Complexity of Sex Differences and Their Impact on Alzheimer’s Disease
Abstract
1. Introduction
2. Alzheimer’s Disease
3. Biological Sex Differences
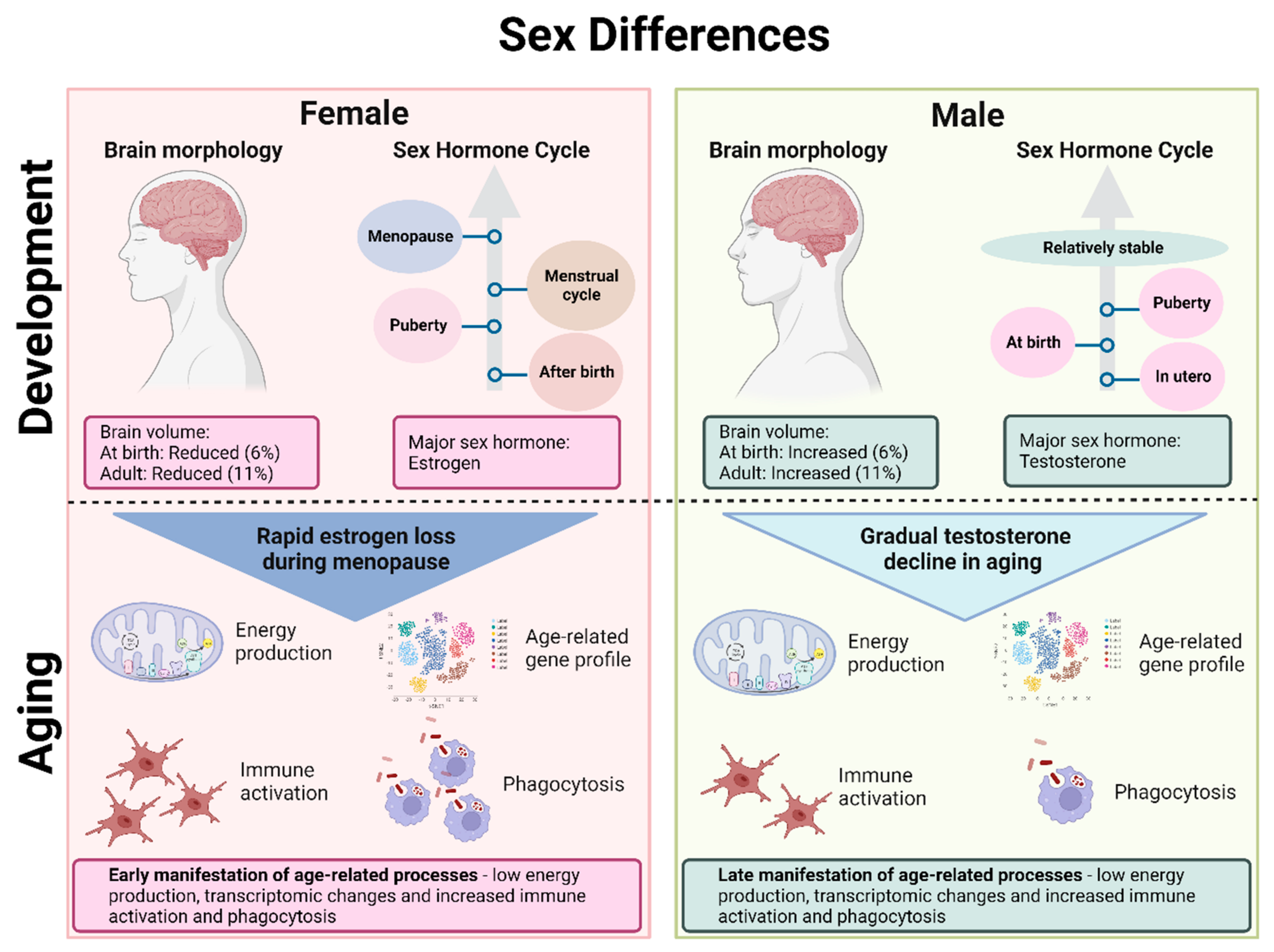
3.1. Brain Morphology
3.2. Sex Hormones
3.3. Aging
3.4. Sex Differences in Alzheimer’s Disease
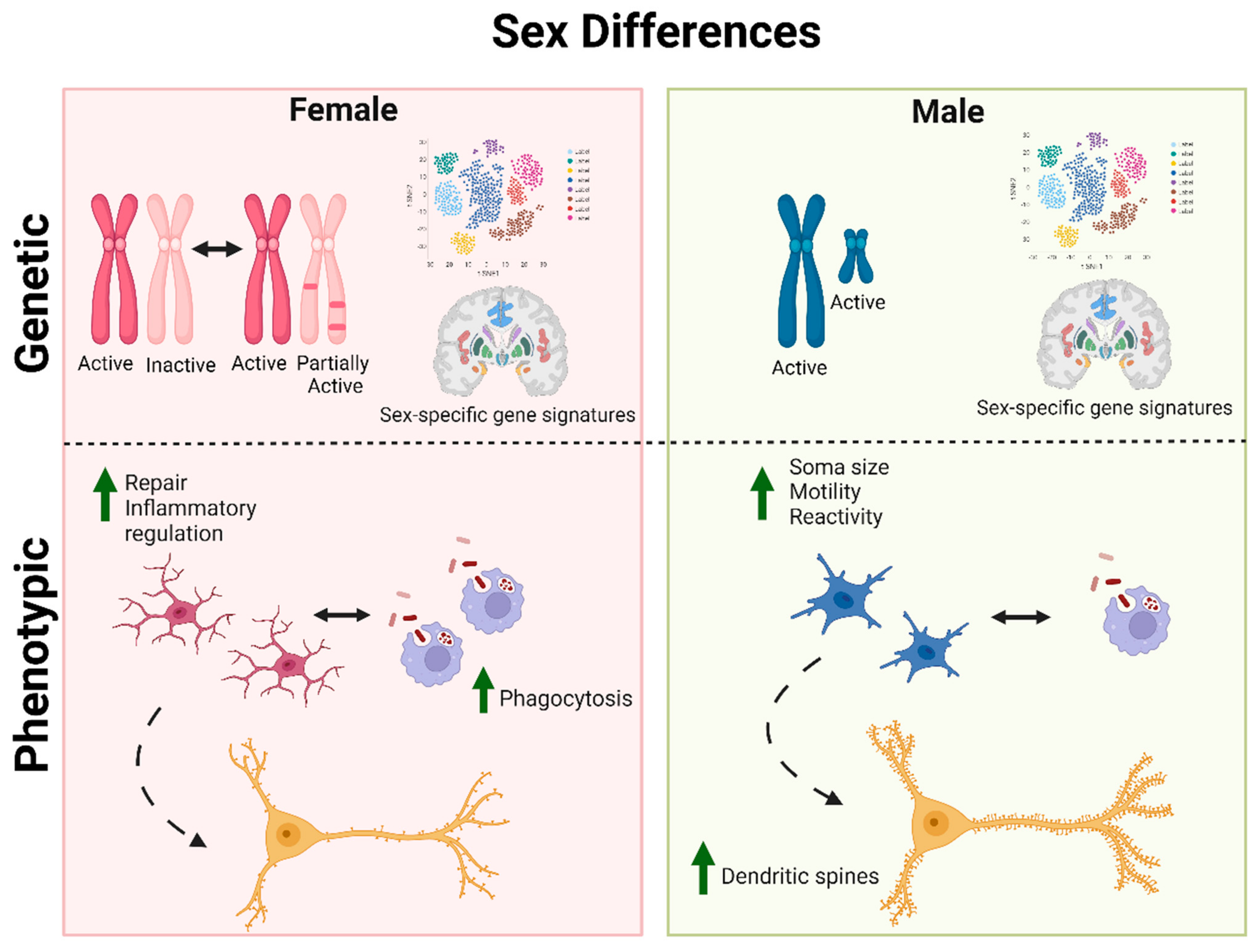
4. Microglia
4.1. Male and Female Microglia
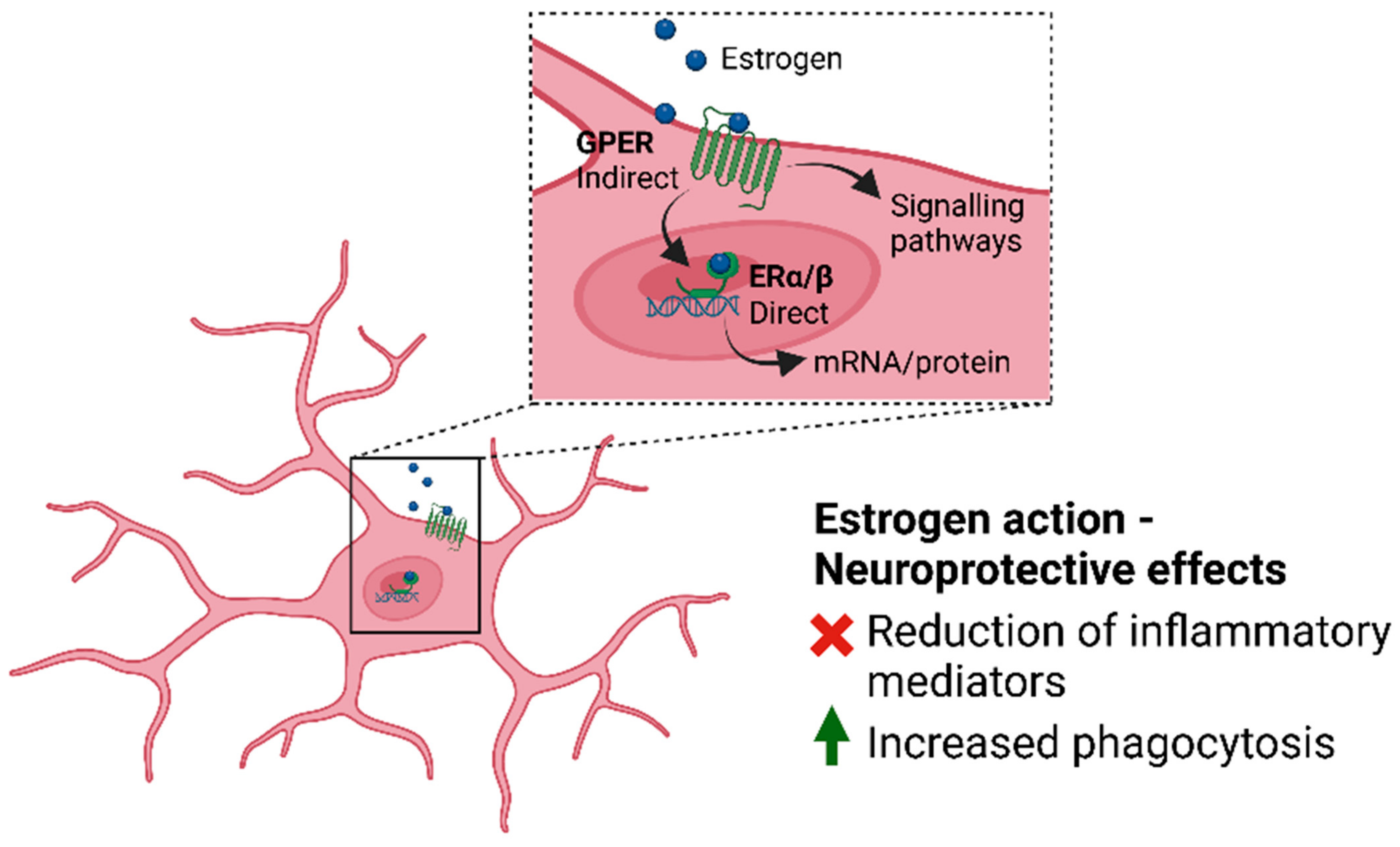
4.2. Microglia and Estrogen
4.3. Microglia, Neuroinflammation and Alzheimer’s Disease
5. Common Model Systems for Studying Alzheimer’s Disease
5.1. Mouse Models
5.2. In Vitro Models
5.2.1. 2D Cellular Models
5.2.2. Cerebral Organoids
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tomaskova, H.; Kuhnova, J.; Cimler, R.; Dolezal, O.; Kuca, K. Prediction of population with Alzheimer’s disease in the European Union using a system dynamics model. Neuropsychiatr. Dis. Treat. 2016, 12, 1589–1598. [Google Scholar] [PubMed]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Henderson, V.W. Alzheimer’s disease: Review of hormone therapy trials and implications for treatment and prevention after menopause. J. Steroid. Biochem. Mol. Biol. 2014, 142, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mills, Z.B.; Faull, R.L.M.; Kwakowsky, A. Is Hormone Replacement Therapy a Risk Factor or a Therapeutic Option for Alzheimer’s Disease? Int. J. Mol. Sci. 2023, 24, 3205. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.R.; Turner, B.E.; Weeks, B.T.; Magnani, C.J.; Wong, B.O.; Rodriguez, F.; Yee, L.M.; Cullen, M.R. Analysis of Female Enrollment and Participant Sex by Burden of Disease in US Clinical Trials Between 2000 and 2020. JAMA Netw. Open 2021, 4, e2113749. [Google Scholar] [CrossRef]
- Baron, S.; Ulstein, I.; Werheid, K. Psychosocial interventions in Alzheimer’s disease and amnestic mild cognitive impairment: Evidence for gender bias in clinical trials. Aging Ment. Health 2015, 19, 290–305. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Will, T.R.; Proaño, S.B.; Thomas, A.M.; Kunz, L.M.; Thompson, K.C.; Ginnari, L.A.; Jones, C.H.; Lucas, S.-C.; Reavis, E.M.; Dorris, D.M.; et al. Problems and Progress regarding Sex Bias and Omission in Neuroscience Research. eNeuro 2017, 4, e0278-17.2017. [Google Scholar] [CrossRef]
- Williams, O.O.F.; Coppolino, M.; Perreault, M.L. Sex differences in neuronal systems function and behaviour: Beyond a single diagnosis in autism spectrum disorders. Transl. Psychiatry 2021, 11, 625. [Google Scholar] [CrossRef]
- Raising the bar on sex and gender reporting in research. Nat. Commun. 2022, 13, 2845. [CrossRef]
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [CrossRef]
- Niikura, T.; Tajima, H.; Kita, Y. Neuronal cell death in Alzheimer’s disease and a neuroprotective factor, humanin. Curr. Neuropharmacol. 2006, 4, 139–147. [Google Scholar] [CrossRef]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef]
- Lumsden, A.L.; Mulugeta, A.; Zhou, A.; Hyppönen, E. Apolipoprotein E (APOE) genotype-associated disease risks: A phenome-wide, registry-based, case-control study utilising the UK Biobank. eBioMedicine 2020, 59, 102954. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Mosconi, L.; Pupi, A.; De Leon, M.J. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2008, 1147, 180–195. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Lee, B.Y.; Hane, F.T. Recent Progress in Alzheimer’s Disease Research, Part 2: Genetics and Epidemiology. J. Alzheimers Dis. 2017, 57, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, M.D.; Hect, J.L.; Hernandez-Andrade, E.; Hassan, S.S.; Romero, R.; Eggebrecht, A.T.; Thomason, M.E. Sex differences in functional connectivity during fetal brain development. Dev. Cogn. Neurosci. 2019, 36, 100632. [Google Scholar] [CrossRef] [PubMed]
- Knickmeyer, R.C.; Wang, J.; Zhu, H.; Geng, X.; Woolson, S.; Hamer, R.M.; Konneker, T.; Styner, M.; Gilmore, J.H. Impact of sex and gonadal steroids on neonatal brain structure. Cereb. Cortex 2014, 24, 2721–2731. [Google Scholar] [CrossRef]
- Chen, X.; Sachdev, P.S.; Wen, W.; Anstey, K.J. Sex differences in regional gray matter in healthy individuals aged 44–48 years: A voxel-based morphometric study. Neuroimage 2007, 36, 691–699. [Google Scholar] [CrossRef]
- Delvecchio, G.; Maggioni, E.; Pigoni, A.; Crespo-Facorro, B.; Nenadić, I.; Benedetti, F.; Gaser, C.; Sauer, H.; Roiz-Santiañez, R.; Poletti, S.; et al. Sexual Regional Dimorphism of Post-Adolescent and Middle Age Brain Maturation. A Multi-center 3T MRI Study. Front. Aging Neurosci. 2021, 13, 622054. [Google Scholar] [CrossRef]
- Lenroot, R.K.; Gogtay, N.; Greenstein, D.K.; Wells, E.M.; Wallace, G.L.; Clasen, L.S.; Blumenthal, J.D.; Lerch, J.; Zijdenbos, A.P.; Evans, A.C.; et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007, 36, 1065–1073. [Google Scholar] [CrossRef]
- Ritchie, S.J.; Cox, S.R.; Shen, X.; Lombardo, M.V.; Reus, L.M.; Alloza, C.; Harris, M.A.; Alderson, H.L.; Hunter, S.; Neilson, E.; et al. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex 2018, 28, 2959–2975. [Google Scholar] [CrossRef]
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef]
- Biswal, B.B.; Mennes, M.; Zuo, X.N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S.; et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739. [Google Scholar] [CrossRef]
- Anticevic, A.; Cole, M.W.; Murray, J.D.; Corlett, P.R.; Wang, X.J.; Krystal, J.H. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012, 16, 584–592. [Google Scholar] [CrossRef]
- Harikumar, A.; Evans, D.W.; Dougherty, C.C.; Carpenter, K.L.H.; Michael, A.M. A Review of the Default Mode Network in Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. Brain Connect. 2021, 11, 253–263. [Google Scholar] [CrossRef]
- Leffa, D.T.; Ferrari-Souza, J.P.; Bellaver, B.; Tissot, C.; Ferreira, P.C.L.; Brum, W.S.; Caye, A.; Lord, J.; Proitsi, P.; Martins-Silva, T.; et al. Genetic Risk for Attention-Deficit/Hyperactivity Disorder Predicts Cognitive Decline and Development of Alzheimer’s Disease Patho-physiology in Cognitively Unimpaired Older Adults. Mol. Psychiatry 2023, 28, 1248–1255. [Google Scholar] [CrossRef]
- Sperling, R.A.; Laviolette, P.S.; O’Keefe, K.; O’Brien, J.; Rentz, D.M.; Pihlajamaki, M.; Marshall, G.; Hyman, B.T.; Selkoe, D.J.; Hedden, T.; et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009, 63, 178–188. [Google Scholar] [CrossRef]
- Ingala, S.; Tomassen, J.; Collij, L.E.; Prent, N.; van’t Ent, D.; Ten Kate, M.; Konijnenberg, E.; Yaqub, M.; Scheltens, P.; de Geus, E.J.C.; et al. Amyloid-driven disruption of default mode network connectivity in cognitively healthy individuals. Brain Commun. 2021, 3, fcab201. [Google Scholar] [CrossRef]
