Preeclampsia Susceptibility Assessment Based on Deep Learning Modeling and Single Nucleotide Polymorphism Analysis
Abstract
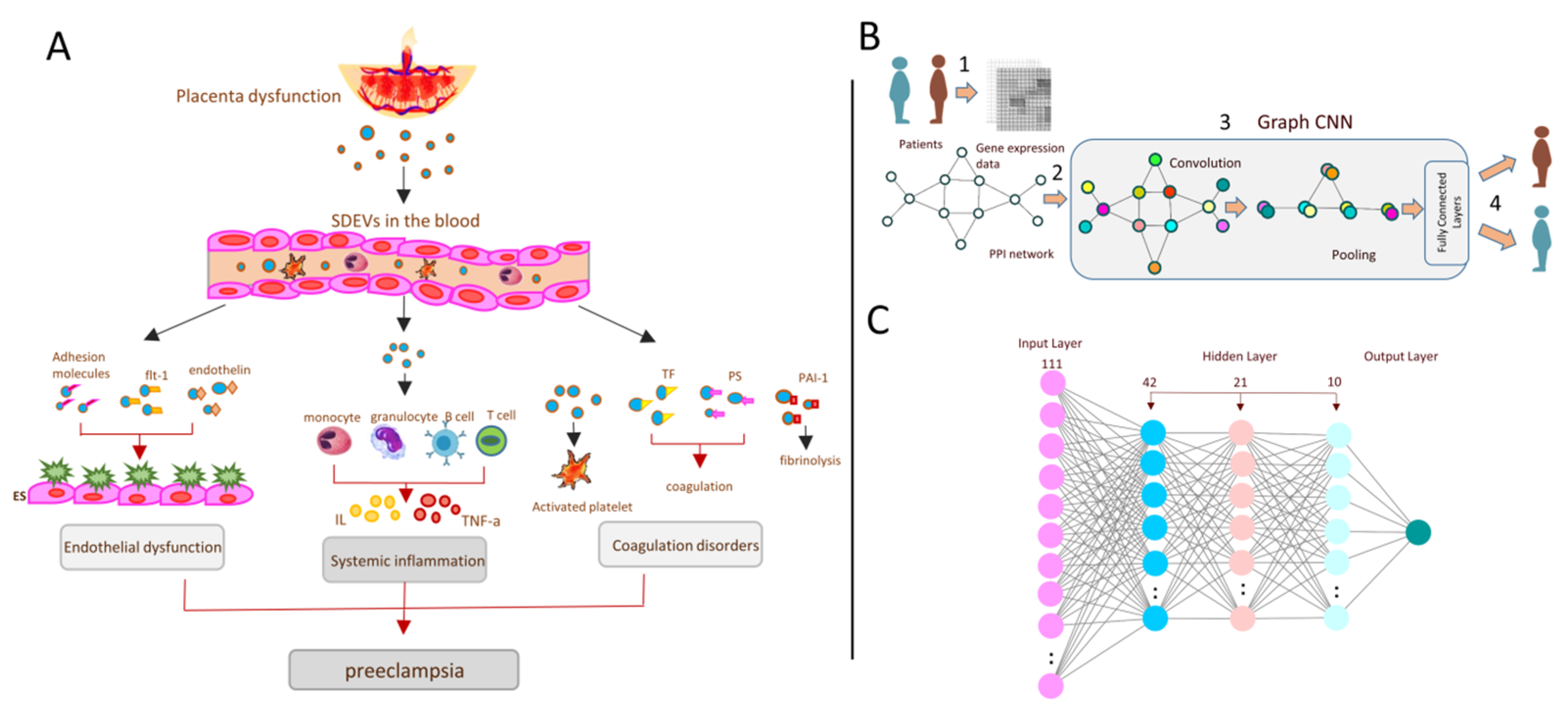
1. Introduction
2. Methods
2.1. Data Collection and Preprocessing
2.2. Deep Learning Neural Network Model Design
2.3. Experimental Design
2.4. DNA Extraction and Genotyping of Polymorphisms
2.5. Software and Statistics
3. Results
3.1. 111 Genes Are Connected to the IL-13 and IL-4 Signalling Pathway
3.2. Two Deep Learning Models to Diagnose Preeclampsia through IL-13 and IL-4 Pathway Expression Data
3.3. The Significant SNPs in IL-13 the Gene Might Improve the Accuracy of the Expression-Based Deep Learning Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SNP | Single nucleotide polymorphism |
| IL | Interleukin |
| MLP | Multilayer perceptron |
| GCNN | Graph convolutional neural network |
| GEO | Gene Expression Omnibus |
| SNAP | Stanford Network Analysis Project |
| ARMS-PCR | Amplification refractory mutation system PCR |
References
- Karrar, S.A.; Hong, P.L.; Preeclampsia. StatPearls Publishing. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570611/ (accessed on 9 September 2022).
