Abstract
Acute myeloid leukemia (AML) is a hematologic malignancy that occurs due to alterations such as genetic mutations, chromosomal translocations, or changes in molecular levels. These alterations can accumulate in stem cells and hematopoietic progenitors, leading to the development of AML, which has a prevalence of 80% of acute leukemias in the adult population. Recurrent cytogenetic abnormalities, in addition to mediating leukemogenesis onset, participate in its evolution and can be used as established diagnostic and prognostic markers. Most of these mutations confer resistance to the traditionally used treatments and, therefore, the aberrant protein products are also considered therapeutic targets. The surface antigens of a cell are characterized through immunophenotyping, which has the ability to identify and differentiate the degrees of maturation and the lineage of the target cell, whether benign or malignant. With this, we seek to establish a relationship according to the molecular aberrations and immunophenotypic alterations that cells with AML present.
1. Introduction
Acute myeloid leukemia (AML) is a malignant disorder of hematopoietic stem cells that emerges due to genetic alterations, such as genetic mutations, chromosomal translocations, or changes in molecular levels. This disease is characterized by clonal expansion of abnormally differentiated blasts of a myeloid lineage [1,2,3]. The increased proliferation of immature myeloid cells causes the accumulation of blasts and impairs normal hematopoiesis, which may lead to hemorrhages, anemia, and severe and persistent infections. These are known to be the most common symptoms in the majority of patients with acute leukemias [3,4].
Although the exact cause of genetic mutations is unclear, the development of AML is associated with some risk factors, such as exposure to radiation, pesticides, chemotherapeutic agents, and smoking. AML can also evolve from other hematological diseases, including myelodysplastic syndrome (MDS), myeloproliferative syndromes (MPS), and aplastic anemia. AML risk also increases for people with some congenital disorders, such as Down syndrome and Bloom syndrome [3,5,6,7].
AML is the most frequent leukemia in adults, accounting for about 80% of acute leukemia cases in this population. It mostly affects male patients, with incidence rates increasing with age. Per year, the number of new AML cases is 4.2 per 100,000 population [3,4,8,9,10].
2. Genetic Alterations in AML
In AML, recurrent cytogenetic abnormalities are established diagnostic and prognostic markers; they can be therapeutically targetable since most of these mutations confer resistance to the therapeutic options used traditionally [11,12,13].
The European Leukemia Net (ELN) and World Health Organization (WHO) guidelines are probably the most widely used sources for assessing the risk of resistance and classifying patients into favorable, intermediate, and adverse groups on the basis of leukemia cell cytogenetics and mutations (Figure 1) [14,15]. The main cytogenetic–molecular entities in AML are translocations 15;17 (t(15;17)(q22,q21)), 8;21 (t(8;21)(q22;q22.1)), and 9;22 (t(9;22)(q34.1;q11.2)); inversions 16 (inv(16)(p13q22) or t(16;16)(p13;q22)) and 3 (inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)); mutations in TP53, NPM1, FLT3, and IDH; complex karyotypes; and others.
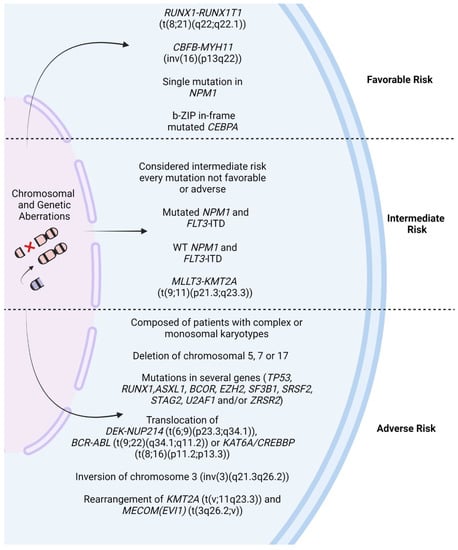
Figure 1.
Cytogenetic and chromosomal abnormalities associated with prognostic risk groups in acute myeloid leukemia (AML). RUNX1: RUNX family transcription factor 1; RUNX1T1: RUNX1 partner transcriptional co-repressor 1; CBFB: core-binding factor subunit beta; MYH11: myosin heavy chain 11; CEBPA: CCAAT enhancer binding protein alpha; NPM1: nucleophosmin 1; bZIP: basic leucine zipper domain; FLT3-ITD: fms-related receptor tyrosine kinase 3—internal tandem duplication; WT: wild-type; KMT2A: lysine methyltransferase 2A; MLLT3: MLLT3 super elongation complex subunit; TP53: tumor protein p53; ASXL1: ASXL transcriptional regulator 1; BCOR: BCL6 corepressor; EZH2: enhancer of zeste 2 polycomb repressive complex 2 subunit; SF3B1: splicing factor 3b subunit 1; SRSF2: serine and arginine rich splicing factor 2; STAG2: stromal antigen 2; U2AF1: U2 small nuclear RNA auxiliary factor 1; ZRSR2: zinc finger CCCH-type, RNA-binding motif and serine/arginine rich 2; DEK: DEK proto-oncogene; NUP214: nucleoporin 214; BCR: BCR activator of RhoGEF and GTPase; ABL: ABL proto-oncogene 1; KAT6A: lysine acetyltransferase 6A; CREBBP: CREB binding protein; KMT2A: histone-lysine N-methyltransferase 2A; MECOM (EVI1): ecotropic virus integration site 1 protein homolog. Created with Biorender.com.
2.1. Favorable Risk Genetic Alterations
Core-binding factor (CBF) is a transcription factor that plays critical roles in hematopoietic stem cell (HSC) maintenance and differentiation. CBF has the ability to regulate the expression of genes associated with cellular proliferation and death. In general, this factor is characterized by the presence of either t(8;21) (q22;q22) or inv(16)(p13q22), which leads to the formation of fusion genes that are associated with a favorable response to treatment (RUNX1-RUNX1T1 and CBFB-MYH11, respectively) [16,17,18].