- Cieri, F.; Zhuang, X.; Cordes, D.; Kaplan, N.; Cummings, J.; Caldwell, J. Relationship of sex differences in cortical thickness and memory among cognitively healthy subjects and individuals with mild cognitive impairment and Alzheimer disease. Alzheimers Res. Ther. 2022, 14, 36. [Google Scholar] [CrossRef]
- Liu, S.; Seidlitz, J.; Blumenthal, J.D.; Clasen, L.S.; Raznahan, A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc. Natl. Acad. Sci. USA 2020, 117, 18788–18798. [Google Scholar] [CrossRef]
- Warling, A.; Yavi, M.; Clasen, L.S.; Blumenthal, J.D.; Lalonde, F.M.; Raznahan, A.; Liu, S. Sex Chromosome Dosage Effects on White Matter Structure in the Human Brain. Cereb. Cortex 2021, 31, 5339–5353. [Google Scholar] [CrossRef]
- Gennatas, E.D.; Avants, B.B.; Wolf, D.H.; Satterthwaite, T.D.; Ruparel, K.; Ciric, R.; Hakonarson, H.; Gur, R.E.; Gur, R.C. Age-Related Effects and Sex Differences in Gray Matter Density, Volume, Mass, and Cortical Thickness from Childhood to Young Adult-hood. J. Neurosci. 2017, 37, 5065–5073. [Google Scholar] [CrossRef]
- Armstrong, N.M.; An, Y.; Beason-Held, L.; Doshi, J.; Erus, G.; Ferrucci, L.; Davatzikos, C.; Resnick, S.M. Sex differences in brain aging and predictors of neurodegeneration in cognitively healthy older adults. Neurobiol. Aging. 2019, 81, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Peper, J.S.; Hulshoff Pol, H.E.; Crone, E.A.; van Honk, J. Sex steroids and brain structure in pubertal boys and girls: A mini-review of neuroimaging studies. Neuroscience 2011, 191, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Camerino, G.; Parma, P.; Radi, O.; Valentini, S. Sex determination and sex reversal. Curr. Opin. Genet. Dev. 2006, 16, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; McArthur, S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 2010, 62, 155–198. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.; Constantinescu, M.; Spencer, D. Early androgen exposure and human gender development. Biol. Sex Differ. 2015, 6, 3. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Haanpää, M.; Turpeinen, U.; Hämäläinen, E.; Seuri, R.; Tyrväinen, E.; Sankilampi, U.; Dunkel, L. Postnatal Ovarian Activation Has Effects in Estrogen Target Tissues in Infant Girls. J. Clin. Endocrinol. Metab. 2013, 98, 4709–4716. [Google Scholar] [CrossRef]
- Hoyt, L.T.; Falconi, A.M. Puberty and perimenopause: Reproductive transitions and their implications for women’s health. Soc. Sci. Med. 2015, 132, 103–112. [Google Scholar] [CrossRef]
- Brann, D.W.; Lu, Y.; Wang, J.; Sareddy, G.R.; Pratap, U.P.; Zhang, Q.; Tekmal, R.R.; Vadlamudi, R.K. Neuron-Derived Estrogen—A Key Neuromodulator in Synaptic Function and Memory. Int. J. Mol. Sci. 2021, 22, 13242. [Google Scholar] [CrossRef]
- Brann, D.W.; Lu, Y.; Wang, J.; Zhang, Q.; Thakkar, R.; Sareddy, G.R.; Pratap, U.P.; Tekmal, R.R.; Vadlamudi, R.K. Brain-derived estrogen and neural function. Neurosci. Biobehav. Rev. 2022, 132, 793–817. [Google Scholar] [CrossRef]
- Garcia-Segura, L.M.; Wozniak, A.; Azcoitia, I.; Rodriguez, J.R.; Hutchison, R.E.; Hutchison, J.B. Aromatase expression by astrocytes after brain injury: Implications for local estrogen formation in brain repair. Neuroscience 1999, 89, 567–578. [Google Scholar] [CrossRef]
- Wang, J.; Sareddy, G.R.; Lu, Y.; Pratap, U.P.; Tang, F.; Greene, K.M.; Meyre, P.L.; Tekmal, R.R.; Vadlamudi, R.K.; Brann, D.W. Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Méndez-Ruette, M.; Ortloff, A.; Luz-Crawford, P.; Rivera, F.J.; Figueroa, C.D.; Molina, L.; Bátiz, L.F. The Impact of Estrogen and Estrogen-Like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful? Front. Cell Neurosci. 2021, 15, 636176. [Google Scholar] [CrossRef]
- McEwen, B.S.; Milner, T.A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 2017, 95, 24–39. [Google Scholar] [CrossRef]
- Sierra, A.; Gottfried-Blackmore, A.; Milner, T.A.; McEwen, B.S.; Bulloch, K. Steroid hormone receptor expression and function in microglia. Glia 2008, 56, 659–674. [Google Scholar] [CrossRef]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef]
- Takahashi, K.; Hosoya, T.; Onoe, K.; Takashima, T.; Tanaka, M.; Ishii, A.; Nakatomi, Y.; Tazawa, S.; Takahashi, K.; Doi, H.; et al. Association between aromatase in human brains and personality traits. Sci. Rep. 2018, 8, 16841. [Google Scholar] [CrossRef]
- Azcoitia, I.P. Mendez, and L.M. Garcia-Segura, Aromatase in the human brain. Androg. Clin. Res. Ther. 2021, 2, 189–202. [Google Scholar]
- Adhya, D.; Annuario, E.; Lancaster, M.A.; Price, J.; Baron-Cohen, S.; Srivastava, D.P. Understanding the role of steroids in typical and atypical brain development: Advantages of using a “brain in a dish” approach. J. Neuroendocrinol. 2018, 30, e12547. [Google Scholar] [CrossRef]
- Frankfurt, M.; Luine, V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm. Behav. 2015, 74, 28–36. [Google Scholar] [CrossRef]
- de Ronde, W.; de Jong, F.H. Aromatase inhibitors in men: Effects and therapeutic options. Reprod. Biol. Endocrinol. 2011, 9, 93. [Google Scholar] [CrossRef]
- Zitzmann, M. Testosterone and the brain. Aging Male 2006, 9, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M.; Kim, J.; Koss, W.A. Estradiol and hippocampal memory in female and male rodents. Curr. Opin. Behav. Sci. 2018, 23, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Maioli, S.; Leander, K.; Nilsson, P.; Nalvarte, I. Estrogen receptors and the aging brain. Essays Biochem. 2021, 65, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Waring, S.C.; Rocca, W.A.; Petersen, R.C.; O’Brien, P.C.; Tangalos, E.G.; Kokmen, E. Postmenopausal estrogen replacement therapy and risk of AD: A population-based study. Neurology 1999, 52, 965. [Google Scholar] [CrossRef]
- Zandi, P.P.; Carlson, M.C.; Plassman, B.L.; Welsh-Bohmer, K.A.; Mayer, L.S.; Steffens, D.C.; Breitner, J.C. Hormone replacement therapy and incidence of Alzheimer disease in older women: The cache county study. JAMA 2002, 288, 2123–2129. [Google Scholar] [CrossRef]
- Vinogradova, Y.; Dening, T.; Hippisley-Cox, J.; Taylor, L.; Moore, M.; Coupland, C. Use of menopausal hormone therapy and risk of dementia: Nested case-control studies using QResearch and CPRD databases. BMJ 2021, 374, n2182. [Google Scholar] [CrossRef]
- Saleh, R.N.M.; Hornberger, M.; Ritchie, C.W.; Minihane, A.M. Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: Results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimer’s Res. Ther. 2023, 15, 10. [Google Scholar] [CrossRef]