- Erez, O.; Romero, R.; Jung, E.; Chaemsaithong, P.; Bosco, M.; Suksai, M.; Gallo, D.M.; Gotsch, F. Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am. J. Obstet. Gynecol. 2022, 226, 786–803. [Google Scholar] [CrossRef] [PubMed]
- Nirupama, R.; Divyashree, S.; Janhavi, P.; Muthukumar, S.P.; Ravindra, P.V. Preeclampsia: Pathophysiology and management. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101975. [Google Scholar] [CrossRef]
- Caplan, M.; Keenan-Devlin, L.S.; Freedman, A.; Grobman, W.; Wadhwa, P.D.; Buss, C.; Miller, G.E.; Borders, A.E.B. Lifetime Psychosocial Stress Exposure Associated with Hypertensive Disorders of Pregnancy. Am. J. Perinatol. 2021, 38, 1412–1419. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Black, K.D.; Horowitz, J.A. Inflammatory Markers and Preeclampsia: A Systematic Review. Nurs. Res. 2018, 67, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Dai, L.; Song, Y.; Wang, Y.; Zhou, B.; Zhou, R. Association between polymorphisms in CXCR2 gene and preeclampsia. Mol. Genet. Genom. Med. 2019, 7, e00578. [Google Scholar] [CrossRef]
- Abedin, D.A.; Esmaeilzadeh, E.; Amin-Beidokhti, M.; Pirjani, R.; Gholami, M.; Mirfakhraie, R. ACE gene rs4343 polymorphism elevates the risk of preeclampsia in pregnant women. J. Hum. Hypertens. 2018, 32, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhong, M. Association between Interleukin-10 gene polymorphisms and risk of early-onset preeclampsia. Int. J. Clin. Exp. Pathol. 2015, 8, 11659–11664. [Google Scholar]
- Liu, H.; Wang, W.; Liu, C. Increased expression of IFN-γ in preeclampsia impairs human trophoblast invasion via a SOCS1/JAK/STAT1 feedback loop. Exp. Ther. Med. 2021, 21, 112. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Smyth, G.K. Limma: Linear Models for Microarray Data. Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Springer: New York, NY, USA, 2005; pp. 397–420. [Google Scholar]
- Slonim, D.K. From patterns to pathways: Gene expression data analysis comes of age. Nat. Genet. 2002, 32, 502–508. [Google Scholar] [CrossRef]
- Chereda, H.; Bleckmann, A.; Kramer, F.; Leha, A.; Beissbarth, T. Utilizing Molecular Network Information via Graph Convolutional Neural Networks to Predict Metastatic Event in Breast Cancer. Stud. Health Technol. Inform. 2019, 267, 181–186. [Google Scholar]
- Eiland, E.; Nzerue, C.; Faulkner, M. Preeclampsia 2012. J. Pregnancy 2012, 2012, 586578. [Google Scholar] [CrossRef] [PubMed]
- Wiles, K.; Chappell, L.C.; Lightstone, L.; Bramham, K. Updates in Diagnosis and Management of Preeclampsia in Women with CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Badriyah, T.; Syarif, I. Classification Algorithms of Maternal Risk Detection For Preeclampsia With Hypertension During Pregnancy Using Particle Swarm Optimization. EMITTER Int. J. Eng. Technol. 2018, 6, 236–253. [Google Scholar] [CrossRef]