According to the ELN guidelines, patients who have mutations in CBF, such as translocation 8;21 (t(8;21)(q22;q22.1)) and inversion 16 (inv(16)(p13q22) or t(16;16)(p13;q22)), are classified as having favorable risk, along with patients with a single mutation in NPM1 or bZIP in-frame mutated CEBPA [14].
The translocation t(8;21)(q22;q22.1) results in a gene fusion between the RUNX1 and RUNX1T1 genes, deriving the RUNX1-RUNXT1 hybrid gene. This cytogenetic aberration is especially identified in patients with M2 AML according to the French–American–British classification (FAB) [19]. Due to high rates of complete remission after conventional treatments, the presence of this translocation came to be considered a good prognostic factor and an indicator of favorable outcomes for patients [16,20,21,22].
The inversion inv(16)(p13q22) and the translocation t(16;16)(p13q22) result in the formation of the chimeric gene CBFB-MYH11, which encodes the fusion protein CBFb-SMMHC. The inv(16) mutation is reported in almost all patients with the M4Eo (M4 with eosinophilia) AML subtype according to FAB, which constitutes about 5–8% of all patients with AML. Just like RUNX1-RUNXT1, CBFB-MYH11 is also considered a cytogenetic alteration that confers a favorable prognosis in afflicted patients [23,24,25,26].
Mutations in the CEBPA gene are present in 10–15% of AML patients. The CEBPA gene is located on chromosome 19 and encodes CCAAT enhancer binding protein alpha (CEBPa), which is a crucial transcription factor for stem and progenitor cell function and the regulation of myeloid cell differentiation. CEBPa mutations in the basic leucine zipper domain (bZIP) have been associated with a favorable prognosis; they are an indicator of a higher chance of achieving complete remission (CR), better overall survival (OS), and a lower risk of relapse [27,28,29,30,31,32,33].
The nucleophosmin (NPM1) gene is located on chromosome 5 and encodes a protein that is involved in critical cell function by playing key roles in ribosome biogenesis, centrosome duplication, genomic stability, cell cycle progression, and apoptosis. NPM1 is the most commonly mutated gene in adult AML, occurring in approximately 30% of all cases, and it is considered to be a prognostic factor of favorable risk [15,34,35,36,37,38,39,40].
2.2. Intermediate Risk Genetic Alterations
The ELN defines intermediate-risk patients as those with cytogenetic abnormalities not qualified as favorable or adverse, with mutated NPM1 co-occurring with a low level of FLT3-ITD or wild-type NPM1 with the co-occurrence of FLT3-ITD and the translocation (t(9;11)(p21.3;q23.3)) [41].
The detection of the FLT3-ITD mutation is documented in about 40% of NPM1-mutated AML cases. The deleterious prognostic effects of FLT3-ITD have previously been found to be most clinically relevant when co-occurring with NPM1 mutations, which are characterized by a higher relapse rate and poorer OS [35,37,42,43].
In AML, translocation 9;11 (t(9;11)(p22;q23)) results in the KMT2A-MLLT3 fusion protein. This translocation is the most common one involving KMT2A, and it can be found in some types of AML, such as de novo and therapy-related AML (t-AML). Usually, the presence of KMT2A-MLLT3 fusion is correlated with a poorer prognosis and more complex cytogenetics [43,44,45].
2.3. Adverse Risk Genetic Alterations
According to the ELN, the high-risk group is composed of patients with complex or monosomal karyotypes; inversion of chromosome 3 (inv(3)(q21.3q26.2)); rearrangement of KMT2A (t(v;11q23.3)) or MECOM(EVI1) (t(3q26.2;v)); translocations 6;9 (1(6;9)(p23.3;q34.1)), 9;22 (t(9;22)(q34.1;q11.2)), and 8;16 (t(8;16)(p11.2;p13.3)); deletions of chromosome 5, 7, or 17; or mutations in several genes, such as TP53, RUNX1, ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, and/or ZRSR2 [14,41,43].
The translocation t(6;9)(p23;q34) generates the fusion gene DEK-NUP214. It is a relatively rare mutation in AML and is associated with a poorer prognosis. Most cases reported in the literature are commonly found in AML M2 and M4 according to FAB classification, although this alteration can also be identified in AML M1 in a few cases [46,47,48].
The t(9;22) chromosome translocation originating from the BCR-ABL1 fusion oncoprotein is detected most commonly in chronic myeloid leukemia (CML), occurring in approximately 95% of the cases and representing the hallmark of this disease. However, t(9;22) can also be found in 10 to 20% of adults and 2 to 5% of children with acute lymphoblastic leukemia (ALL), as well as in rare cases (1% approximately) of AML. The prognosis of BCR-ABL-mutated AML seems to depend on the cytogenetic and/or molecular background rather than BCR-ABL itself. Cases of AML with this mutation can be often challenging to distinguish from the presentation of CML in a blast crisis [49,50,51].
The TP53 protein has an impact on various cellular functions, especially DNA repair and cellular senescence, and the inactivation of TP53 by either mutation or deletion increases the proliferation of cancer cells. In AML, mutated TP53 is reported in patients with older age, lower blast counts, adverse risk karyotypes, and exposure to antecedent chemotherapy. Overall, this genetic aberration may occur across nearly all FAB subtypes. The TP53 mutation associated with AML represents a distinct subgroup associated with a poorer prognosis since this mutation predicts inferior OS and resistance to cytotoxic chemotherapies [52,53,54].