- Branigan, G.L.; Soto, M.; Neumayer, L.; Rodgers, K.; Brinton, R.D. Association Between Hormone-Modulating Breast Cancer Therapies and Incidence of Neurodegenerative Outcomes for Women With Breast Cancer. JAMA Netw. Open 2020, 3, e201541. [Google Scholar] [CrossRef]
- Sun, L.-M.; Chen, H.-J.; Liang, J.-A.; Kao, C.-H. Long-term use of tamoxifen reduces the risk of dementia: A nationwide population-based cohort study. QJM Int. J. Med. 2015, 109, 103–109. [Google Scholar] [CrossRef]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef]
- Ohnishi, T.; Matsuda, H.; Tabira, T.; Asada, T.; Uno, M. Changes in brain morphology in Alzheimer disease and normal aging: Is Alzheimer disease an exaggerated aging process? Am. J. Neuroradiol. 2001, 22, 1680–1685. [Google Scholar]
- Goyal, M.S.; Blazey, T.M.; Su, Y.; Couture, L.E.; Durbin, T.J.; Bateman, R.J.; Benzinger, T.L.-S.; Morris, J.C.; Raichle, M.E.; Vlassenko, A.G. Persistent metabolic youth in the aging female brain. Proc. Natl. Acad. Sci. USA 2019, 116, 3251–3255. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Y.P.; Boyd-Kirkup, J.; Khaitovich, P.; Somel, M. Accelerated aging-related transcriptome changes in the female prefrontal cortex. Aging Cell 2012, 11, 894–901. [Google Scholar] [CrossRef]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; d’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef]
- Marriott, R.J.; Murray, K.; Hankey, G.J.; Manning, L.; Dwivedi, G.; Wu, F.C.W.; Yeap, B.B. Longitudinal changes in serum testosterone and sex hormone-binding globulin in men aged 40–69 years from the UK Biobank. Clin. Endocrinol. 2022, 96, 589–598. [Google Scholar] [CrossRef]
- Stanworth, R.D.; Jones, T.H. Testosterone for the aging male; current evidence and recommended practice. Clin. Interv. Aging 2008, 3, 25–44. [Google Scholar]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef]
- Higaki, S.; Takumi, K.; Itoh, M.; Watanabe, G.; Taya, K.; Shimizu, K.; Hayashi, M.; Oishi, T. Response of ERβ and aromatase expression in the monkey hippocampal formation to ovariectomy and menopause. Neurosci. Res. 2012, 72, 148–154. [Google Scholar] [CrossRef]
- Wilson, M.E.; Rosewell, K.L.; Kashon, M.L.; Shughrue, P.J.; Merchenthaler, I.; Wise, P.M. Age differentially influences estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) gene expression in specific regions of the rat brain. Mech. Ageing Dev. 2002, 123, 593–601. [Google Scholar] [CrossRef]
- Osterlund, M.K.; Gustafsson, J.A.; Keller, E.; Hurd, Y.L. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: Distinct distribution pat-tern to ERalpha mRNA. J. Clin. Endocrinol. Metab. 2000, 85, 3840–3846. [Google Scholar]
- González, M.; Cabrera-Socorro, A.; Pérez-García, C.G.; Fraser, J.D.; López, F.J.; Alonso, R.; Meyer, G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J. Comp. Neurol. 2007, 503, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.W.; Hoskin, E.; Yudkovitz, J.; Pear, L.; Wilkinson, H.A.; Hayashi, S.; Pfaff, D.W.; Ogawa, S.; Rohrer, S.P.; Schaeffer, J.M.; et al. Immunolocalization of estrogen receptor beta in the mouse brain: Comparison with estrogen receptor alpha. Endocrinology 2003, 144, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Orikasa, C.; McEwen, B.S.; Hayashi, H.; Sakuma, Y.; Hayashi, S. Estrogen receptor alpha, but not beta, is expressed in the interneurons of the hippocampus in prepubertal rats: An in situ hybridization study. Dev. Brain Res. 2000, 120, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Raga, S.; Specchio, N.; Rheims, S.; Wilmshurst, J.M. Developmental and epileptic encephalopathies: Recognition and approaches to care. Epileptic Disord. 2021, 23, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Yanguas-Casás, N.; Crespo-Castrillo, A.; Arevalo, M.A.; Garcia-Segura, L.M. Aging and sex: Impact on microglia phagocytosis. Aging Cell 2020, 19, e13182. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q.; et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, M.; Hrabovszky, E.; Kalló, I.; Solymosi, N.; Likó, I.; Berchtold, N.; Cotman, C.; Liposits, Z. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: Rat and human studies identify strikingly similar changes. J. Neuroinflam. 2012, 9, 264. [Google Scholar] [CrossRef]
- Habib, P.; Beyer, C. Regulation of brain microglia by female gonadal steroids. J. Steroid Biochem. Mol. Biol. 2015, 146, 3–14. [Google Scholar] [CrossRef]
- Yang, H.; Xu, H.; Li, Q.; Jin, Y.; Jiang, W.; Wang, J.; Wu, Y.; Li, W.; Yang, C.; Li, X.; et al. Study of brain morphology change in Alzheimer’s disease and amnestic mild cognitive impairment compared with normal controls. Gen. Psychiatry 2019, 32, e100005. [Google Scholar] [CrossRef]
- Rahman, A.; Jackson, H.; Hristov, H.; Isaacson, R.S.; Saif, N.; Shetty, T.; Etingin, O.; Henchcliffe, C.; Brinton, R.D.; Mosconi, L. Sex and Gender Driven Modifiers of Alzheimer’s: The Role for Estrogenic Control Across Age, Race, Medical, and Lifestyle Risks. Front. Aging Neurosci. 2019, 11, 315. [Google Scholar] [CrossRef]
- Mosconi, L.; Brinton, R.D. How would we combat menopause as an Alzheimer’s risk factor? Expert Rev. Neurother 2018, 18, 689–691. [Google Scholar] [CrossRef]
- Irvine, K.; Laws, K.R.; Gale, T.M.; Kondel, T.K. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. J. Clin. Exp. Neuropsychol. 2012, 34, 989–998. [Google Scholar] [CrossRef]
- Mosconi, L.; Berti, V.; Quinn, C.; McHugh, P.; Petrongolo, G.; Varsavsky, I.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; Isaacson, R.S.; et al. Sex differences in Alzheimer risk: Brain imaging of endocrine vs. chronologic aging. Neurology 2017, 89, 1382–1390. [Google Scholar] [CrossRef]
- Altmann, A.; Tian, L.; Henderson, V.W.; Greicius, M.D. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014, 75, 563–573. [Google Scholar] [CrossRef]
- Zhu, D.; Montagne, A.; Zhao, Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell Mol. Life Sci. 2021, 78, 4907–4920. [Google Scholar] [CrossRef]
- Hogervorst, E.; Temple, S.; O’Donnell, E. Sex differences in dementia. Curr. Top. Behav. Neurosci. 2023, 62, 309–331. [Google Scholar]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef]
- Tong, C.K.; Vidyadaran, S. Role of microglia in embryonic neurogenesis. Exp. Biol. Med. 2016, 241, 1669–1675. [Google Scholar] [CrossRef]
- Menassa, D.A.; Gomez-Nicola, D. Microglial dynamics during human brain development. Front. Immunol. 2018, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Chu, Y.; Jin, X.; Parada, I.; Pesic, A.; Stevens, B.; Barres, B.; Prince, D.A. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7975–7980. [Google Scholar] [CrossRef]
