Impact of Hyper- and Hypo-Uricemia on Kidney Function
Abstract
1. Introduction to Hyperuricemia and Hypouricemia: Epidemiology and Genetic Mutation
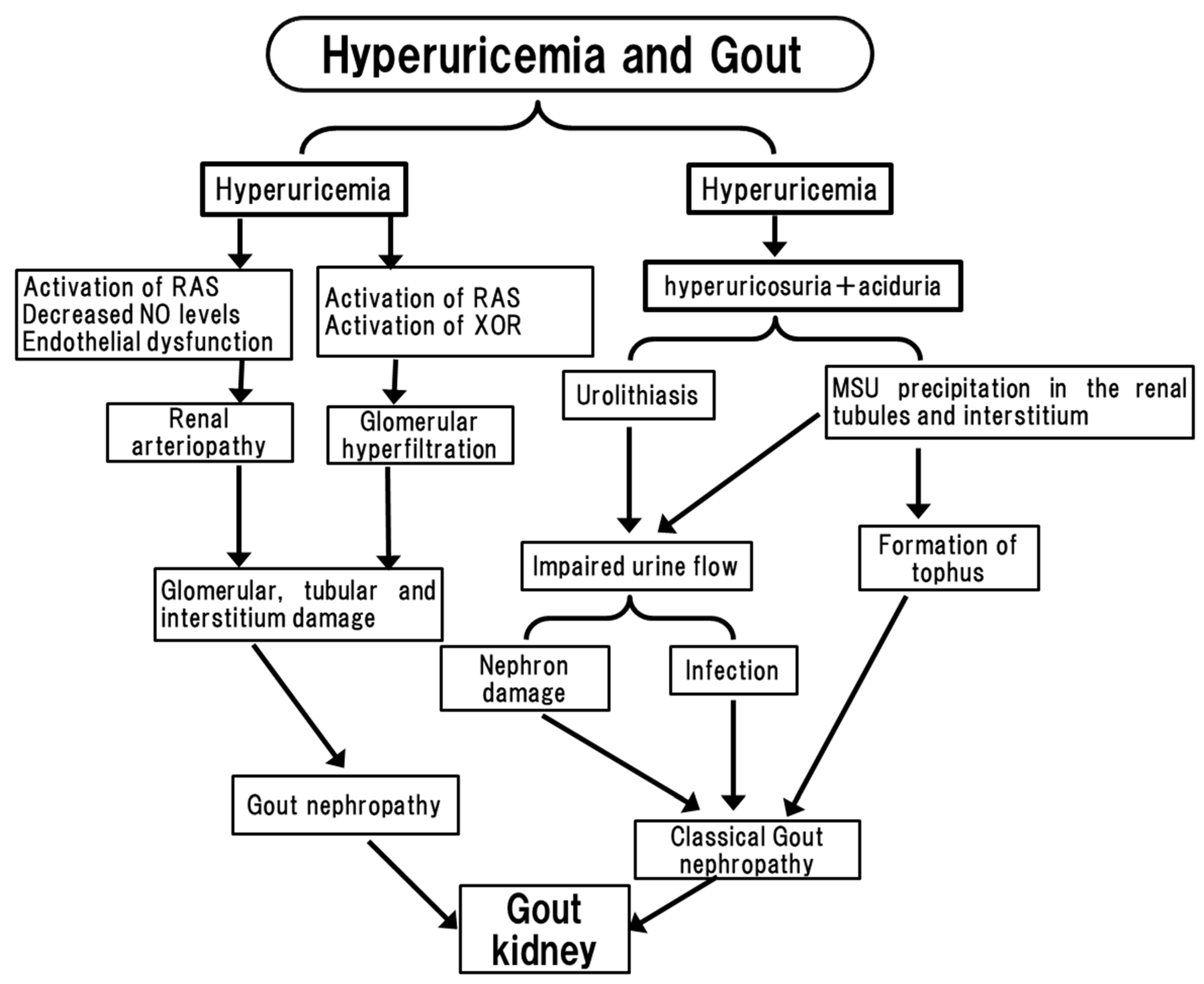
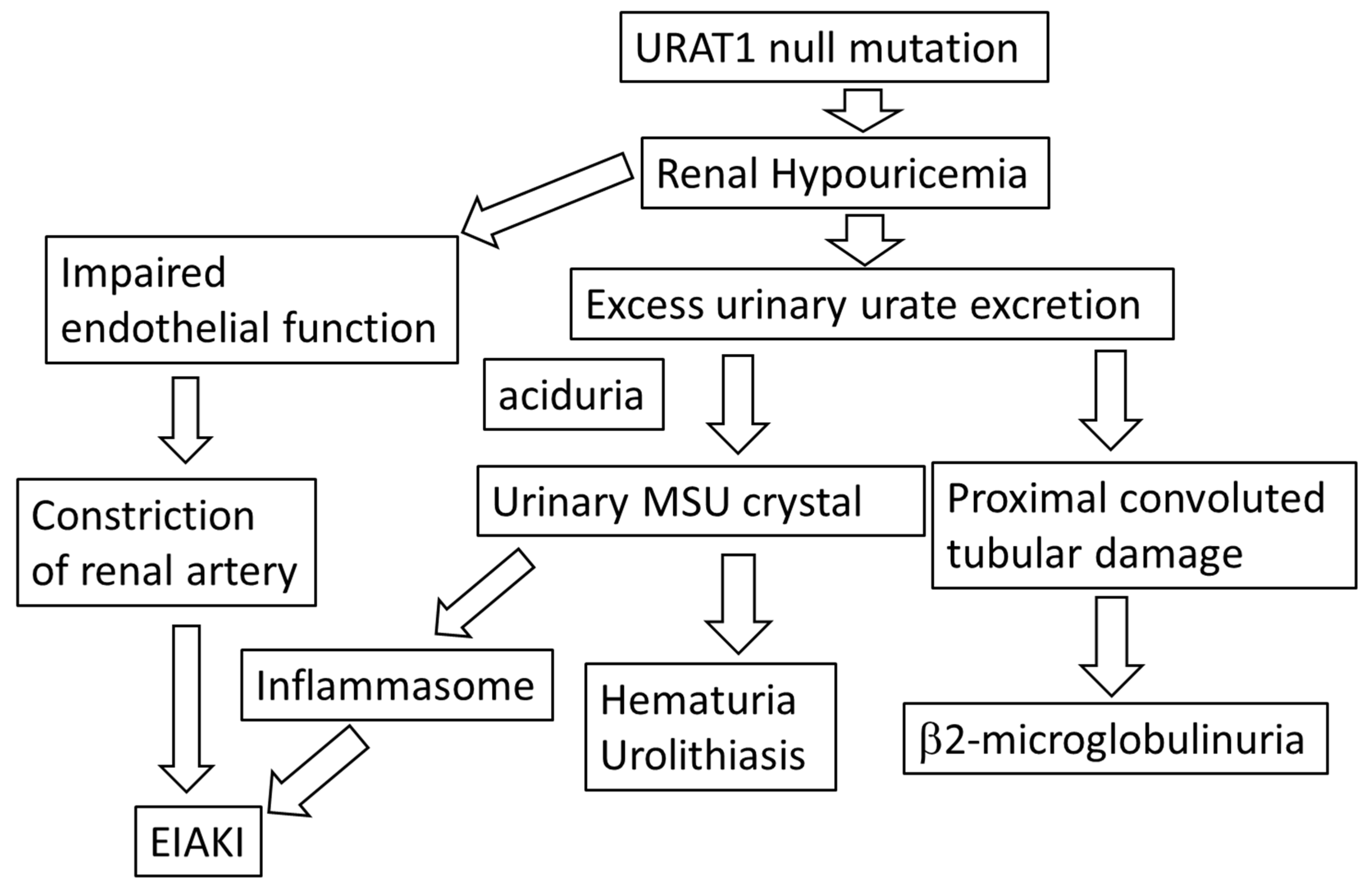
2. Clinical Evidence of Hyper- and Hypo-Uricemia May Cause an Adverse Effect on Kidney
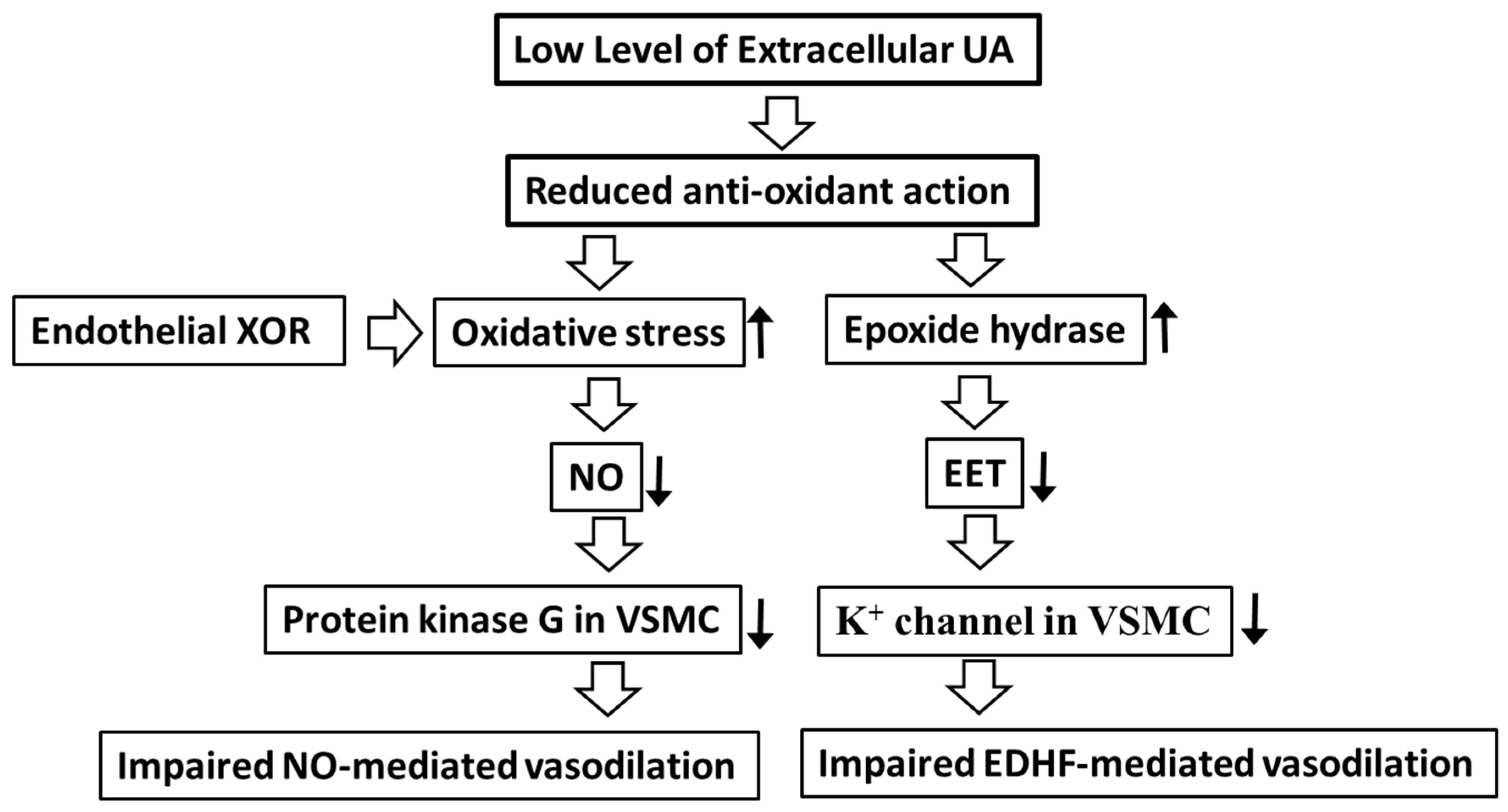
3. Molecular Mechanism of Hype-, Hypo-, Transient Hypo-Uricemia May Cause Endothelial Dysfunction
4. Treatment of Hyper- and Hypo Uricemia and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hisatome, I.; Li, P.; Miake, J.; Taufiq, F.; Mahati, E.; Maharani, N.; Utami, S.B.; Kuwabara, M.; Bahrudin, U.; Ninomiya, H. Uric Acid as a Risk Factor for Chronic Kidney Disease and Cardiovascular Disease—Japanese Guideline on the Management of Asymptomatic Hyperuricemia. Circ. J. 2021, 85, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elela, A. Epidemiology, pathophysiology, and management of uric acid urolithiasis: A narrative review. J. Adv. Res. 2017, 8, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, B.; Li, Y.; Huang, Y.; Li, J.; Yao, H.; Jing, X.; Chen, J.; Wang, J. Uric acid and risk of heart failure: A systematic review and meta-analysis. Eur. J. Heart Fail. 2014, 16, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Niwa, K.; Nishihara, S.; Nishi, Y.; Takahashi, O.; Kario, K.; Yamamoto, K.; Yamashita, T.; Hisatome, I. Hyperuricemia is an independent competing risk factor for atrial fibrillation. Int. J. Cardiol. 2017, 231, 137–142. [Google Scholar] [CrossRef]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci. Rep. 2016, 6, 19520. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Nakashima, A.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Hidaka, T.; et al. Hyperuricemia is independently associated with endothelial dysfunction in postmenopausal women but not in premenopausal women. BMJ Open 2013, 3, e003659. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Ichida, K.; Hosoyamada, M.; Hisatome, I.; Enomoto, A.; Hikita, M.; Endou, H.; Hosoya, T. Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J. Am. Soc. Nephrol. 2004, 15, 164–173. [Google Scholar] [CrossRef]
- Tabara, Y.; Kohara, K.; Kawamoto, R.; Hiura, Y.; Nishimura, K.; Morisaki, T.; Kokubo, Y.; Okamura, T.; Tomoike, H.; Iwai, N.; et al. Association of four genetic loci with uric acid levels and reduced renal function: The J-SHIPP suita study. Am. J. Nephrol. 2010, 32, 279–286. [Google Scholar] [CrossRef]
- Kanda, E.; Muneyuki, T.; Kanno, Y.; Suwa, K.; Nakajima, K. Uric acid level has a U-shaped association with loss of kidney function in healthy people: A prospective cohort study. PLoS ONE 2015, 10, e0118031. [Google Scholar] [CrossRef]