Notably, all these genetic aberrations have an impact on the patient’s diagnosis, treatment, and prognosis. Thus, the development of novel targeted therapies that may help deal with these cytogenetic alterations in a specific and individualized way is essential.
3. Immunophenotyping in Acute Myeloid Leukemia Cells
The antigens that summarize the immunophenotype of a cell are the indicators of its identity, making it possible to characterize its degree of maturation and lineage; the main surface antigens of myeloid lines are CD11b, CD13, CD14, CD15, and CD33. From the phenotypic alterations that occur in a neoplastic clone, the surface antigens also undergo changes that can be used to identify and characterize these cells. Due to the variability of immunophenotypic changes present in leukemic cells, they can be categorized into groups: expression of crossline antigens, asynchronous expression of maturational markers, expression of decreased or absent antigens, and overexpression of antigens [21,55,56].
For AML, diagnostic tools include cytomorphology, cytochemistry, immunophenotyping, cytogenetics, and molecular genetics techniques, all of which complement each other to classify the disease in view of its intrinsic heterogeneity. However, among these techniques, immunophenotyping is of great importance, as it is crucial for the detection, characterization, and quantification of cells and can provide a useful line of markers for differential diagnosis and classification. Noting that leukemic cells have immunophenotypic aberrations, this technique allows the identification of acute leukemias of mixed lineage while also being able to suggest possible genetic changes [57,58,59].
Regarding the identification of antigens for the diagnosis of AML, they are classified as precursors (CD34, CD117, HLA-DR), myeloid markers (cMPO, CD33, CD13), myeloid maturation markers (CD11b, CD15, CD64, CD4, CD38, CD11c), monocytic markers (CD14, CD36, CD64), megakaryocytic markers (CD41, CD36, CD61), and erythrocyte markers (CD235, CD71, CD36) [14,57,60,61].
Immunophenotyping also plays a fundamental role in the evaluation of treatment response and the detection of minimal/measurable residual disease (MRD), for which the identification of antigens defined at the time of diagnosis is sought and their presence is evaluated during and/or after treatment [3,62].
MRD can be performed at two moments: right after the beginning of the induction of treatment (to evaluate the kinetics of the response to the disease) and after the end of treatment (to evaluate the possibility of recurrence). The means of detection of MRD have evolved with advances in therapies, and today, the approaches used for its evaluation are multiparametric flow cytometry (MFC) and molecular approaches, such as real-time quantitative PCR (qPCR), digital PCR (dPCR), and next-generation sequencing (NGS) [63,64,65].
Advances in diagnostic techniques have led to improvements in not only the prognosis and monitoring of the disease but also the classification of AML with the precise identification of mutations. However, the application of MFC and qRT-PCR/NGS in combination still needs to be better investigated in order for it to become a feasible approach in AML [57,64].
4. Immunophenotyping x Genetics in AML
Identifying correlations between immunophenotypic markers and genetic changes may provide more a precise diagnosis and better targeted treatment strategies for AML patients. In this context, Table 1 comprises a series of clinical trials with published results that, in the past 10 years, described genetic alterations and/or clinical features and immunophenotypic characteristics at diagnosis. These studies were found in the PubMed database from research using the descriptors “acute myeloid leukemia”, “immunophenotyping”, “genetic alterations”, and “prognosis”.
Currently, the wide application of immunophenotyping and genetic characterization has become critical for clinicopathological evaluations, diagnostic refinement, risk group classification, prognosis prediction, and promotion of targeted therapy in AML patients [66,67,68].


Table 1.
Studies from the last 10 years indicating relationships between immunophenotyping and genetic changes in AML patients.
Table 1.
Studies from the last 10 years indicating relationships between immunophenotyping and genetic changes in AML patients.
| Number of Patients | Mean Age | Immunophenotypic Characteristics | Genetic Alterations | References |
|---|---|---|---|---|
| 41 (71% males and 29% females) | 66 | CD45, CD34, CD117, CD36, HLA-DR, CD13, CD33, CD7, CD71 | Deletions involving chromosome 5/5q; deletions involving chromosome 7/7q; deletion of the TP53 locus (17p13) | [69] |
| 90 patients | Aged > 60 years | CD123, CD34, CD7, CD117, CD64, CD38, CD11b, CD33, CD56 | TET2, DNMT3A, IDH2, NPM1, FLT3-ITD, CEBPA, ASXL1, IDH1, SRSF2, BCOR, TP53, NRAS, RUNX1, U2AF1, BCORL1, WT1, and FLT3-TKD. | [70] |
| 67 (26 males and 41 females) | 64 (19–84) | CD34, CD117, HLA-DR | NPM1 p. W288fs DNMT3A, FLT3, TET2, IDH2, PTPN11, IDH1, NRAS, SRSF2, RAD21, WT1, ASXL1, and NF1 | [71] |
| 1040 (51.92% males and 48.08% females) | 10.3 | CD123 | t(8;21), inv(16), CEBPA, NPM1, FLT3-ITD; monosomy 7, monosomy 5/del(5q), t(6;9) with DEK-NUP214 fusion, KMT2A rearrangements | [72] |
| 37 Males: 20 (54%), Females: 17 (46%) | 54 (24–70) | CD8+CD45RA− CD27+/int CD28+ PD1+ TCF1+ | NPM1, DNMT3A, ASXL1, IDH2, TP53, CEBPA, NRAS, WT1 | [73] |
| 84 (51 females and 33 males) | 65.5 (22–89) | CD34-, HLA-DR-, CD117+, CD11b-, CD56+, CD13 | FLT3-ITD, FLT3-TKD, DNMT3A, NPM1, TET2, IDH1, IDH2 | [74] |
| 769 patients | NR | CD56, CD34, CD7, CD11b, CD117, CD13, SSC, CD33, CD45, CD38, FSC, HLA-DR | FAB, inv(16), t(8;21), cKit, 11q23, FLIT3, NPM1, CEBPA, HT1, GLIS2 | [75] |
| 217 patients | NR | CD34+ CD38− CD90− CD45RA+ | RUNX1 DNMT3A IDH2 TET2 | [76] |
| 174 (153 adults and 21 children | Adults aged 20–85 years and children aged < 20 years | CD34, CD7, HLA-DR | NPM1, FLT3-ITD, FLT3-D835, DNMT3A, NPM1 | [77] |
| 13 (11 males and 2 females) | 68 | CD13+, CD34+, HLA-DR+, CD33 | addX, del7, del5/5q, and add19 | [78] |
AML: acute myeloid leukemia; CD: cluster of differentiation; HLA-DR: human leukocyte antigens; NR: not reported; ITD: internal tandem domain; TKD: tyrosine kinase domain; ADD: addition; DEL: deletion; NT/T: near-tetraploidy/tetraploidy; APL: acute promyelocytic leukemia.