- Javanmehr, N.; Saleki, K.; Alijanizadeh, P.; Rezaei, N. Microglia dynamics in aging-related neurobehavioral and neuroinflammatory diseases. J. Neuroinflammation 2022, 19, 273. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Perry, V.H. Microglial dynamics and role in the healthy and diseased brain: A paradigm of functional plasticity. Neuroscientist 2015, 21, 169–184. [Google Scholar] [CrossRef]
- Leyh, J.; Paeschke, S.; Mages, B.; Michalski, D.; Nowicki, M.; Bechmann, I.; Winter, K. Classification of Microglial Morphological Phenotypes Using Machine Learning. Front. Cell Neurosci. 2021, 15, 701673. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Bennett, F.C.; Bennett, M.L.; Yaqoob, F.; Mulinyawe, S.B.; Grant, G.A.; Hayden Gephart, M.; Plowey, E.D.; Barres, B.A. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 2018, 98, 1170–1183.e8. [Google Scholar] [CrossRef]
- Gosselin, D.; Skola, D.; Coufal, N.G.; Holtman, I.R.; Schlachetzki, J.C.M.; Sajti, E.; Jaeger, B.N.; O’Connor, C.; Fitzpatrick, C.; Pasillas, M.P.; et al. An environment-dependent transcriptional network specifies human microglia identity. Science 2017, 356, eaal3222. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R.; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef]
- Nissen, J.C. Microglial Function across the Spectrum of Age and Gender. Int. J. Mol. Sci. 2017, 18, 561. [Google Scholar] [CrossRef]
- Edler, M.K.; Mhatre-Winters, I.; Richardson, J.R. Microglia in Aging and Alzheimer’s Disease: A Comparative Species Review. Cells 2021, 10, 1138. [Google Scholar] [CrossRef]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Lynch, M.A. Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front. Aging Neurosci. 2022, 14, 868448. [Google Scholar] [CrossRef]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Souyris, M.; Cenac, C.; Azar, P.; Daviaud, D.; Canivet, A.; Grunenwald, S.; Pienkowski, C.; Chaumeil, J.; Mejía, J.E.; Guéry, J.C. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018, 3, eaap8855. [Google Scholar] [CrossRef]
- Youness, A.; Miquel, C.H.; Guéry, J.C. Escape from X Chromosome Inactivation and the Female Predominance in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 1114. [Google Scholar] [CrossRef]
- Gentilini, D.; Castaldi, D.; Mari, D.; Monti, D.; Franceschi, C.; Di Blasio, A.M.; Vitale, G. Age-dependent skewing of X chromosome inactivation appears delayed in centenarians’ offspring. Is there a role for allelic imbalance in healthy aging and longevity? Aging Cell 2012, 11, 277–283. [Google Scholar] [CrossRef]
- Qi, S.; Al Mamun, A.; Ngwa, C.; Romana, S.; Ritzel, R.; Arnold, A.P.; McCullough, L.D.; Liu, F. X chromosome escapee genes are involved in ischemic sexual dimorphism through epigenetic modification of inflammatory signals. J. Neuroinflammation 2021, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Bordt, E.A.; Ceasrine, A.M.; Bilbo, S.D. Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors. Glia 2020, 68, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- VanRyzin, J.W.; Pickett, L.A.; McCarthy, M.M. Microglia: Driving critical periods and sexual differentiation of the brain. Dev. Neurobiol. 2018, 78, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nugent, B.M.; Haliyur, R.; McCarthy, M.M. Microglia are essential to masculinization of brain and behavior. J. Neurosci. 2013, 33, 2761–2772. [Google Scholar] [CrossRef]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Alter, M.D.; Block, C.S.; Sullivan, H.; Bolton, J.L.; Bilbo, S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 2017, 65, 1504–1520. [Google Scholar] [CrossRef]
- Lenz, K.M.; McCarthy, M.M. A starring role for microglia in brain sex differences. Neuroscientist 2015, 21, 306–321. [Google Scholar] [CrossRef]
- Yanguas-Casás, N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol. Neuroinflammation 2020, 7, 13–22. [Google Scholar] [CrossRef]
- Mangold, C.A.; Wronowski, B.; Du, M.; Masser, D.R.; Hadad, N.; Bixler, G.V.; Brucklacher, R.M.; Ford, M.M.; Sonntag, W.E.; Freeman, W.M. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J. Neuroinflammation 2017, 14, 141. [Google Scholar] [CrossRef]
- Kerr, N.; Dietrich, D.W.; Bramlett, H.M.; Raval, A.P. Sexually dimorphic microglia and ischemic stroke. CNS Neurosci. Ther. 2019, 25, 1308–1317. [Google Scholar] [CrossRef]
- Acosta-Martínez, M. Shaping Microglial Phenotypes Through Estrogen Receptors: Relevance to Sex-Specific Neuroinflammatory Responses to Brain Injury and Disease. J. Pharmacol. Exp. Ther. 2020, 375, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Loiola, R.A.; Wickstead, E.S.; Solito, E.; McArthur, S. Estrogen Promotes Pro-resolving Microglial Behavior and Phagocytic Cell Clearance Through the Actions of Annexin A1. Front. Endocrinol. 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Purvis, G.S.D.; Solito, E.; Thiemermann, C. Annexin-A1: Therapeutic Potential in Microvascular Disease. Front. Immunol. 2019, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Chen, Y.-W.; Tsai, S.-F.; Wu, S.-N.; Shih, Y.-H.; Jiang-Shieh, Y.-F.; Yang, T.-T.; Kuo, Y.-M. Estrogen ameliorates microglial activation by inhibiting the Kir2.1 inward-rectifier K+ channel. Sci. Rep. 2016, 6, 22864. [Google Scholar] [CrossRef] [PubMed]
- Benedek, G.; Zhang, J.; Nguyen, H.; Kent, G.; Seifert, H.; Vandenbark, A.A.; Offner, H. Novel feedback loop between M2 macrophages/microglia and regulatory B cells in estrogen-protected EAE mice. J. Neuroimmunol. 2017, 305, 59–67. [Google Scholar] [CrossRef]
- Thakkar, R.; Wang, R.; Wang, J.; Vadlamudi, R.K.; Brann, D.W. 17β-estradiol regulates microglia activation and polarization in the hippocampus following global cerebral ischemia. Oxidative Med. Cell. Longev. 2018, 2018, 4248526. [Google Scholar] [CrossRef]
- Loram, L.C.; Sholar, P.W.; Taylor, F.R.; Wiesler, J.L.; Babb, J.A.; Strand, K.A.; Berkelhammer, D.; Day, H.E.; Maier, S.F.; Watkins, L.R. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 2012, 37, 1688–1699. [Google Scholar] [CrossRef]
- Benedusi, V.; Meda, C.; Della Torre, S.; Monteleone, G.; Vegeto, E.; Maggi, A. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology 2012, 153, 2777–2788. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Edison, P.; Donat, C.K.; Sastre, M. In vivo Imaging of Glial Activation in Alzheimer’s Disease. Front. Neurol. 2018, 9, 625. [Google Scholar] [CrossRef]