- Kuwabara, M.; Hisatome, I.; Niwa, K.; Bjornstad, P.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Kanbay, M.; Johnson, R.J.; Lanaspa, M.A. The Optimal Range of Serum Uric Acid for Cardiometabolic Diseases: A 5-Year Japanese Cohort Study. J. Clin. Med. 2020, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H. Japanese guideline for the management of hyperuricemia and gout: Second edition. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Matsuo, H.; Ohtahara, A.; Ogino, K.; Hakoda, M.; Hamada, T.; Hosoyamada, M.; Yamaguchi, S.; Hisatome, I.; Ichida, K.; et al. Clinical practice guideline for renal hypouricemia (1st edition). Hum. Cell 2019, 32, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, M.; Kazama, J.J.; Narita, I.; Konta, T.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Asahi, K.; et al. Association between hypouricemia and reduced kidney function: A cross-sectional population-based study in Japan. Am. J. Nephrol. 2015, 41, 138–146. [Google Scholar] [CrossRef]
- Matsuo, H.; Chiba, T.; Nagamori, S.; Nakayama, A.; Domoto, H.; Phetdee, K.; Wiriyasermkul, P.; Kikuchi, Y.; Oda, T.; Nishiyama, J.; et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am. J. Hum. Genet. 2008, 83, 744–751. [Google Scholar] [CrossRef]
- Kuwabara, M.; Niwa, K.; Ohtahara, A.; Hamada, T.; Miyazaki, S.; Mizuta, E.; Ogino, K.; Hisatome, I. Prevalence and complications of hypouricemia in a general population: A large-scale cross-sectional study in Japan. PLoS ONE 2017, 12, e0176055. [Google Scholar] [CrossRef] [PubMed]
- Hisatome, I.; Ogino, K.; Kotake, H.; Ishiko, R.; Saito, M.; Hasegawa, J.; Mashiba, H.; Nakamoto, S. Cause of persistent hypouricemia in outpatients. Nephron 1989, 51, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Tabe, A.; Sakai, N. Pathophysiology of hypouricemia. J. Jikei Med. Sch. 2015, 111, 821–839. [Google Scholar]
- Kaneko, K.; Fujimori, S. Hypouricemia: Prevalence and clinical significance of hypouricemia. Med. Pract. 1995, 12, 659–662. [Google Scholar]
- Nakayama, A.; Nakatochi, M.; Kawamura, Y.; Yamamoto, K.; Nakaoka, H.; Shimizu, S.; Higashino, T.; Koyama, T.; Hishida, A.; Kuriki, K.; et al. Subtype-specific gout susceptibility loci and enrichment of selection pressure on ABCG2 and ALDH2 identified by subtype genome-wide meta-analyses of clinically defined gout patients. Ann. Rheum. Dis. 2020, 79, 657–665. [Google Scholar] [CrossRef]
- Sugihara, S.; Hisatome, I.; Kuwabara, M.; Niwa, K.; Maharani, N.; Kato, M.; Ogino, K.; Hamada, T.; Ninomiya, H.; Higashi, Y.; et al. Depletion of uric acid due to SLC22A12 (URAT1) loss-of-function mutation causes endothelial dysfunction in hypouricemia. Circ. J. 2015, 79, 1125–1132. [Google Scholar] [CrossRef]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T.; et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Nakayama, A.; Ikebuchi, Y.; Ito, K.; Kusanagi, Y.; Chiba, T.; Tadokoro, S.; et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: A function-based genetic analysis in a Japanese population. Sci. Transl. Med. 2009, 1, 5ra11. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.I.; Kang, J.H.; Lee, J.H.; Ha, I.S.; Kim, S.; Komoda, F.; Sekine, T.; Igarashi, T.; Choi, Y. Mutational analysis of idiopathic renal hypouricemia in Korea. Pediatr. Nephrol. 2005, 20, 886–890. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, H.J.; Lee, B.H.; Kang, H.K.; Chin, H.J.; Yoon, H.J.; Ha, I.S.; Kim, S.; Choi, Y.; Cheong, H.I. Prevalence of hypouricaemia and SLC22A12 mutations in healthy Korean subjects. Nephrology 2008, 13, 661–666. [Google Scholar] [CrossRef]
- Ichida, K.; Hosoyamada, M.; Kamatani, N.; Kamitsuji, S.; Hisatome, I.; Shibasaki, T.; Hosoya, T. Age and origin of the G774A mutation in SLC22A12 causing renal hypouricemia in Japanese. Clin. Genet. 2008, 74, 243–251. [Google Scholar] [CrossRef]
- Miyazaki, S.; Hamada, T.; Isoyama, T.; Okada, S.; Tomita, K.; Endo, Y.; Kuwabara, M.; Sugihara, S.; Ogino, K.; Ninomiya, H.; et al. Characterization of Urate Metabolism and Complications of Patients with Renal Hypouricemia. Intern. Med. 2023, 0457-22. [Google Scholar] [CrossRef]
- Prien, E.L. Crystallographic analysis of urinary calculi: A 23-year survey study. J. Urol. 1963, 89, 917–924. [Google Scholar] [CrossRef]
- Yü, T.; Gutman, A.B. Uric acid nephrolithiasis in gout. Predisposing factors. Ann. Intern. Med. 1967, 67, 1133–1148. [Google Scholar] [CrossRef]
- Gutman, A.B.; Yue, T.F. Urinary ammonium excretion in primary gout. J. Clin. Investig. 1965, 44, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Hisatome, I.; Tanaka, Y.; Kotake, H.; Kosaka, H.; Hirata, N.; Fujimoto, Y.; Yoshida, A.; Shigemasa, C.; Mashiba, H.; Sato, R.; et al. Renal hypouricemia due to enhanced tubular secretion of urate associated with urolithiasis; successful treatment of urolithiasis by alkalization of urine K+, Na+-citrate. Nephron 1993, 65, 578–582. [Google Scholar] [CrossRef]
- Hisatome, I.; Tanaka, Y.; Ogino, K.; Shimoyama, M.; Hiroe, K.; Tsuboi, M.; Yamamoto, Y.; Hamada, N.; Kato, T.; Manabe, I.; et al. Hematuria in patients with renal hypouricemia. Intern. Med. 1998, 37, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hisatome, I.; Tanaka, Y.; Tsuboi, M.; Yatsuhashi, T.; Ogino, K.; Uchida, T.; Yamanouchi, Y.; Shimoyama, M.; Fujita, S.; Kinugawa, T.; et al. Excess urate excretion correlates with severely acidic urine in patients with renal hypouricemia. Intern. Med. 1998, 37, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Dong, B.; Geng, Z.; Xu, L. Excess Uric Acid Induces Gouty Nephropathy Through Crystal Formation: A Review of Recent Insights. Front. Endocrinol. 2022, 13, 911968. [Google Scholar] [CrossRef] [PubMed]