- Sakinah, N.; Tahir, M.; Badriyah, T.; Syarif, I. LSTM with adam optimization-powered high accuracy preeclampsia classification. In Proceedings of the 2019 International Electronics Symposium (IES), Surabaya, Indonesia, 27–28 September 2019; pp. 314–319. [Google Scholar]
- Wang, Q.; Liu, D.; Liu, G. Value of Ultrasonic Image Features in Diagnosis of Perinatal Outcomes of Severe Preeclampsia on account of Deep Learning Algorithm. Comput. Math. Methods Med. 2022, 2022, 4010339. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.; Mulla, Z.D.; Parikh, P.; Hauspurg, A.; Razzaghi, T. An imbalance-aware deep neural network for early prediction of preeclampsia. PLoS ONE 2022, 17, e0266042. [Google Scholar] [CrossRef]
- Jonsson, Y.; Rubèr, M.; Matthiesen, L.; Berg, G.; Nieminen, K.; Sharma, S.; Ernerudh, J.; Ekerfelt, C. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J. Reprod. Immunol. 2006, 70, 83–91. [Google Scholar] [CrossRef]
- Salazar Garcia, M.D.; Mobley, Y.; Henson, J.; Davies, M.; Skariah, A.; Dambaeva, S.; Gilman-Sachs, A.; Beaman, K.; Lampley, C.; Kwak-Kim, J. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J. Reprod. Immunol. 2018, 125, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakova, P.A.; Volodina, M.A.; Tarasova, N.V.; Marey, M.V.; Tsvirkun, D.V.; Vavina, O.V.; Khodzhaeva, Z.S.; Kan, N.E.; Menon, R.; Vysokikh, M.Y.; et al. Mitochondrial role in adaptive response to stress conditions in preeclampsia. Sci. Rep. 2016, 6, 32410. [Google Scholar] [CrossRef]
- Choi, S.J.; Oh, S.Y.; Kim, J.H.; Sadovsky, Y.; Roh, C.R. Increased expression of N-myc downstream-regulated gene 1 (NDRG1) in placentas from pregnancies complicated by intrauterine growth restriction or preeclampsia. Am. J. Obstet. Gynecol. 2007, 196, e1–e7. [Google Scholar] [CrossRef]
- Nuzzo, A.M.; Giuffrida, D.; Zenerino, C.; Piazzese, A.; Olearo, E.; Todros, T.; Rolfo, A. JunB/cyclin-D1 imbalance in placental mesenchymal stromal cells derived from preeclamptic pregnancies with fetal-placental compromise. Placenta 2014, 35, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Y.; Cao, G.; Ma, Y.; Li, Y.X.; Zhao, Y.; Shao, X.; Wang, Y.L. Hypoxic stress disrupts HGF/Met signaling in human trophoblasts: Implications for the pathogenesis of preeclampsia. J. Biomed. Sci. 2022, 29, 8. [Google Scholar] [CrossRef] [PubMed]
- Gumusoglu, S.; Scroggins, S.; Vignato, J.; Santillan, D.; Santillan, M. The Serotonin-Immune Axis in Preeclampsia. Curr. Hypertens. Rep. 2021, 23, 37. [Google Scholar] [CrossRef]
- Cecati, M.; Giannubilo, S.R.; Saccucci, F.; Sartini, D.; Ciavattini, A.; Emanuelli, M.; Tranquilli, A.L. Potential Role of Placental Klotho in the Pathogenesis of Preeclampsia. Cell Biochem. Biophys. 2016, 74, 49–57. [Google Scholar] [CrossRef]