- Kater, M.S.J.; Huffels, C.F.M.; Oshima, T.; Renckens, N.S.; Middeldorp, J.; Boddeke, E.W.G.M.; Smit, A.B.; Eggen, B.J.L.; Hol, E.M.; Verheijen, M.H.G. Prevention of microgliosis halts early memory loss in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2023, 107, 225–241. [Google Scholar] [CrossRef]
- Takata, K.; Kitamura, Y.; Saeki, M.; Terada, M.; Kagitani, S.; Kitamura, R.; Fujikawa, Y.; Maelicke, A.; Tomimoto, H.; Taniguchi, T.; et al. Galantamine-induced amyloid-β clearance mediated via stimulation of microglial nicotinic acetylcholine receptors. J. Biol. Chem. 2010, 285, 40180–40191. [Google Scholar] [CrossRef]
- Parhizkar, S.; Arzberger, T.; Brendel, M.; Kleinberger, G.; Deussing, M.; Focke, C.; Nuscher, B.; Xiong, M.; Ghasemigharagoz, A.; Katzmarski, N.; et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 2019, 22, 191–204. [Google Scholar] [CrossRef]
- Jana, M.; Palencia, C.A.; Pahan, K. Fibrillar amyloid-beta peptides activate microglia via TLR2 Implications for Alzheimer’s disease. J. Immunol. 2008, 181, 7254–7262. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Y.; Hwang, S.; Archuleta, K.; Huang, H.; Campos, A.; Murad, R.; Piña-Crespo, J.; Xu, H.; Huang, T.Y. Trem2 deletion enhances tau dispersion and pathology through microglia exosomes. Mol. Neurodegener. 2022, 17, 58. [Google Scholar] [CrossRef]
- Lee, D.C.; Rizer, J.; Selenica, M.L.; Reid, P.; Kraft, C.; Johnson, A.; Blair, L.; Gordon, M.N.; Dickey, C.A.; Morgan, D. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J. Neuroinflammation 2010, 7, 56. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.-V.; Araiz, A.R.; Mela, V.; Gaban, A.S.; O’Neill, E.; Joshi, L.; Chouchani, E.T.; Mills, E.L.; Lynch, M.A. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun. Biol. 2021, 4, 711. [Google Scholar] [CrossRef]
- Vinet, J.; van Weering, H.R.J.; Heinrich, A.; Kälin, R.E.; Wegner, A.; Brouwer, N.; Heppner, F.L.; van Rooijen, N.; Boddeke, H.W.G.M.; Biber, K. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J. Neuroinflammation 2012, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Franco-Bocanegra, D.K.; Gourari, Y.; McAuley, C.; Chatelet, D.S.; Johnston, D.A.; Nicoll, J.A.R.; Boche, D. Microglial morphology in Alzheimer’s disease and after Aβ immunotherapy. Sci. Rep. 2021, 11, 15955. [Google Scholar] [CrossRef] [PubMed]
- Coales, I.; Tsartsalis, S.; Fancy, N.; Weinert, M.; Clode, D.; Owen, D.; Matthews, P.M. Alzheimer’s disease-related transcriptional sex differences in myeloid cells. J. Neuroinflammation 2022, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Belcredito, S.; Ghisletti, S.; Meda, C.; Etteri, S.; Maggi, A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 2006, 147, 2263–2272. [Google Scholar] [CrossRef]
- Spence, R.D.; Voskuhl, R.R. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front. Neuroendocrinol. 2012, 33, 105–115. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Shay, T.; Jojic, V.; Zuk, O.; Rothamel, K.; Puyraimond-Zemmour, D.; Feng, T.; Wakamatsu, E.; Benoist, C.; Koller, D.; Regev, A. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl. Acad. Sci. USA 2013, 110, 2946–2951. [Google Scholar] [CrossRef]
- Yamada, T.; Sasaki, H.; Furuya, H.; Miyata, T.; Goto, I.; Sakaki, Y. Complementary DNA for the mouse homolog of the human amyloid beta protein precursor. Biochem. Biophys. Res. Commun. 1987, 149, 665–671. [Google Scholar] [CrossRef]
- Masuda, A.; Kobayashi, Y.; Kogo, N.; Saito, T.; Saido, T.C.; Itohara, S. Cognitive deficits in single App knock-in mouse models. Neurobiol. Learn. Mem. 2016, 135, 73–82. [Google Scholar] [CrossRef]
- Kawasumi, M.; Chiba, T.; Yamada, M.; Miyamae-Kaneko, M.; Matsuoka, M.; Nakahara, J.; Tomita, T.; Iwatsubo, T.; Kato, S.; Aiso, S.; et al. Targeted introduction of V642I mutation in amyloid precursor protein gene causes functional abnormality resembling early stage of Alzheimer’s disease in aged mice. Eur. J. Neurosci. 2004, 19, 2826–2838. [Google Scholar] [CrossRef]
- Reaume, A.G.; Howland, D.S.; Trusko, S.P.; Savage, M.J.; Lang, D.M.; Greenberg, B.D.; Siman, R.; Scott, R.W. Enhanced Amyloidogenic Processing of the β-Amyloid Precursor Protein in Gene-targeted Mice Bearing the Swedish Familial Alzhei-mer’s Disease Mutations and a “Humanized” Aβ Sequence. J. Biol. Chem. 1996, 271, 23380–23388. [Google Scholar] [CrossRef]
- Yokoyama, M.; Kobayashi, H.; Tatsumi, L.; Tomita, T. Mouse models of Alzheimer’s disease. Front. Mol. Neurosci. 2022, 15, 912995. [Google Scholar] [CrossRef]
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 1996, 274, 99–102. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Rademakers, R.; Cruts, M.; van Broeckhoven, C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum. Mutat. 2004, 24, 277–295. [Google Scholar] [CrossRef]
- Eriksen, J.L.; Janus, C.G. Plaques, Tangles, and Memory Loss in Mouse Models of Neurodegeneration. Behav. Genet. 2007, 37, 79–100. [Google Scholar] [CrossRef]
- Hall, A.M.; Roberson, E.D. Mouse models of Alzheimer’s disease. Brain Res. Bull. 2012, 88, 3–12. [Google Scholar] [CrossRef]
- Foidl, B.M.; Humpel, C. Can mouse models mimic sporadic Alzheimer’s disease? Neural Regen. Res. 2020, 15, 401–406. [Google Scholar]
- Mullane, K.; Williams, M. Alzheimer’s therapeutics: Continued clinical failures question the validity of the amyloid hypothesis—But what lies beyond? Biochem. Pharmacol. 2013, 85, 289–305. [Google Scholar] [CrossRef]
- Sabbagh, J.J.; Kinney, J.W.; Cummings, J.L. Animal systems in the development of treatments for Alzheimer’s disease: Challenges, methods, and implications. Neurobiol. Aging 2013, 34, 169–183. [Google Scholar] [CrossRef]
- Barak, M.; Fedorova, V.; Pospisilova, V.; Raska, J.; Vochyanova, S.; Sedmik, J.; Hribkova, H.; Klimova, H.; Vanova, T.; Bohaciakova, D. Human iPSC-Derived Neural Models for Studying Alzheimer’s Disease: From Neural Stem Cells to Cerebral Organoids. Stem Cell Rev. Rep. 2022, 18, 792–820. [Google Scholar] [CrossRef] [PubMed]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In vitro models of neurodegenerative diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.R.; Holst, B.; Mau-Holzmann, U.A.; Freude, K.; Schmid, B. Generation of two isogenic iPSC lines with either a heterozygous or a homozygous E280A mutation in the PSEN1 gene. Stem Cell Res. 2019, 35, 101403. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.R.; Holst, B.; Ramakrishna, S.; Muddashetty, R.; Schmid, B.; Freude, K. Generation of two ipsc lines with either a heterozygous V717I or a heterozygous KM670/671NLmutation in the app gene. Stem Cell Res. 2019, 34, 101368. [Google Scholar] [CrossRef] [PubMed]