- Lusco, M.A.; Chen, Y.-P.; Cheng, H.; Dong, H.-R.; Najafian, B.; Alpers, C.E.; Fogo, A.B. AJKD Atlas of Renal Pathology: Fibronectin Glomerulopathy. Am. J. Kidney Dis. 2017, 70, e21–e22. [Google Scholar] [CrossRef]
- Bardin, T.; Nguyen, Q.D.; Tran, K.M.; Le, N.H.; Do, M.D.; Richette, P.; Letavernier, E.; Correas, J.-M.; Resche-Rigon, M. A cross-sectional study of 502 patients found a diffuse hyperechoic kidney medulla pattern in patients with severe gout. Kidney Int. 2021, 99, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Roughley, M.J.; Belcher, J.; Mallen, C.D.; Roddy, E. Gout and risk of chronic kidney disease and nephrolithiasis: Meta-analysis of observational studies. Arthritis Res. Ther. 2015, 17, 90–101. [Google Scholar] [CrossRef]
- Krishnan, E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS ONE 2012, 7, e50046. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Comorbidities of gout and hyperuricemia in the us general population: NHANES 2007–2008. Am. J. Med. 2012, 125, 679–687.e1. [Google Scholar] [CrossRef]
- Kohagura, K.; Kochi, M.; Miyagi, T.; Kinjyo, T.; Maehara, Y.; Nagahama, K.; Sakima, A.; Iseki, K.; Ohya, Y. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: A biopsy-based study. Hypertens. Res. 2013, 36, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Berni, A.; Boddi, M.; Fattori, E.B.; Cecioni, I.; Berardino, S.; Montuschi, F.; Chiostri, M.; Poggesi, L. Serum uric acid levels and renal damage in hyperuricemic hypertensive patients treated with renin-angiotensin system blockers. Am. J. Hypertens. 2010, 23, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Nishizawa, M.; Kiuchi, M.; Kiyosue, A.; Tomita, F.; Ohtani, H.; Abe, Y.; Kuga, H.; Miyazaki, S.; Kasai, T.; et al. Comparative effects of topiroxostat and febuxostat on arterial properties in hypertensive patients with hyperuricemia. J. Clin. Hypertens. 2021, 23, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Go, S.; Son, H.E.; Ryu, J.Y.; Lee, H.; Heo, N.J.; Chin, H.J.; Park, J.H. Association between Serum Uric Acid Level and ESRD or Death in a Korean Population. J. Korean Med. Sci. 2020, 35, e254. [Google Scholar] [CrossRef]
- Masi, S.; Georgiopoulos, G.; Alexopoulos, G.; Pateras, K.; Rosada, J.; Seravalle, G.; De Ciuceis, C.; Taddei, S.; Borghi, C.; Grassi, G.; et al. The Complex Relationship Between Serum Uric Acid, Endothelial Function and Small Vessel Remodeling in Humans. J. Clin. Med. 2020, 9, 2027. [Google Scholar] [CrossRef]
- Mannarino, M.R.; Pirro, M.; Gigante, B.; Savonen, K.; Kurl, S.; Giral, P.; Smit, A.; Veglia, F.; Tremoli, E.; Baldassarre, D.; et al. Association Between Uric Acid, Carotid Intima-Media Thickness, and Cardiovascular Events: Prospective Results From the IMPROVE Study. J. Am. Heart Assoc. 2021, 10, e020419. [Google Scholar] [CrossRef]