- Espino, Y. Sosa.S.; Flores-Pliego, A.; Espejel-Nuñez, A.; Medina-Bastidas, D.; Vadillo-Ortega, F.; Zaga-Clavellina, V.; Estrada-Gutierrez, G. New Insights into the Role of Matrix Metalloproteinases in Preeclampsia. Int. J. Mol. Sci. 2017, 18, 1448. [Google Scholar]
- Qu, H.; Yu, Q.; Jia, B.; Zhou, W.; Zhang, Y.; Mu, L. HIF-3α affects preeclampsia development by regulating EVT growth via activation of the Flt-1/JAK/STAT signaling pathway in hypoxia. Mol. Med. Rep. 2021, 23, 68. [Google Scholar] [CrossRef]
- Mannon, P.; Reinisch, W. Interleukin 13 and its role in gut defence and inflammation. Gut 2012, 61, 1765–1773. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Yao, Y.; Li, H.; Liang, H.; Xin, M.; Wang, L.; Zhao, L.; Lin, J.; Liu, S. Polymorphisms of the IL27 gene in a Chinese Han population complicated with pre-eclampsia. Sci. Rep. 2016, 6, 23029. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.M.; Wang, Y.; Liu, X.L.; Zhang, A.; Xu, Q. Polymorphisms in interleukin-6 and interleukin-10 may be associated with risk of preeclampsia. Genet. Mol. Res. 2017, 16, gmr16018588. [Google Scholar] [CrossRef] [PubMed]
- Raguema, N.; Gannoun, M.B.A.; Zitouni, H.; Meddeb, S.; Benletaifa, D.; Lavoie, J.L.; Almawi, W.Y.; Mahjoub, T. Interleukin-10 rs1800871 (-819C/T) and ATA haplotype are associated with preeclampsia in a Tunisian population. Gestation. Hypertens. 2018, 11, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Quach, K.; Grover, S.A.; Kenigsberg, S.; Librach, C.L. A combination of single nucleotide polymorphisms in the 3′untranslated region of HLA-G is associated with preeclampsia. Hum. Immunol. 2014, 75, 1163–1170. [Google Scholar] [CrossRef]
- Salas-Pacheco, J.; Vazquez-Alaniz, F.; Martínez, S.; Quiñones, A.M.; Aguilar-Durán, M. Combined genotypes-509CT/869TC of the TGFB1 gene associated with preeclampsia. Rev. Int. De Contam. Ambient. 2014, 30, 45–51. [Google Scholar]
- Pfab, T.; Chen, Y.P.; Slowinski, T.; Richter, C.M.; Godes, M.; Arck, P.C.; Halle, H.; Hocher, B. Impact of genes related to immune tolerance and inflammation (tumour necrosis factor-alpha, interleukin-6) on blood pressure, protein excretion and oedema in pregnancy. J. Hypertens. 2005, 23, 2187–2191. [Google Scholar] [CrossRef]
- Lisi, V.; Paternoster, D.M.; Stecca, A.; Micciché, F.; Fantinato, S.; Leon, A.; Damante, G.; Fabbro, D.; Clementi, M. Investigation of endothelin-1 type A receptor gene polymorphism (−231 G > A) in preeclampsia susceptibility. J. Matern. Fetal Neonatal Med. 2007, 20, 145–149. [Google Scholar] [CrossRef]
| Variable | Preeclampsia | Control | p-Value |
|---|---|---|---|
| N = (150) | N = (150) | ||
| Age, years | 30.09 ± 6.87 | 27.23 ± 4.86 | 0.239 |
| Range | 16–48 | 16–38 | – |
| BMI, kg/m2 | 26.19 ± 3.60 | 25.83 ± 3.47 | 0.214 |
| Gestational age, median (range), wk (at the time of sampling) | 32 (28–33) | 32 (28–33) | – |
| Mode of delivery (at the time of sampling) Emergency caesarean section Pre-labour caesarean section | 7 (4.67) 3 (2) | – | – |
