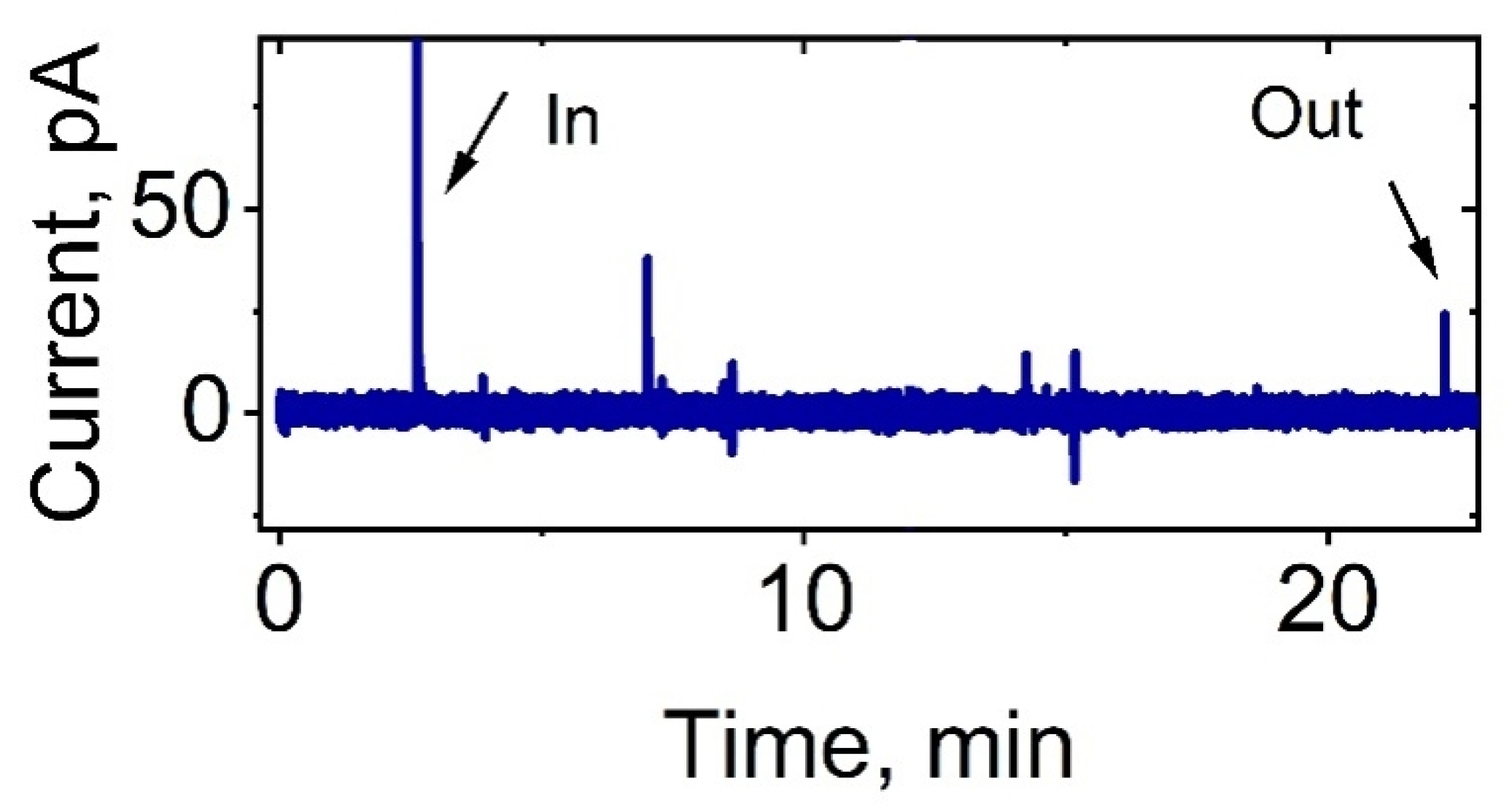


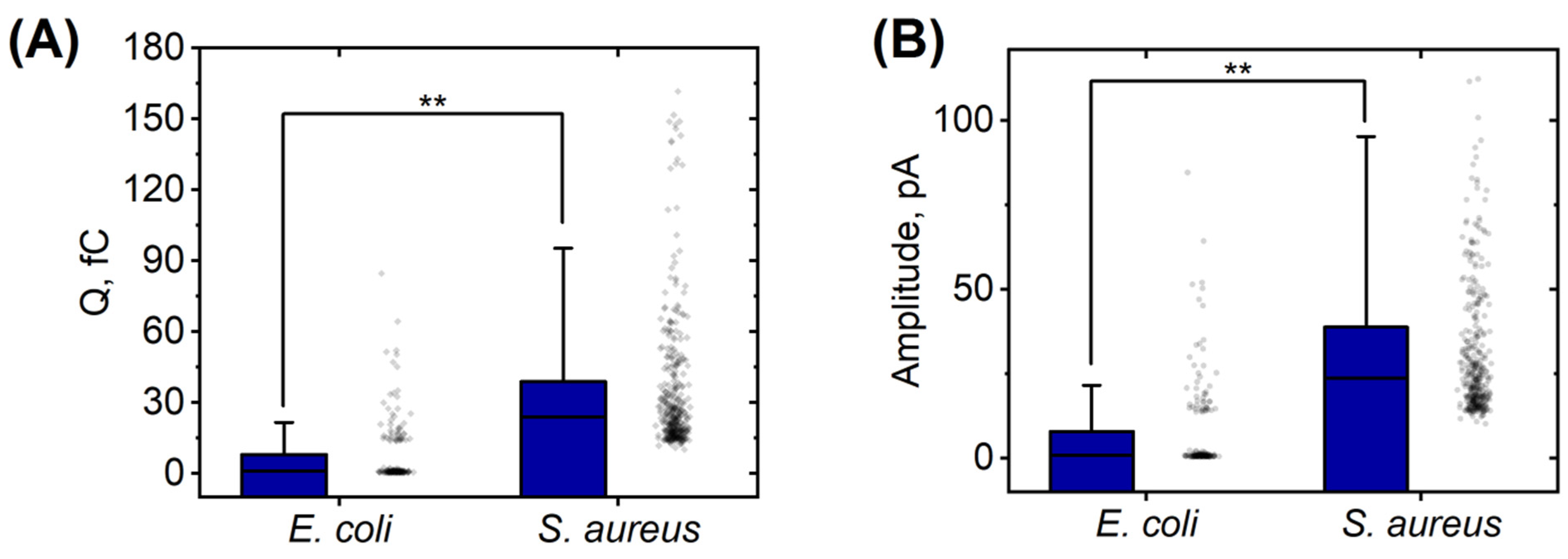
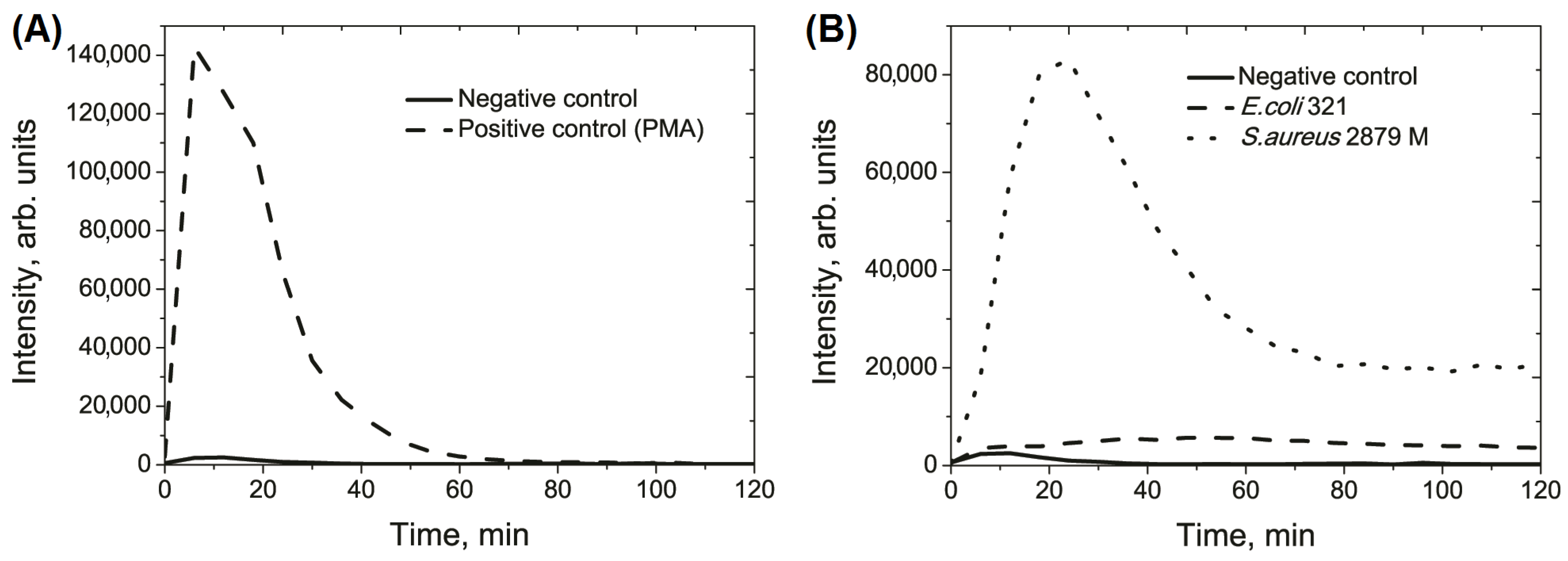
| Strain | Total Cells | Activated Cells | Silent Cells | Average Time of Appearance of Peaks, min | Average Duration of Peaks Appearance, min |
|---|---|---|---|---|---|
| E. coli 321 | 6 | 5 | 1 | 8.7 ± 3.3 | 9.6 ± 3.0 |
| S. aureus 2879 M | 10 | 8 | 2 | 5.4 ± 4.6 | 8.4 ± 4.2 |
| Parameter | Negative Control | E. coli 321 | S. aureus 2879 M |
|---|---|---|---|
| Integral value of the light sum, arb. units, ×105 | 1.5 ± 0.4 | 5.2 ± 1.3 a | 39.7 ± 4.9 a |
| Height of curve peak, arb. units, ×103 | 5.1 ± 1.7 | 5.9 ± 1.3 | 81.1 ± 12.8 a |