All 10 articles described in Table 1 reported the main genetic alterations and the immunophenotyping results of AML patients enrolled in each study. Of all the reported cytogenetic abnormalities, the most frequent were mutations involving NPM1 (7), FLT3 (7), and DNMT3A (6). Regarding immunophenotyping results, the most frequent immunophenotyping markers were CD34 (7), HLA-DR (5), CD45 (4), and CD13 (4) [69,70,71,72,73,74,75,76,77,78].
The NPM1 mutation was one of the most frequent genetic aberrations detected in the populations of the different studies (7/10), and it is indeed the most frequent in AML cases, usually representing a favorable prognosis when presented as a single mutation [14,70,71,72,73,74,75,77]. However, as previously stated, these mutations can be commonly associated with mutations in other genes, such as DNMT3A, FLT3, TET2, IDH1, and IDH2, all of which directly influence AML leukemogenesis progression [71,76]. It was possible to observe a significant relationship between the mutation of the NPM1 gene and a lower expression of CD34+ or CD34 expression, indicating a favorable prognosis in patients with this profile, as observed by Dohner et al. in 2005 [79].
In addition, CD34+ expression in more than 10% of blast cells and/or leukemic stem cells (LSCs) is reported in approximately 75% of cases of AML. However, in other cases in which this expression does not occur, it is possible to observe NPM1 mutations [61,70,71,77,79]. Some studies have associated NPM1-mutated AML with CD117+ blasts with increased expression of CD33 and/or CD123 and decreased expression or absence of CD13, HLA-DR, CD15, and CD64, along with CD34 [80,81].
Mutated FLT3 was reported in 7 of the 10 studies in Table 1 [70,71,72,73,74,75,77]. Although mutations may manifest as either internal tandem duplication of the juxta membrane domain (FLT3-ITD) or point mutations in the tyrosine kinase domain (FLT3TKD), FLT3-ITD is the manifestation more highly associated with a worse prognosis in AML patients, predicting shorter remission and decreased OS [82,83]. Some studies have identified that the phenotypes CD34, CD123, CD25, and CD99 predict both FLT3-ITD and FLT3-ITDmut. This correlation could lead to the utilization of targeted therapies with specific inhibitors [84,85]. Furthermore, higher rates of FLT3-ITD mutations were associated with a higher expression of the CD33 phenotype in the study of Feldman et al. [86], leading to a shorter duration of remission [87,88,89].
Alterations in DNMT3A were present in patients in five different studies described in Table 1, and they are also common mutations in early leukemic stages that confer proliferative advantages to pre-leukemic cells [56]. DNMT3A activity on gene methylation directly influences AML development, as either hypermethylation of tumor suppressors or hypomethylation of oncogenes might act as drivers of leukemogenesis onset [90]. However, although many mutations in DNMT3A are cataloged, few have been thoroughly investigated, with point mutations in the catalytic domain being the major players associated with AML drivers [91]. Other studies have shown that patients with DNMT3A mutation present higher levels of HLA- DR, CLIP, PD-L1, and TIM-3 and lower levels of CD34 (Figure 2) [89,92,93].
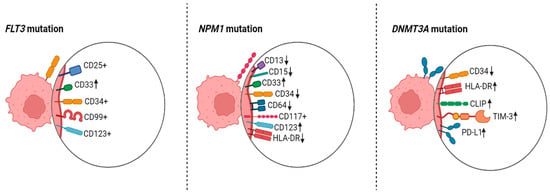
Figure 2.
Commonly reported genetic mutations and associated immunophenotypic characteristics in acute myeloid leukemia (AML) neoplastic clones. CD: cluster of differentiation; DNMT3A: DNA methyltransferase 3 alpha; FLT3: fms-related receptor tyrosine kinase; HLA-DR: major histocompatibility complex, class II, DR; NPM1: nucleophosmin 1; PD-L1: programmed cell death 1 ligand 1; TIM-3: T-cell immunoglobulin mucin family member 3. Created with Biorender.
The majority of studies in Table 1 (7/10) reported the presence of CD34+ immunophenotypic markers in most patients. Its presence is associated with immature cell populations, worse prognosis due to increased cell proliferation, blockage in differentiation, cell traffic and adhesion pathways, and increased resistance to induced apoptosis when compared with CD34- cells [69,70,71,75,76,77,78]. In a study by Loghavi et al. [71], for example, it was demonstrated that CD34+ expression combined with a CD117 increase and a CD38 and/or HLA-DR decrease may characterize complications in AML. Both the HLA-DR and CD117 markers are found in myeloid precursor cells and activate metabolization by inducing cycle progression; CD117 is a primitive myeloid marker associated with a worse prognosis. In turn, the negative expression of HLA-DR is associated with a favorable evolution and complete remission unless it is associated with CD117+, which is then correlated with unfavorable cytogenetics [94,95].