| Systolic blood pressure, mmHg | 151.84 ± 10.78 | 109.37 ± 11.07 | <0.001 |
| Diastolic blood pressure, mmHg | 94.95 ± 6.88 | 64.85 ± 9.02 | <0.001 |
| SNP | Primer Sequence | Annealing Temperature (°C) | Fragment Length |
|---|---|---|---|
| rs2069740(T/A) | FO:CCTCTGCACAGTTTGGAC RO:TCTGTCCAGCAATCCAGG FI:AATGCCGTGGCCTCTGCT RI:CAGCCTTAGTCCAGGTCAGAGA | 58.2 °C | T:151 bp A:384 bp |
| rs34255686(C/A) | FO: CCTCTGCACAGTTTGGAC RO: TCTGTCCAGCAATCCAGG FI: CCTTCTCAATAAGTCCAT RI: CTGGTTCTGGGTGATGTTT | 56.8 °C | C:186 bp A:220 bp |
| Group | Subgroup | Total Number |
|---|---|---|
| Control | TNL(176) | 314 |
| TL(138) | ||
| Early Preeclampsia | None | 66 |
| Preterm Birth | None | 355 |
| Model | Accuracy | F1-Score (Weighted) |
|---|---|---|
| Graph Convolutional Neural Network (GCNN) | 83.16% | 75.51% |
| Multalayer Perceptron (MLP) | 82.11% | 82.32% |
| Genotype and Allele | Control Group | Patient Group | p-Value |
|---|---|---|---|
| n (%) | n (%) | ||
| rs2069740(T/A) | |||
| AA | 20(13.3) | 9(6) | 0.099 |
| TT | 123(82) | 133(88.7) | |
| AT | 7(4.7) | 8(5.3) | |
| A | 47(15.7) | 26(8.7) | 0.009 |
| T | 253(84.3) | 274(91.3) | |
| rs34255686(C/A) | |||
| CC | 121(80.7) | 141(94) | 0.002 |
| AA | 15(10) | 3(2) | |
| CA | 14(9.3) | 6(4) | |
| A | 12(4) | 44(14.7) | 0.00 |
| C | 288(96) | 256(85.3) | |
| Genotype combination (rs2069740–rs34255686) | |||
| AA–CC | 18(12) | 9(6) | 0.06 |
| AA–AA | 2(1.3) | 0(0) | 0.1 |
| TT–CC | 117(78) | 106(70.7) | 0.14 |
| TT–AA | 0(0) | 13(8.7) | 0.001 |
| TT–CA | 6(4) | 14(9.3) | 0.06 |
| AT–CC | 6(4) | 7(4.7) | 0.7 |
| AT–AA | 1(7) | 1(7) | 1 |
| rs2069740 (T/A) | Genotype n (%) | p-Value | rs34255686 (C/A) | Genotype n (%) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| AA | TT | AT | CC | AA | CA | ||||
| Seizures Present | 0 (0) | 1 (100) | 0 (0) | 0.9 | Seizures Present | 1 (100) | 0 (0) | 0 (0) | 0.88 |
| Absent | 9 (6) | 132 (88) | 8 (5.4) | Absent | 120 (80.5) | 15 (10) | 14 (9.4) | ||
| Proteinuria Absent | 3 (5.7) | 47 (88.7) | 3 (5.7) | 0.9 | Proteinuria Absent | 43 (81) | 4 (7) | 6 (11) | 0.5 |
| Trace | 1 (5.6) | 16 (88) | 1 (5.6) | Trace | 16 (88.9) | 0 (0) | 2 (11) | ||
| 1 | 3 (6.5) | 41 (89.3) | 2 (4.3) | 1 | 35 (76) | 7 (15) | 4 (8.7) | ||
| 2 | 2 (13) | 12 (80) | 1 (6.7) | 2 | 11 (73) | 3 (20) | 1 (6.7) | ||
| 3 | 0 (0) | 13 (92) | 1 (7.1) | 3 | 13 (93.1) | 0 (0) | 1 (6.7) | ||
| 4 | 0 (0) | 4 (100) | 0 (0) | 4 | 3 (75) | 1 (25) | 0 (0) | ||
| Edema Absent | 0 (0) | 23 (100) | 0 (0) | 0.131 | Edema Absent | 15 (65) | 4 (17) | 4 (17) | 0.047 |
| 1 | 8 (8.8) | 78 (85) | 5 (5.5) | 1 | 79 (86) | 4 (4.4) | 8 (8.8) | ||
| 2 | 0 (0) | 24 (96) | 1 (4) | 2 | 18 (72) | 6 (24) | 1 (4) | ||
| 3 | 1 (9.1) | 8 (72.7) | 2 (18.2) | 3 | 9 (81) | 1 (9.1) | 1 (9.1) | ||
| Diabetes Present | 1 (11) | 8 (88) | 0 (0) | 0.629 | Diabetes Present | 7 (77.8) | 2 (22.2) | 0 (0) | 0.311 |