- Israel, M.A.; Yuan, S.H.; Bardy, C.; Reyna, S.M.; Mu, Y.; Herrera, C.; Hefferan, M.P.; Van Gorp, S.; Nazor, K.L.; Boscolo, F.S.; et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 2012, 482, 216–220. [Google Scholar] [CrossRef]
- Kondo, T.; Asai, M.; Tsukita, K.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Imamura, K.; Egawa, N.; Yahata, N.; Okita, K.; et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell 2013, 12, 487–496. [Google Scholar] [CrossRef]
- Muratore, C.R.; Rice, H.C.; Srikanth, P.; Callahan, D.G.; Shin, T.; Benjamin, L.N.; Walsh, D.M.; Selkoe, D.J.; Young-Pearse, T.L. The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum. Mol. Genet. 2014, 23, 3523–3536. [Google Scholar] [CrossRef]
- Yagi, T.; Ito, D.; Okada, Y.; Akamatsu, W.; Nihei, Y.; Yoshizaki, T.; Yamanaka, S.; Okano, H.; Suzuki, N. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum. Mol. Genet. 2011, 20, 4530–4539. [Google Scholar] [CrossRef]
- Papaspyropoulos, A.; Tsolaki, M.; Foroglou, N.; Pantazaki, A.A. Modeling and targeting Alzheimer’s disease with organoids. Front. Pharmacol. 2020, 11, 396. [Google Scholar] [CrossRef]
- Ochalek, A.; Mihalik, B.; Avci, H.X.; Chandrasekaran, A.; Téglási, A.; Bock, I.; Giudice, M.L.; Táncos, Z.; Molnár, K.; László, L.; et al. Neurons derived from sporadic Alzheimer’s disease iPSCs reveal elevated tau hyperphosphorylation, increased amyloid levels, and gsk3b activation. Alzheimer’s Res. Ther. 2017, 9, 90. [Google Scholar] [CrossRef]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. Ipsc-derived human microglia-like cells to study neurological diseases. Neuron 2017, 94, 278–293.e279. [Google Scholar] [CrossRef]
- Luchena, C.; Zuazo-Ibarra, J.; Valero, J.; Matute, C.; Alberdi, E.; Capetillo-Zarate, E. A neuron, microglia, and astrocyte triple co-culture model to study Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 271. [Google Scholar] [CrossRef]
- Guttikonda, S.R.; Sikkema, L.; Tchieu, J.; Saurat, N.; Walsh, R.M.; Harschnitz, O.; Ciceri, G.; Sneeboer, M.; Mazutis, L.; Setty, M.; et al. Fully defined human pluripotent stem cell-derived microglia and tri-culture system model c3 production in Alzheimer’s disease. Nat. Neurosci. 2021, 24, 343–354. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Qian, X.; Song, H.; Ming, G.-L. Brain organoids: Advances, applications and challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef]
- Meyer, K.; Feldman, H.M.; Lu, T.; Drake, D.; Lim, E.T.; Ling, K.H.; Bishop, N.A.; Pan, Y.; Seo, J.; Lin, Y.T.; et al. Rest and neural gene network dysregulation in ipsc models of Alzheimer’s disease. Cell Rep. 2019, 26, 1112–1127. [Google Scholar] [CrossRef]
- Lin, Y.T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. Apoe4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human ipsc-derived brain cell types. Neuron 2018, 98, 1141–1154.e7. [Google Scholar] [CrossRef]
- Gonzalez, C.; Armijo, E.; Bravo-Alegria, J.; Becerra-Calixto, A.; Mays, C.E.; Soto, C. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol. Psychiatry 2018, 23, 2363–2374. [Google Scholar] [CrossRef]
- Raja, W.K.; Mungenast, A.E.; Lin, Y.T.; Ko, T.; Abdurrob, F.; Seo, J.; Tsai, L.H. Self-organizing 3d human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS ONE 2016, 11, e0161969. [Google Scholar] [CrossRef]
- Ghatak, S.; Dolatabadi, N.; Trudler, D.; Zhang, X.; Wu, Y.; Mohata, M.; Ambasudhan, R.; Talantova, M.; Lipton, S.A. Mechanisms of hyperexcitability in Alzheimer’s disease hiPSC-derived neurons and cerebral organoids vs isogenic controls. eLife 2019, 8, e50333. [Google Scholar] [CrossRef]
- Park, J.C.; Jang, S.Y.; Lee, D.; Lee, J.; Kang, U.; Chang, H.; Kim, H.J.; Han, S.H.; Seo, J.; Choi, M.; et al. A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids. Nat. Commun. 2021, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Wörsdörfer, P.; Dalda, N.; Kern, A.; Krüger, S.; Wagner, N.; Kwok, C.K.; Henke, E.; Ergün, S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019, 9, 15663. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, I.; Dougalis, A.; Shakirzyanova, A.; Gómez-Budia, M.; Pelkonen, A.; Konttinen, H.; Ohtonen, S.; Fazaludeen, M.F.; Koskuvi, M.; Kuusisto, J.; et al. Microglia-like cells promote neuronal functions in cerebral organoids. Cells 2021, 11, 124. [Google Scholar] [CrossRef]
- Xu, R.; Boreland, A.J.; Li, X.; Erickson, C.; Jin, M.; Atkins, C.; Pang, Z.P.; Daniels, B.P.; Jiang, P. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Rep. 2021, 16, 1923–1937. [Google Scholar] [CrossRef]
- Ormel, P.R.; Vieira de Sá, R.; van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis van Berlekom, A.; et al. Microglia innately develop within cerebral organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar] [CrossRef]
- Ashraf, H.; Solla, P.; Sechi, L.A. Current advancement of immunomodulatory drugs as potential pharmacotherapies for autoimmunity based neurological diseases. Pharmaceuticals 2022, 15, 1077. [Google Scholar] [CrossRef]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef]
- Dubbelaar, M.L.; Kracht, L.; Eggen, B.J.L.; Boddeke, E. The kaleidoscope of microglial phenotypes. Front. Immunol. 2018, 9, 1753. [Google Scholar] [CrossRef]
- Boche, D.; Gordon, M.N. Diversity of transcriptomic microglial phenotypes in aging and Alzheimer’s disease. Alzheimers Dement. 2022, 18, 360–376. [Google Scholar] [CrossRef]
- Bundy, J.L.; Vied, C.; Badger, C.; Nowakowski, R.S. Sex-biased hippocampal pathology in the 5XFAD mouse model of Alzheimer’s disease: A multi-omic analysis. J. Comp. Neurol. 2019, 527, 462–475. [Google Scholar] [CrossRef]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009, 48, A.4I.1–A.4I.8. [Google Scholar] [CrossRef]
- Staley, K.; Scharfman, H. A woman’s prerogative. Nat. Neurosci. 2005, 8, 697–699. [Google Scholar] [CrossRef]
- Diaz Brinton, R. Minireview: Translational animal models of human menopause: Challenges and emerging opportunities. Endocrinology 2012, 153, 3571–3578. [Google Scholar] [CrossRef]
- Arnold, A.P. Four Core Genotypes and XY* mouse models: Update on impact on SABV research. Neurosci. Biobehav. Rev. 2020, 119, 1–8. [Google Scholar] [CrossRef]
- Liu, L.P.; Zheng, Y.W. Predicting differentiation potential of human pluripotent stem cells: Possibilities and challenges. World J. Stem Cells 2019, 11, 375–382. [Google Scholar] [CrossRef]
- Yokobayashi, S.; Okita, K.; Nakagawa, M.; Nakamura, T.; Yabuta, Y.; Yamamoto, T.; Saitou, M. Clonal variation of human induced pluripotent stem cells for induction into the germ cell fate. Biol. Reprod. 2017, 96, 1154–1166. [Google Scholar] [CrossRef]