| Time of maximal peak, min | 11.5 ± 2.0 | 57.0 ± 9.3 a | 25.5 ± 2.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleskova, S.N.; Erofeev, A.S.; Vaneev, A.N.; Gorelkin, P.V.; Bobyk, S.Z.; Kolmogorov, V.S.; Bezrukov, N.A.; Lazarenko, E.V. ROS Production by a Single Neutrophil Cell and Neutrophil Population upon Bacterial Stimulation. Biomedicines 2023, 11, 1361. https://doi.org/10.3390/biomedicines11051361
Pleskova SN, Erofeev AS, Vaneev AN, Gorelkin PV, Bobyk SZ, Kolmogorov VS, Bezrukov NA, Lazarenko EV. ROS Production by a Single Neutrophil Cell and Neutrophil Population upon Bacterial Stimulation. Biomedicines. 2023; 11(5):1361. https://doi.org/10.3390/biomedicines11051361
Chicago/Turabian StylePleskova, Svetlana N., Alexander S. Erofeev, Alexander N. Vaneev, Petr V. Gorelkin, Sergey Z. Bobyk, Vasilii S. Kolmogorov, Nikolay A. Bezrukov, and Ekaterina V. Lazarenko. 2023. "ROS Production by a Single Neutrophil Cell and Neutrophil Population upon Bacterial Stimulation" Biomedicines 11, no. 5: 1361. https://doi.org/10.3390/biomedicines11051361
APA StylePleskova, S. N., Erofeev, A. S., Vaneev, A. N., Gorelkin, P. V., Bobyk, S. Z., Kolmogorov, V. S., Bezrukov, N. A., & Lazarenko, E. V. (2023). ROS Production by a Single Neutrophil Cell and Neutrophil Population upon Bacterial Stimulation. Biomedicines, 11(5), 1361. https://doi.org/10.3390/biomedicines11051361