- Verdecchia, P.; Schillaci, G.; Reboldi, G.; Santeusanio, F.; Porcellati, C.; Brunetti, P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000, 36, 1072–1078. [Google Scholar] [CrossRef]
- Mazza, A.; Zamboni, S.; Rizzato, E.; Pessina, A.C.; Tikhonoff, V.; Schiavon, L.; Casiglia, E. Serum uric acid shows a J-shaped trend with coronary mortality in non-insulin-dependent diabetic elderly people. The CArdiovascular STudy in the ELderly (CASTEL). Acta Diabetol. 2007, 44, 99–105. [Google Scholar] [CrossRef]
- White, W.B.; Saag, K.G.; Becker, M.A.; Borer, J.S.; Gorelick, P.B.; Whelton, A.; Hunt, B.; Castillo, M.; Gunawardhana, L. Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. N. Engl. J. Med. 2018, 378, 1200–1210. [Google Scholar] [CrossRef]
- de Leeuw, P.W.; Thijs, L.; Birkenhäger, W.H.; Voyaki, S.M.; Efstratopoulos, A.D.; Fagard, R.H.; Leonetti, G.; Nachev, C.; Petrie, J.C.; Rodicio, J.L.; et al. Prognostic significance of renal function in elderly patients with isolated systolic hypertension. J. Am. Soc. Nephrol. 2002, 13, 2213–2222. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, W.; Suh, Y.J.; Lim, M.J.; Kwon, S.-R.; Lee, J.-H.; Bin Joo, Y.; Oh, Y.-K.; Jung, K.-H. Association of Serum Uric Acid with Cardiovascular Disease Risk Scores in Koreans. Int. J. Environ. Res. Public Health 2019, 16, 4632. [Google Scholar] [CrossRef] [PubMed]
- Warning, W.S.; Webb, D.J.; Maxwell, S.R.J. Effect of local hyperuricemia on endothelial function in the human vascular bed. Br. J. Clin. Pharmacol. 2000, 59, 511. [Google Scholar]
- Kato, M.; Hisatome, I.; Tomikura, Y.; Kotani, K.; Kinugawa, T.; Ogino, K.; Ishida, K.; Igawa, O.; Shigemasa, C.; Somers, V.K. Status of endothelial dependent vasodilation in patients with hyperuricemia. Am. J. Cardiol. 2005, 96, 1576–1578. [Google Scholar] [CrossRef]
- Mishima, M.; Hamada, T.; Maharani, N.; Ikeda, N.; Onohara, T.; Notsu, T.; Ninomiya, H.; Miyazaki, S.; Mizuta, E.; Sugihara, S.; et al. Effects of Uric Acid on the NO Production of HUVECs and its Restoration by Urate Lowering Agents. Drug Res. 2016, 66, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Anzai, N.; Ichida, K.; Jutabha, P.; Kimura, T.; Babu, E.; Jin, C.J.; Srivastava, S.; Kitamura, K.; Hisatome, I.; Endou, H.; et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J. Biol. Chem. 2008, 283, 26834–26838. [Google Scholar] [CrossRef]
- Nakamura, M.; Anzai, N.; Jutabha, P.; Sato, H.; Sakurai, H.; Ichida, K. Concentration-dependent inhibitory effect of irbesartan on renal uric acid transporters. J. Pharmacol. Sci. 2010, 114, 115–118. [Google Scholar] [CrossRef]