CD123 is associated with high-risk disease in AML, with greater expression in patients with KMT2A rearrangements and FLT3-ITD mutations, as observed by Lamble et al. [72]. As an interleukin 3 (IL-3) receptor, the CD123 plays an important role in the production and function of healthy hematopoietic cells, but its overexpression in leukemic patients results in an adverse prognosis, with leukocytosis due to increased susceptibility to IL-3 and higher chemoresistance rates due to the high replication rate. In addition, mutations in DNMT3A and WT1 can also be strongly associated with CD123 [70,96,97,98,99,100].
Some cytogenetic alterations already have a well-established immunophenotype in the literature, such as AML patients with PML-RARA, RUNX1-RUNX1T1, and CBFB-MYH11, as shown in Table 2, where red and green are consistent with the increase and decrease in the markers’ expression, respectively. Studies on PML-RARA AML have identified the positive expression of CD33, CD13, and CD19 antigens; the absence of HLA-DR expression; and low-frequency CD7, CD11b, and CD14 expression. Moreover, aberrant surface antigens, including CD2 and CD34, have also been described in some variations of APL [101,102,103]. The common blast immunophenotypes of RUNX1-RUNX1T1 AML were CD34, CD117, CD13, CD33, CD19, CD56, CD38, HLA-DR, and MPO [104,105,106]. Regarding the CBFB-MYH11 fusion gene, studies have reported a high frequency of CD33, CD34, CD117, HLA-DR, CD15, CD64, and CD14 expression [107,108].

Table 2.
Correlation between mutations and immunophenotypic profiles.
5. Conclusions
Studies have pointed out that it is possible to have a correlation between genetic alterations and surface antigens in patients with AML; however, further studies are needed to establish a complete correlation between alterations and markers. Such information may provide a faster diagnosis for AML patients due to the easy access to immunophenotyping tools, which could help steer investigators in clinical practice toward a preset cohort of genetic alterations while also highlighting which targeted therapeutic strategies may potentially be more effective for patient treatment.
Author Contributions
Invitation received, C.A.M.-N.; Conceptualization., F.M.C.d.P.P., G.F.S., I.V.B., C.B.M. and C.A.M.-N.; Provision of data and sub-sequent analysis and interpretation, I.V.B., C.B.M., F.M.C.d.P.P., D.d.S.O., R.M.R., G.S.L., M.O.d.M.F., M.E.A.d.M., L.E.B.d.S. and A.S.K.; Writing—original draft preparation, F.M.C.d.P.P., G.F.S., I.V.B., C.B.M. and C.A.M.-N.; Writing—review and editing, F.M.C.d.P.P., I.V.B. and C.A.M.-N.; Funding acquisition, C.A.M.-N. and A.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Brazilian funding agencies: Coordination for the Improvement of Higher Education Personnel (CAPES; to C.B.M), the National Council of Technological and Scientific Development (CNPq grant number 404213/2021-9 to CAM-N; Productivity in Research PQ scholarships to M.O.d.M.F., M.E.A.d.M., C.A.M.-N. and A.S.K.), and the Cearense Foundation of Scientific and Technological Support (FUNCAP grant number P20-0171-00078.01.00/20 to F.M.C.d.P.P, M.O.d.M.F. and C.A.M.-N.); we also thank PROPESP/UFPA for the publication payment.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or data interpretation; in the writing of the manuscript; or in the decision to publish the results.
References
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute myeloid leukaemia. Lancet 2018, 392, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Pelcovits, A.; Niroula, R. Acute Myeloid Leukemia: A Review. Rhode Isl. Med. J. 2020, 103, 38–40. [Google Scholar]
- Vakiti, A.; Mewawalla, P.; Wood, S.K. Acute Myeloid Leukemia (Nursing). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK568718/ (accessed on 15 August 2022).
- Tran, T.H.; Hunger, S.P. The genomic landscape of pediatric acute lymphoblastic leukemia and precision medicine opportunities. Semin. Cancer Biol. 2022, 84, 144–152. [Google Scholar] [CrossRef]
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Park, Y.; Mayne, S.T.; Wang, R.; Sinha, R.; Hollenbeck, A.R.; Schatzkin, A.; Cross, A.J. Diet, lifestyle, and acute myeloid leukemia in the NIH-AARP cohort. Am. J. Epidemiol. 2010, 171, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Quessada, J.; Cuccuini, W.; Saultier, P.; Loosveld, M.; Harrison, C.J.; Lafage-Pochitaloff, M. Cytogenetics of Pediatric Acute Myeloid Leukemia: A Review of the Current Knowledge. Genes 2021, 12, 924. [Google Scholar] [CrossRef]
- Oran, B.; Weisdorf, D.J. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica 2012, 97, 1916–1924. [Google Scholar] [CrossRef]
- INCA Atlas de Mortalidade. Available online: https://www.gov.br/inca/pt-br/assuntos/gestor-e-profissional-de-saude/controle-do-cancer-de-mama/dados-e-numeros/mortalidade (accessed on 1 February 2023).