- Rohani, L.; Johnson, A.A.; Arnold, A.; Stolzing, A. The aging signature: A hallmark of induced pluripotent stem cells? Aging Cell 2014, 13, 2–7. [Google Scholar] [CrossRef]
- Waldhorn, I.; Turetsky, T.; Steiner, D.; Gil, Y.; Benyamini, H.; Gropp, M.; Reubinoff, B.E. Modeling sex differences in humans using isogenic induced pluripotent stem cells. Stem Cell Rep. 2022, 17, 2732–2744. [Google Scholar] [CrossRef]
- Bruck, T.; Benvenisty, N. Meta-analysis of the heterogeneity of X chromosome inactivation in human pluripotent stem cells. Stem Cell Res. 2011, 6, 187–193. [Google Scholar] [CrossRef]
- Mekhoubad, S.; Bock, C.; de Boer, A.S.; Kiskinis, E.; Meissner, A.; Eggan, K. Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell 2012, 10, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Ronen, D.; Benvenisty, N. Sex-dependent gene expression in human pluripotent stem cells. Cell Rep. 2014, 8, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Mizee, M.R.; Miedema, S.S.M.; van der Poel, M.; Adelia; Schuurman, K.G.; van Strien, M.E.; Melief, J.; Smolders, J.; Hendrickx, D.A.; Heutinck, K.M.; et al. Isolation of primary microglia from the human post-mortem brain: Effects of ante- and post-mortem variables. Acta Neuropathol. Commun. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- McFarland, K.N.; Ceballos, C.; Rosario, A.; Ladd, T.; Moore, B.; Golde, G.; Wang, X.; Allen, M.; Ertekin-Taner, N.; Funk, C.C.; et al. Microglia show differential transcriptomic response to Aβ peptide aggregates ex vivo and in vivo. Life Sci. Alliance 2021, 4, e202101108. [Google Scholar] [CrossRef] [PubMed]
- Speicher, A.M.; Wiendl, H.; Meuth, S.G.; Pawlowski, M. Generating microglia from human pluripotent stem cells: Novel in vitro models for the study of neurodegeneration. Mol. Neurodegener. 2019, 14, 46. [Google Scholar] [CrossRef]
- Haenseler, W.; Sansom, S.N.; Buchrieser, J.; Newey, S.E.; Moore, C.S.; Nicholls, F.J.; Chintawar, S.; Schnell, C.; Antel, J.P.; Allen, N.D.; et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and In-flammatory Response. Stem Cell Rep. 2017, 8, 1727–1742. [Google Scholar] [CrossRef]
- Camp, J.G.; Badsha, F.; Florio, M.; Kanton, S.; Gerber, T.; Wilsch-Bräuninger, M.; Lewitus, E.; Sykes, A.; Hevers, W.; Lancaster, M.; et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl. Acad. Sci. USA 2015, 112, 15672–15677. [Google Scholar] [CrossRef]
- Hernández, D.; Rooney, L.A.; Daniszewski, M.; Gulluyan, L.; Liang, H.H.; Cook, A.L.; Hewitt, A.W.; Pébay, A. Culture Variabilities of Human iPSC-Derived Cerebral Organoids Are a Major Issue for the Modelling of Phenotypes Observed in Alz-heimer’s Disease. Stem Cell Rev. Rep. 2022, 18, 718–731. [Google Scholar] [CrossRef]
- Kelava, I.; Lancaster, M.A. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev. Biol. 2016, 420, 199–209. [Google Scholar] [CrossRef]
- Kelava, I.; Chiaradia, I.; Pellegrini, L.; Kalinka, A.T.; Lancaster, M.A. Male sex hormones increase excitatory neuron production in developing human neocortex. bioRxiv 2020. bioRxiv:2020.10.24.353359. [Google Scholar]
- Popova, G.; Soliman, S.S.; Kim, C.N.; Keefe, M.G.; Hennick, K.M.; Jain, S.; Li, T.; Tejera, D.; Shin, D.; Chhun, B.B.; et al. Human microglia states are conserved across experimental models and regulate neural stem cell responses in chimeric organoids. Cell Stem Cell 2021, 28, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Coluccio, M.L.; Perozziello, G.; Malara, N.; Parrotta, E.; Zhang, P.; Gentile, F.; Limongi, T.; Raj, P.M.; Cuda, G.; Candeloro, P.; et al. Microfluidic platforms for cell cultures and investigations. Microelectron. Eng. 2019, 208, 14–28. [Google Scholar] [CrossRef]
- Schulla, L.S.; Alupoaie, E.D.; De Silva, L.; Gawlitta, D.; Middendorp, S.; Coffer, P.J.; Roukens, M.G. Development of a Novel Microfluidic Co-culture model to study Organoid Vascularization. bioRxiv 2022. bioRxiv:2022.03.25.485813. [Google Scholar]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef]
- Gordon, A.; Yoon, S.J.; Tran, S.S.; Makinson, C.D.; Park, J.Y.; Andersen, J.; Valencia, A.M.; Horvath, S.; Xiao, X.; Huguenard, J.R.; et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 2021, 24, 331–342. [Google Scholar] [CrossRef]
- Porciúncula, L.O.; Goto-Silva, L.; Ledur, P.F.; Rehen, S.K. The Age of Brain Organoids: Tailoring Cell Identity and Functionality for Normal Brain Development and Disease Modeling. Front. Neurosci. 2021, 15, 674563. [Google Scholar] [CrossRef]


| SNP ID | Gene | Variant | Frequency | Function | Brain Expression |
|---|---|---|---|---|---|
| rs3752246 | ABCA7 | G > C/C > T | G = 0.15755 (C = 0.84245) | Lipid homeostasis | Neurons, astrocytes, microglia and oligodendrocytes |
| rs3764650 | ABCA7 | T > G | G = 0.102719 (T = 0.897281) | Lipid homeostasis | Neurons, astrocytes, microglia and oligodendrocytes |
| rs1800764 | ACE | C > G/C > T | C = 0.481027 (T = 0.518973) | Blood pressure and electrolyte balance | Neurons, microglia and oligodendrocytes |
| rs442495 | ADAM10 | T > C/T > G | C = 0.40762 (T = 0.59238) | Lipid transport and phagocytosis | Oligodendrocytes, microglia, neurons and astrocytes |
| rs7412 | APOC1 | C > T | T = 0.083116 (C = 0.916884) | Lipid transport | Microglia, astrocytes and neurons |
| rs429358 | APOC1 | T > C | C = 0.074418 (T = 0.925582) | Lipid transport | Microglia, astrocytes and neurons |
| rs4663105 | BIN1 | A > C | C = 0.44298 (A = 0.55702) | Endocytosis | Oligodendrocytes, microglia, neurons and astrocytes |
| rs744373 | BIN1 | A > C/A > G/A > T | G = 0.297771 (A = 0.702229) | Endocytosis | Oligodendrocytes, microglia, neurons and astrocytes |
| rs9381563 | CD2AP | C > A/C > G/C > T | C = 0.39767 (T = 0.60233) | Cytoskeleton regulation | Microglia, neurons, astrocytes and oligodendrocytes |
| rs9349407 | CD2AP | G > C | C = 0.24780 (G = 0.75220) | Cytoskeleton regulation | Microglia, neurons, astrocytes and oligodendrocytes |
| rs10948363 | CD2AP | A > G | G = 0.24596 (A = 0.75404) | Cytoskeleton regulation | Microglia, neurons, astrocytes and oligodendrocytes |
| rs1354106 | CD33 | T > G | G = 0.34719 (T = 0.65281) | Cell adhesion and immune cell homeostasis | Microglia |
| rs3865444 | CD33 | C > A | A = 0.302324 (C = 0.697676) | Cell adhesion and immune cell homeostasis | Microglia |
| rs12459419 | CD33 | C > A/C > G/C > T | T = 0.26380 (C = 0.73620) | Cell adhesion and immune cell homeostasis | Microglia |
| rs4845378 | CHRNB2 | G > A/G > T | T = 0.08354 (G = 0.91646) | Ion transport | Neurons, oligodendrocytes and astrocytes, microglia |