- Stamp, L.K.; Farquhar, H.; Pisaniello, H.L.; Vargas-Santos, A.B.; Fisher, M.; Mount, D.B.; Choi, H.K.; Terkeltaub, R.; Hill, C.L.; Gaffo, A.L. Management of gout in chronic kidney disease: A G-CAN Consensus Statement on the research priorities. Nat. Rev. Rheumatol. 2021, 17, 633–641. [Google Scholar] [CrossRef]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.E.; Hare, J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004, 555, 589–606. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Matsumoto, M.; Tanaka, M.; Moniwa, N.; Murase, T.; Nakamura, T.; Ohnishi, H.; Saitoh, S.; Shimamoto, K.; Miura, T. Plasma Xanthine Oxidoreductase Activity as a Novel Biomarker of Metabolic Disorders in a General Population. Circ. J. 2018, 82, 1892–1899. [Google Scholar] [CrossRef]
- Houston, M.; Estevez, A.; Chumley, P.; Aslan, M.; Marklund, S.; Parks, D.A.; Freeman, B.A. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide-dependent signaling. J. Biol. Chem. 1999, 274, 4985–4994. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Brock, T.A.; Chang, L.Y.; Crapo, J.; Briscoe, P.; Ku, D.; Bradley, W.A.; Gianturco, S.H.; Gore, J.; Freeman, B.A. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. USA 1994, 91, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Washio, K.; Kusunoki, Y.; Tsunoda, T.; Osugi, K.; Ohigashi, M.; Murase, T.; Nakamura, T.; Matsuo, T.; Konishi, K.; Katsuno, T.; et al. Xanthine oxidoreductase activity correlates with vascular endothelial dysfunction in patients with type 1 diabetes. Acta Diabetol. 2020, 57, 31–39. [Google Scholar] [CrossRef]
- Terawaki, H.; Hayashi, T.; Murase, T.; Iijima, R.; Waki, K.; Tani, Y.; Nakamura, T.; Yoshimura, K.; Uchida, S.; Kazama, J.J. Relationship between Xanthine Oxidoreductase Redox and Oxidative Stress among Chronic Kidney Disease Patients. Oxidative Med. Cell. Longev. 2018, 2018, 9714710. [Google Scholar] [CrossRef]
- Miyazaki, S.; Hamada, T.; Sugihara, S.; Mizuta, E.; Endo, Y.; Ohtahara, A.; Komatsu, K.; Kuwabara, M.; Fukuuchi, T.; Kaneko, K.; et al. Xanthinuria Type 1 with a Novel Mutation in Xanthine Dehydrogenase and a Normal Endothelial Function. Intern. Med. 2022, 61, 1383–1386. [Google Scholar] [CrossRef]
- Bhasin, B.; Stiburkova, B.; De Castro-Pretelt, M.; Beck, N.; Bodurtha, J.N.; Atta, M.G. Hereditary renal hypouricemia: A new role for allopurinol? Am. J. Med. 2014, 127, e3–e4. [Google Scholar] [CrossRef]
- Yeun, J.Y.; Hasbargen, J.A. Renal hypouricemia: Prevention of exercise-induced acute renal failure and a review of the literature. Am. J. Kidney Dis. 1995, 25, 937–946. [Google Scholar] [CrossRef]
- Hosoya, T.; Uchida, S.; Shibata, S.; Tomioka, N.H.; Matsumoto, K.; Hosoyamada, M. Xanthine Oxidoreductase Inhibitors Suppress the Onset of Exercise-Induced AKI in High HPRT Activity Urat1-Uox Double Knockout Mice. J. Am. Soc. Nephrol. 2022, 33, 326–341. [Google Scholar] [CrossRef]