- Creutzig, U.; Kutny, M.A.; Barr, R.; Schlenk, R.F.; Ribeiro, R.C. Acute myelogenous leukemia in adolescents and young adults. Pediatr. Blood Cancer 2018, 65, e27089. [Google Scholar] [CrossRef]
- Kantarjian, H.; Kadia, T.; DiNardo, C.; Daver, N.; Borthakur, G.; Jabbour, E.; Garcia-Manero, G.; Konopleva, M.; Ravandi, F. Acute myeloid leukemia: Current progress and future directions. Blood Cancer J. 2021, 11, 41. [Google Scholar] [CrossRef]
- Estey, E.H. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol. 2018, 93, 1267–1291. [Google Scholar] [CrossRef]
- Estey, E.H. Acute myeloid leukemia: 2021 update on risk-stratification and management. Am. J. Hematol. 2020, 95, 1368–1398. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Borthakur, G.; Kantarjian, H. Core binding factor acute myelogenous leukemia-2021 treatment algorithm. Blood Cancer J. 2021, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Surapally, S.; Tenen, D.G.; Pulikkan, J.A. Emerging therapies for inv(16) AML. Blood 2021, 137, 2579–2584. [Google Scholar] [CrossRef]
- Pulikkan, J.A.; Castilla, L.H. Preleukemia and Leukemia-initiating cell activity in inv(16) acute myeloid Leukemia. Front. Oncol. 2018, 8, 129. [Google Scholar] [CrossRef]
- Bennett, J.M.; Catovsky, D.; Daniel, M.-T.; Flandrin, G.; Galton, D.A.G.; Gralnick, H.R.; Sultan, C. Proposals for the Classification of the Acute Leukaemias French-American-British (FAB) Co-operative Group. Br. J. Haematol. 1976, 33, 451–458. [Google Scholar] [CrossRef]
- Marosi, C.; Köller, U.; Koller-Weber, E.; Schwarzinger, I.; Schneider, B.; Jäger, U.; Vahls, P.; Nowotny, H.; Pirc-Danoewinata, H.; Steger, G.; et al. Prognostic impact of karyotype and immunologic phenotype in 125 adult patients with de novo AML. Cancer Genet. Cytogenet. 1992, 61, 14–25. [Google Scholar] [CrossRef]
- Bain, B.J.; Béné, M.C. Morphological and Immunophenotypic Clues to the WHO Categories of Acute Myeloid Leukaemia. Acta Haematol. 2019, 141, 232–244. [Google Scholar] [CrossRef]
- Al-Harbi, S.; Aljurf, M.; Mohty, M.; Almohareb, F.; Ahmed, S.O.A. An update on the molecular pathogenesis and potential therapeutic targeting of AML with t(8;21)(q22;q22.1);RUNX1-RUNX1T1. Blood Adv. 2020, 4, 229–238. [Google Scholar] [CrossRef]
- Campone, M.; Im, S.A.; Iwata, H.; Clemons, M.; Ito, Y.; Awada, A.; Chia, S.; Jagiełło-Gruszfeld, A.; Pistilli, B.; Tseng, L.M.; et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: Overall survival results from BELLE-2. Eur. J. Cancer 2018, 103, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Boisset, J.C.; Robin, C. On the origin of hematopoietic stem cells: Progress and controversy. Stem Cell Res. 2012, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Béné, M.C.; Bernier, M.; Casasnovas, R.O.; Castoldi, G.; Doekharan, D.; Van Der Holt, B.; Knapp, W.; Lemež, P.; Ludwig, W.D.; Matutes, E.; et al. Acute myeloid leukaemia M0: Haematological, immunophenotypic and cytogenetic characteristics and their prognostic significance: An analysis in 241 patients. Br. J. Haematol. 2001, 113, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Lenvatinib-StatPearls-NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567768/ (accessed on 1 February 2023).
- Schmidt, L.; Heyes, E.; Grebien, F. Gain-of-Function Effects of N-Terminal CEBPA Mutations in Acute Myeloid Leukemia. BioEssays 2020, 42, e1900178. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Hahn, C.N.; Scott, H.S. Secondary leukemia in patients with germline transcription factor mutations (RUNX1, GATA2, CEBPA). Blood 2020, 136, 24–35. [Google Scholar] [CrossRef]
- Su, L.; Shi, Y.Y.; Liu, Z.Y.; Gao, S.J. Acute Myeloid Leukemia With CEBPA Mutations: Current Progress and Future Directions. Front. Oncol. 2022, 12, 12. [Google Scholar] [CrossRef]
- Wakita, S.; Sakaguchi, M.; Oh, I.; Kako, S.; Toya, T.; Najima, Y.; Doki, N.; Kanda, J.; Kuroda, J.; Mori, S.; et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. 2022, 6, 238–247. [Google Scholar] [CrossRef]
- Tarlock, K.; Lamble, A.J.; Wang, Y.C.; Gerbing, R.B.; Ries, R.E.; Loken, M.R.; Brodersen, L.E.; Pardo, L.; Leonti, A.; Smith, J.L.; et al. CEBPA-bZip mutations are associated with favorable prognosis in de novo AML: A report from the Children’s Oncology Group. Blood 2021, 138, 1137–1147. [Google Scholar] [CrossRef]
- Sierra, J.; Nomdedeu, J.F. CEBPA bZip mutations: Just a single shot. Blood 2021, 138, 1091–1092. [Google Scholar] [CrossRef]