| rs11136000 | CLU | T > A/T > C | C = 0.607739 (T = 0.392261) | Apoptosis, complement and immunity | Astrocytes, microglia, neurons and oligodendrocytes |
| rs9331896 | CLU | C > A/C > G/C > T | C = 0.42127 (T = 0.57873) | Apoptosis, complement and immunity | Astrocytes, microglia, neurons and oligodendrocytes |
| rs1532278 | CLU | T > A/T > C | T = 0.374146 (C = 0.625854) | Apoptosis, complement and immunity | Astrocytes, microglia, neurons and oligodendrocytes |
| rs4236673 | CLU | A > C/A > G | A = 0.33623 (G = 0.66377) | Apoptosis, complement and immunity | Astrocytes, microglia, neurons and oligodendrocytes |
| rs3818361 | CR1 | A > C/A > G | A = 0.201980 (G = 0.798020) | Complement and immunity | Oligodendrocytes and microglia |
| rs6656401 | CR1 | A > G/A > T | A = 0.179434 (G = 0.820566) | Complement and immunity | Oligodendrocytes and microglia |
| rs4844609 | CR1 | A > G/A > T | A = 0.021785 (T = 0.978215) | Complement and immunity | Oligodendrocytes and microglia |
| rs1064039 | CST3 | C > G/C > T | T = 0.19579 (C = 0.80421) | Protease inhibitor | Astrocytes, oligodendrocytes and microglia |
| rs11771145 | EPHA1 | G > A/G > T | A = 0.351605 (G = 0.648395) | Angiogenesis and cell adhesion | Neurons and microglia |
| rs11763230 | EPHA1 | C > T | T = 0.20866 (C = 0.79134) | Angiogenesis and cell adhesion | Neurons and microglia |
| rs11767557 | EPHA1 | T > A/T > C | C = 0.194037 (T = 0.805963) | Angiogenesis and cell adhesion | Neurons and microglia |
| rs10793294 | GAB2 | C > A/C > G | C = 0.251454 (A = 0.748546) | Cell proliferation and growth | Oligodendrocytes, microglia, neurons and astrocytes |
| rs3745833 | GALP | C > A/C > G/C > T | G = 0.33124 (C = 0.66875) | Neuropeptide | Neurons |
| rs6931277 | HLA-DQA1 | A > T | T = 0.14976 (A = 0.85024) | Adaptive immunity | Microglia |
| rs1143634 | IL1B | G > A | A = 0.227942 (G = 0.772058) | Inflammatory response | Microglia |
| rs35349669 | INPP5D | C > T | T = 0.38287 (C = 0.61713) | Apoptosis, lipid metabolism and immunity | Microglia |
| rs10933431 | INPPD5 | G > C | G = 0.30651 (C = 0.69349) | Apoptosis, lipid metabolism and immunity | Microglia |
| rs190982 | MEF2C | G > A/G > C | G = 0.376709 (A = 0.623291) | Apoptosis, differentiation, neurogenesis and immune proliferation | Neurons, microglia, oligodendrocytes and astrocytes |
| rs190982 | MEF2C | G > A/G > C | G = 0.376709 (A = 0.623291) | Apoptosis, differentiation, neurogenesis and immune proliferation | Neurons, microglia, oligodendrocytes and astrocytes |
| rs558678 | MS4A2 | T > G | G = 0.24086 (T = 0.75914) | Immune responses | Neurons |
| rs4938933 | MS4A4A | C > G/C > T | C = 0.404463 (T = 0.595537) | Signal transduction and immune responses | Microglia |
| rs610932 | MS4A4A | T > C/T > G | T = 0.430441 (G = 0.569559) | Signal transduction and immune responses | Microglia |
| rs10897011 | MS4A4E | G > A | A = 0.33764 (G = 0.66236) | Innate immunity | Microglia |
| rs610932 | MS4A6A | T > C/T > G | T = 0.430441 (G = 0.569559) | Signal transduction and immune responses | Microglia |
| rs7935829 | MS4A6A | A > G | G = 0.35879 (A = 0.64121) | Signal transduction and immune responses | Microglia |
| rs2081545 | MS4A6A | C > A/C > T | A = 0.21413 (C = 0.78587) | Signal transduction and immune responses | Microglia |
| rs11754661 | MTHFD1L | G > A/G > T | A = 0.061583 (G = 0.938417) | Metabolism | Microglia, neurons, oligodendrocytes and astrocytes |
| rs3800324 | PGBD1 | G > A | A = 0.054414 (G = 0.945586) | Unknown | Neurons, oligodendrocytes, astrocytes and microglia |
| rs3851179 | PICALM | T > C | T = 0.361052 (C = 0.638948) | Endocytosis | Oligodendrocytes, microglia, neurons and astrocytes |
| rs561655 | PICALM | G > A/G > T | G = 0.348713 (A = 0.651287) | Endocytosis | Oligodendrocytes, microglia, neurons and astrocytes |
| rs541458 | PICALM | C > T | C = 0.315402 (T = 0.684598) | Endocytosis | Oligodendrocytes, microglia, neurons and astrocytes |
| rs10792832 | PICALM | A > C/A > G/A > T | A = 0.29879 (G = 0.70121) | Endocytosis | Oligodendrocytes, microglia, neurons and astrocytes |
| rs2058716 | PRKD3 | G > A/G > C | G = 0.31978 (C = 0.68022) | Apoptosis and lipid transport | Microglia, oligodendrocytes, neurons and astrocytes |
| rs165932 | PSEN1 | G > A/G > T | G = 0.424510 (T = 0.575490) | Amyloidogenic- and Notch processing | Oligodendrocytes, neurons, microglia and astrocytes |
| rs28834970 | PTK2B | T > C | C = 0.31596 (T = 0.68404) | Adaptive immunity | Neurons, microglia, oligodendrocytes and astrocytes |
| rs2301275 | PVR | A > C/A > G | G = 0.23252 (A = 0.76748) | Cell adhesion | Neurons, oligodendrocytes, astrocytes and microglia |
| rs142787485 | RAB10 | A > G | G = 0.02890 (A = 0.97110) | Protein transport | Oligodendrocytes, neurons, microglia and astrocytes |
| rs2376866 | RELB | C > T | T = 0.14727 (C = 0.85273) | Apoptosis, cell growth and immunity | Microglia, neurons, oligodendrocytes and astrocytes |
| rs11218343 | SORL1 | T > A/T > C | C = 0.02728 (T = 0.97272) | Endocytosis | Microglia, neurons, astrocytes and oligodendrocytes |
| rs2282649 | SORL1 | C > A/C > T | T = 0.28928 (C = 0.71072) | Endocytosis | Microglia, neurons, astrocytes and oligodendrocytes |
| rs2306604 | TFAM | A > C/A > G/A > T | G = 0.41883 (A = 0.58117) | Transcription regulation | Oligodendrocytes, neurons, astrocytes and microglia |
| rs5011436 | TMEM106B | A > C/A > G/A > T | C = 0.27232 (A = 0.72768) | Lysosomal transport | Neurons, oligodendrocytes, astrocytes and microglia |
| rs187370608 | TREM2 | G > A | A = 0.00201 (G = 0.99799) | Phagocytosis and immune activation | Microglia |
| rs75932628 | TREM2 | C > A/C > T | T = 0.00232 (C = 0.99768) | Phagocytosis and immune activation | Microglia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadlecova, M.; Freude, K.; Haukedal, H. Complexity of Sex Differences and Their Impact on Alzheimer’s Disease. Biomedicines 2023, 11, 1261. https://doi.org/10.3390/biomedicines11051261
Kadlecova M, Freude K, Haukedal H. Complexity of Sex Differences and Their Impact on Alzheimer’s Disease. Biomedicines. 2023; 11(5):1261. https://doi.org/10.3390/biomedicines11051261
Chicago/Turabian StyleKadlecova, Marion, Kristine Freude, and Henriette Haukedal. 2023. "Complexity of Sex Differences and Their Impact on Alzheimer’s Disease" Biomedicines 11, no. 5: 1261. https://doi.org/10.3390/biomedicines11051261
APA StyleKadlecova, M., Freude, K., & Haukedal, H. (2023). Complexity of Sex Differences and Their Impact on Alzheimer’s Disease. Biomedicines, 11(5), 1261. https://doi.org/10.3390/biomedicines11051261