| Absent | 8 (5.7) | 125 (88) | 8 (5.7) | Absent | 114 (80.7) | 13 (9.2) | 14 (9.9) | ||
| Multipara Primary | 11 (8) | 115 (83) | 12 (8.7) | 0.02 | Multipara Primary | 122 (88.4) | 10 (7.2) | 6 (4.3) | 0.252 |
| Multiple | 18 (11.1) | 141 (87) | 3 (1.9) | Multiple | 140 (86.6) | 8 (4.9) | 14 (8.6) | ||
| Hypertension Mild | 8 (6.9) | 101 (87.7) | 7 (6.4) | 0.7 | Hypertension Mild | 93 (80) | 10 (8.6) | 13 (11) | 0.38 |
| Moderate | 1 (0) | 24 (33.3) | 1 (17.1) | Moderate | 22 (84.6) | 3 (11) | 1 (3.8) | ||
| Severe | 0 (0) | 8 (100) | 0 (0) | Severe | 6 (75) | 2 (25) | 0 (0) | ||
| Gravida Present | 2 (33.3) | 4 (66.7) | 0 (0) | 0.015 | Gravida Present | 5 (83.3) | 1 (16.7) | 0 (0) | 0.64 |
| Absent | 7 (4.9) | 129 (89.6) | 8 (5.6) | Absent | 116 (80.6) | 14 (9.7) | 14 (9.7) | ||
| Hyperthyroidism Present | 0 (0) | 16 (94) | 1 (5.9) | 0.54 | Hyperthyroidism Present | 14 (82.4) | 2 (11.8) | 1 (5.9) | 0.85 |
| Absent | 9 (6.8) | 117 (80) | 7 (5.3) | Absent | 107 (80.1) | 13 (9.8) | 13 (9.8) | ||
| HELLP syndrome Present | 0 (0) | 1 (100) | 0 (0) | 0.9 | HELLP syndrome Present | 0 (0) | 0 (0) | 1 (100) | 0.008 |
| Absent | 9 (6) | 132 (88.6) | 8 (5.4) | Absent | 121 (81.2) | 15 (10) | 13 (8.7) | ||
| History of abortion Present | 1 (3.3) | 26 (86) | 3 (10) | 0.36 | History of abortion Present | 26 (86.7) | 4 (13.3) | 0 (0) | 0.13 |
| Absent | 8 (6.7) | 107 (89.2) | 5 (4.2) | Absent | 95 (79.3) | 11 (9.2) | 14 (11.7) | ||
| History of preeclampsia Present | 1 (7.7) | 10 (76.9) | 2 (15) | 0.225 | History of preeclampsia Present | 11 (84) | 1 (7.7) | 1 (7.7) | 0.9 |
| Absent | 8 (5.8) | 123 (89) | 6 (4.4) | Absent | 110 (80.5) | 14 (10) | 13 (9.5) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadaty, A.; Parhoudeh, S.; Khashei Varnamkhasti, K.; Moghanibashi, M.; Naeimi, S. Preeclampsia Susceptibility Assessment Based on Deep Learning Modeling and Single Nucleotide Polymorphism Analysis. Biomedicines 2023, 11, 1257. https://doi.org/10.3390/biomedicines11051257
Saadaty A, Parhoudeh S, Khashei Varnamkhasti K, Moghanibashi M, Naeimi S. Preeclampsia Susceptibility Assessment Based on Deep Learning Modeling and Single Nucleotide Polymorphism Analysis. Biomedicines. 2023; 11(5):1257. https://doi.org/10.3390/biomedicines11051257
Chicago/Turabian StyleSaadaty, Aida, Sara Parhoudeh, Khalil Khashei Varnamkhasti, Mehdi Moghanibashi, and Sirous Naeimi. 2023. "Preeclampsia Susceptibility Assessment Based on Deep Learning Modeling and Single Nucleotide Polymorphism Analysis" Biomedicines 11, no. 5: 1257. https://doi.org/10.3390/biomedicines11051257
APA StyleSaadaty, A., Parhoudeh, S., Khashei Varnamkhasti, K., Moghanibashi, M., & Naeimi, S. (2023). Preeclampsia Susceptibility Assessment Based on Deep Learning Modeling and Single Nucleotide Polymorphism Analysis. Biomedicines, 11(5), 1257. https://doi.org/10.3390/biomedicines11051257