- Ruecker, F.G.; Corbacioglu, A.; Theis, F.; Christopeit, M.; Germing, U.; Wulf, G.; Abu Samra, M.; Teichmann, L.; Lübbert, M.; Kühn, M.W.; et al. P448: Prognostic Impact of Somatic Cebpa Bzip Domain Mutations in Acute Myeloid Leukemia. HemaSphere 2022, 6, 348–349. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Sportoletti, P.; Paola Martelli, M. NPM1-mutated acute myeloid leukemia: From bench to bedside. Blood 2020, 136, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Comoli, P.; Marasca, R.; Potenza, L.; Luppi, M. Minimal/Measurable Residual Disease Monitoring in NPM1-Mutated Acute Myeloid Leukemia: A Clinical Viewpoint and Perspectives. Int. J. Mol. Sci. 2018, 19, 3492. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Riva, G.; Lagreca, I.; Barozzi, P.; Bettelli, F.; Paolini, A.; Nasillo, V.; Lusenti, B.; Pioli, V.; Giusti, D.; et al. Neoantigen-specific T-cell immune responses: The paradigm of NPM1-mutated acute myeloid leukemia. Int. J. Mol. Sci. 2021, 22, 9159. [Google Scholar] [CrossRef]
- Hindley, A.; Catherwood, M.A.; McMullin, M.F.; Mills, K.I. Significance of npm1 gene mutations in aml. Int. J. Mol. Sci. 2021, 22, 10040. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.M.; Chan, S.M.; Minden, M.D.; Murphy, T.; Shlush, L.I.; Schimmer, A.D. Biological and clinical consequences of NPM1 mutations in AML. Leukemia 2017, 31, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Rau, R.; Brown, P. Nucleophosmin (NPM1) mutations in adult and childhood acute myeloid leukaemia: Towards definition of a new leukaemia entity. Hematol. Oncol. 2009, 27, 171–181. [Google Scholar] [CrossRef]
- Thiede, C.; Koch, S.; Creutzig, E.; Steudel, C.; Illmer, T.; Schaich, M.; Ehninger, G. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood 2006, 107, 4011–4020. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.-P.; et al. Ivosidenib and Azacitidine in IDH1 -Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- Chi, S.G.; Minami, Y. Emerging Targeted Therapy for Specific Genomic Abnormalities in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 2362. [Google Scholar] [CrossRef]
- Roloff, G.W.; Griffiths, E.A. When to obtain genomic data in acute myeloid leukemia (AML) and which mutations matter. Blood Adv. 2018, 2, 3070–3080. [Google Scholar] [CrossRef]
- Kayser, S.; Döhner, K.; Krauter, J.; Köhne, C.H.; Horst, H.A.; Held, G.; Von Lilienfeld-Toal, M.; Wilhelm, S.; Kündgen, A.; Götze, K.; et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood 2011, 117, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Kühn, M.W.M.; Bullinger, L.; Gröschel, S.; Krönke, J.; Edelmann, J.; Rücker, F.G.; Eiwen, K.; Paschka, P.; Gaidzik, V.I.; Holzmann, K.; et al. Genome-wide genotyping of acute myeloid leukemia with translocation t(9;11)(p22;q23) reveals novel recurrent genomic alterations. Haematologica 2014, 99, 133–135. [Google Scholar] [CrossRef]
- Alsabeh, R.; Brynes, R.K.; Slovak, M.L.; Arber, D.A. Acute Myeloid Leukemia With t (6; 9) (p23; q34) Association with Myelodysplasia, Basophilia, and Initial CD34 Negative Immunophenotype. Am. J. Clin. Pathol. 1996, 107, 430–437. [Google Scholar] [CrossRef]
- von Lindern, M.; Fornerod, M.; van Baal, S.; Jaegle, M.; de Wit, T.; Buijs, A.; Grosveld, G. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol. Cell. Biol. 1992, 12, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Maze, D.; Xia, D.; Capo-Chichi, J.M.; King, I.; Sibai, H. A t(6;14;9)(p22;q22;q34) three-way translocation: Description of a cytogenetically visible variant t(6;9) in acute myeloid leukemia. Am. J. Hematol. 2022, 97, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Sabine Kayser, M.J.L. Clinical implications of molecular markers in acute myeloid leukemia. Eur. J. Haematol. 2019, 102, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, D.; Chi, S.; Uchiyama, S.; Nakamura, H.; Guo, Y.M.; Yamauchi, N.; Yuda, J.; Minami, Y. Molecular Classification and Overcoming Therapy Resistance for Acute Myeloid Leukemia with Adverse Genetic Factors. Int. J. Mol. Sci. 2022, 23, 5950. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, M.; Ottone, T.; Alfonso, V.; Ferrari, A.; Conte, E.; Divona, M.; Bianchi, M.P.; Ricciardi, M.R.; Mirabilii, S.; Licchetta, R.; et al. A rare BCR-ABL1 transcript in Philadelphia-positive acute myeloid leukemia: Case report and literature review. BMC Cancer 2019, 19, 50. [Google Scholar] [CrossRef]
- George, B.; Kantarjian, H.; Baran, N.; Krocker, J.D.; Rios, A. Tp53 in acute myeloid leukemia: Molecular aspects and patterns of mutation. Int. J. Mol. Sci. 2021, 22, 10782. [Google Scholar] [CrossRef]
- Welch, J.S. Patterns of mutations in TP53 mutated AML. Best Pract. Res. Clin. Haematol. 2018, 31, 379–383. [Google Scholar] [CrossRef]
- Cluzeau, T.; Loschi, M.; Fenaux, P.; Komrokji, R.; Sallman, D.A. Personalized medicine for tp53 mutated myelodysplastic syndromes and acute myeloid leukemia. Int. J. Mol. Sci. 2021, 22, 10105. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Steidl, U. Targeting Immunophenotypic Markers on Leukemic Stem Cells: How Lessons from Current Approaches and Advances in the Leukemia Stem Cell (LSC) Model Can Inform Better Strategies for Treating Acute Myeloid Leukemia (AML). Cold Spring Harb. Perspect. Med 2020, 10, a036251. [Google Scholar] [CrossRef]