- De Becker, B.; Coremans, C.; Chaumont, M.; Delporte, C.; Van Antwerpen, P.; Franck, T.; Rousseau, A.; Zouaoui Boudjeltia, K.; Cullus, P.; van de Borne, P. Severe Hypouricemia Impairs Endothelium-Dependent Vasodilatation and Reduces Blood Pressure in Healthy Young Men: A Randomized, Placebo-Controlled, and Crossover Study. J. Am. Heart Assoc. 2019, 8, e013130. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, C.M.; Kelley, W.N. The clinical significance of hypouricemia. Ann. Intern. Med. 1973, 78, 239–242. [Google Scholar] [CrossRef]
- Ogino, K.; Hisatome, I.; Saitoh, M.; Miyamoto, J.; Ishiko, R.; Hasegawa, J.; Kotake, H.; Mashiba, H. Clinical significance of hypouricemia in hospitalized patients. J. Med. 1991, 22, 76–82. [Google Scholar]
- Bugdayci, G.; Balaban, Y.; Sahin, O. Causes of hypouricemia among outpatients. Lab. Med. 2008, 39, 550–552. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Tazawa, C.; Ono, T.; Sugitani, S.; Tomita KHisatome, I. Hypouricemia in the admitted patients. In Proceedings of the 76th Annual Meeting of Japanese Society of National Medical Services, Kumamoto, Japan, 7–8 October 2022; Volume 76, p. 56. [Google Scholar]
- Cang, Y.; Xu, S.; Zhang, J.; Ju, J.; Chen, Z.; Wang, K.; Li, J.; Xu, Y. Serum Uric Acid Revealed a U-Shaped Relationship With All-Cause Mortality and Cardiovascular Mortality in High Atherosclerosis Risk Patients: The ASSURE Study. Front. Cardiovasc. Med. 2021, 8, 641513. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincón, A.; Arroyo, D.; Luño, J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, W.; Jalal, D.; Li, Z.; Chen, W.; Mao, H.; Yang, Q.; Johnson, R.J.; Yu, X. Clinical outcome of hyperuricemia in iga nephropathy: A retrospective cohort study and randomized controlled trial. Kidney Blood Press. Res. 2011, 35, 153–160. [Google Scholar] [CrossRef]
- Hosoya, T.; Ohno, I.; Nomura, S.; Hisatome, I.; Uchida, S.; Fujimori, S.; Yamamoto, T.; Hara, S. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin. Exp. Nephrol. 2014, 18, 876–884. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Wang, B.; Zhang, F.; Wang, D.; Wang, Y. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin. Endocrinol. 2015, 83, 475–482. [Google Scholar] [CrossRef]
- Kojima, S.; Matsui, K.; Hiramitsu, S.; Hisatome, I.; Waki, M.; Uchiyama, K.; Yokota, N.; Tokutake, E.; Wakasa, Y.; Jinnouchi, H.; et al. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur. Heart J. 2019, 40, 1778–1786. [Google Scholar] [CrossRef]
- Sircar, D.; Chatterjee, S.; Waikhom, R.; Golay, V.; Raychaudhury, A.; Chatterjee, S.; Pandey, R. Efficacy of Febuxostat for Slowing the GFR Decline in Patients With CKD and Asymptomatic Hyperuricemia: A 6-Month, Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Kidney Dis. 2015, 66, 945–950. [Google Scholar] [CrossRef]
- Siu, Y.-P.; Leung, K.-T.; Tong, M.K.-H.; Kwan, T.-H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Whelton, A.; Becker, M.A.; MacDonald, P.; Hunt, B.; Gunawardhana, L. Impact of Febuxostat on Renal Function in Gout Patients With Moderate-to-Severe Renal Impairment. Arthritis Rheumatol. 2016, 68, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.; Galecki, A.T.; Spino, C.; Pop-Busui, R.; Cherney, D.Z.; Lingvay, I.; Parsa, A.; Rossing, P.; Sigal, R.J.; Afkarian, M.; et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N. Engl. J. Med. 2020, 382, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.V.; Pascoe, E.M.; Tiku, A.; Boudville, N.; Brown, F.G.; Cass, A.; Clarke, P.; Dalbeth, N.; Day, R.O.; de Zoysa, J.R.; et al. Effects of allopurinol on the progression of chronic kidney disease. N. Engl. J. Med. 2020, 382, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Pyne, L.; Walsh, M. Allopurinol: Good for gout but not for preventing loss of kidney function. Am. J. Kidney Dis. 2021, 77, 459–461. [Google Scholar] [CrossRef]

| Reports | Subjects | Total | Male | Female | Transient | Persistent |
|---|---|---|---|---|---|---|
| Wakasugi et al. (2015) [14] | Health checkup | 0.21% (n = 90,710) | 0.39% (n = 136,935) | |||
| Tabe et al. (2015) [18] | Medical checkup | 0.14% (n = 17,603) | 0.4% (n = 3544) | |||
| Kaneko et al. (1995) [19] | Medical checkup | 0.09~0.12% | 0.36~0.51% | |||
| Matsuo et al. (2008) [15] | Self-defense forces | 0.18% (n = 21,260) | ||||
| Hisatome et al. (1989) [17] | Outpatients | 0.4% (n = 3258) | 0.25% | 0.15% |
| Reports | Objects | Total | Male | Female | Transient | Persistent | Unclassified |
|---|---|---|---|---|---|---|---|
| Ramsdel et al. [72] | Admitted patients | 0.97% (n = 6629) | |||||
| Ogino et al. [73] | Admitted patients | 2.54% (n = 1220) | 2.26% (n = 708) | 2.93% (n = 512) | |||
| Matsumoto et al. [74] | Admitted patients | 11.1% (n = 675) | 11.5% (n = 419) | 10.5% (n = 256) | 3.3% (22/675) | 1.0% (7/675) | 6.8% (46/675) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miake, J.; Hisatome, I.; Tomita, K.; Isoyama, T.; Sugihara, S.; Kuwabara, M.; Ogino, K.; Ninomiya, H. Impact of Hyper- and Hypo-Uricemia on Kidney Function. Biomedicines 2023, 11, 1258. https://doi.org/10.3390/biomedicines11051258
Miake J, Hisatome I, Tomita K, Isoyama T, Sugihara S, Kuwabara M, Ogino K, Ninomiya H. Impact of Hyper- and Hypo-Uricemia on Kidney Function. Biomedicines. 2023; 11(5):1258. https://doi.org/10.3390/biomedicines11051258
Chicago/Turabian StyleMiake, Junichiro, Ichiro Hisatome, Katsuyuki Tomita, Tadahiro Isoyama, Shinobu Sugihara, Masanari Kuwabara, Kazuhide Ogino, and Haruaki Ninomiya. 2023. "Impact of Hyper- and Hypo-Uricemia on Kidney Function" Biomedicines 11, no. 5: 1258. https://doi.org/10.3390/biomedicines11051258
APA StyleMiake, J., Hisatome, I., Tomita, K., Isoyama, T., Sugihara, S., Kuwabara, M., Ogino, K., & Ninomiya, H. (2023). Impact of Hyper- and Hypo-Uricemia on Kidney Function. Biomedicines, 11(5), 1258. https://doi.org/10.3390/biomedicines11051258