- Barreto, I.V.; Pessoa, F.M.C.d.P.; Machado, C.B.; Pantoja, L.d.C.; Ribeiro, R.M.; Lopes, G.S.; Amaral de Moraes, M.E.; de Moraes Filho, M.O.; de Souza, L.E.B.; Burbano, R.M.R.; et al. Leukemic Stem Cell: A Mini-Review on Clinical Perspectives. Front. Oncol. 2022, 12, 931050. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, T.; Schmidts, I. The power and potential of integrated diagnostics in acute myeloid leukaemia. Br. J. Haematol. 2020, 188, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Zeisig, B.B.; Fung, T.K.; Troadec, E.; So, C.W.E. Reconstruction of Human AML Using Functionally and Immunophenotypically Defined Human Haematopoietic Stem and Progenitor Cells as Targeted Populations. Bio-Protocol 2021, 11, e4262. [Google Scholar] [CrossRef] [PubMed]
- Tiso, F.; Koorenhof-Scheele, T.N.; Huys, E.; Martens, J.H.A.; de Graaf, A.O.; van der Reijden, B.A.; Langemeijer, S.M.C.; Preijers, F.W.M.B.; Kroeze, L.I.; Jansen, J.H. Genetic diversity within leukemia-associated immunophenotype-defined subclones in AML. Ann. Hematol. 2022, 101, 571–579. [Google Scholar] [CrossRef]
- Basharat, M.; Khan, S.A.; Din, N.U.; Ahmed, D. Immunophenotypic characterisation of morphologically diagnosed cases of acute myeloid Leukaemia (AML). Pakistan J. Med. Sci. 2019, 35, 470–476. [Google Scholar] [CrossRef]
- Thomas, D.; Majeti, R. Biology and relevance of human acute myeloid leukemia stem cells. Blood 2017, 129, 1577–1585. [Google Scholar] [CrossRef]
- Riva, G.; Nasillo, V.; Ottomano, A.M.; Bergonzini, G.; Paolini, A.; Forghieri, F.; Lusenti, B.; Barozzi, P.; Lagreca, I.; Fiorcari, S.; et al. Multiparametric flow cytometry for MRD monitoring in hematologic malignancies: Clinical applications and new challenges. Cancers 2021, 13, 4582. [Google Scholar] [CrossRef]
- Ferrari, B.; Peyvandi, F. How I treat thrombotic thrombocytopenic purpura in pregnancy. Blood 2020, 136, 2125–2132. [Google Scholar] [CrossRef]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.C.; et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef] [PubMed]
- Voso, M.T.; Ottone, T.; Lavorgna, S.; Venditti, A.; Maurillo, L.; Lo-Coco, F.; Buccisano, F. MRD in AML: The Role of New Techniques. Front. Oncol. 2019, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Varotto, E.; Munaretto, E.; Stefanachi, F.; Torre, F.D.; Buldini, B. Diagnostic challenges in acute monoblastic/monocytic leukemia in children. Front. Pediatr. 2022, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Dubose-Marchenay, N.; Lacombe, F.; Dumain, P.; Belloc, F.; Boisseau, M.R.; Reiffers, J. Role of blast cell immunophenotyping for the diagnosis and prognosis of acute myeloid leukemia. Biol. Cell 1992, 76, 283. [Google Scholar] [CrossRef]
- Pinheiro, L.H.S.; Trindade, L.D.; Costa, A.F.d.O.; Silva, N.d.L.; Sandes, A.F.; Nunes, M.A.P.; Correa, C.B.; Almeida, C.A.C.; da Cruz, G.S.; de Lyra Junior, D.P.; et al. Aberrant phenotypes in acute myeloid leukemia and its relationship with prognosis and survival: A systematic review and meta-analysis. Int. J. Hematol. Stem Cell Res. 2020, 14, 274–288. [Google Scholar]
- Reichard, K.K.; Tefferi, A.; Abdelmagid, M.; Orazi, A.; Alexandres, C.; Haack, J.; Greipp, P.T. Pure (acute) erythroid leukemia: Morphology, immunophenotype, cytogenetics, mutations, treatment details, and survival data among 41 Mayo Clinic cases. Blood Cancer J. 2022, 12, 147. [Google Scholar] [CrossRef]
- Li, F.; Li, N.; Wang, A.; Liu, X. Correlation Analysis and Prognostic Impacts of Biological Characteristics in Elderly Patients with Acute Myeloid Leukemia. Clin. Interv. Aging 2022, 17, 1187–1197. [Google Scholar] [CrossRef]
- Loghavi, S.; DiNardo, C.D.; Furudate, K.; Takahashi, K.; Tanaka, T.; Short, N.J.; Kadia, T.; Konopleva, M.; Kanagal-Shamanna, R.; Farnoud, N.R.; et al. Flow cytometric immunophenotypic alterations of persistent clonal haematopoiesis in remission bone marrows of patients with NPM1-mutated acute myeloid leukaemia. Br. J. Haematol. 2021, 192, 1054–1063. [Google Scholar] [CrossRef]
- Lamble, A.J.; Brodersen, L.E.; Alonzo, T.A.; Wang, J.; Pardo, L.; Sung, L.; Cooper, T.M.; Kolb, E.A.; Aplenc, R.; Tasian, S.K.; et al. CD123 Expression Is Associated With High-Risk Disease Characteristics in Childhood Acute Myeloid Leukemia: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2022, 40, 252–261. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Vincent, B.G.; Ivanova, A.; Moore, D.; McKinnon, K.P.; Wilkinson, A.D.; Mukhopadhyay, R.; Mazziotta, F.; Knaus, H.A.; Foster, M.C.; et al. Phase II Trial of Pembrolizumab after High-Dose Cytarabine in Relapsed/Refractory Acute Myeloid Leukemia. Blood Cancer Discov. 2021, 2, 616–629. [Google Scholar] [CrossRef]
- Mason, E.F.; Kuo, F.C.; Hasserjian, R.P.; Seegmiller, A.C.; Pozdnyakova, O. A distinct immunophenotype identifies a subset of NPM1-mutated AML with TET2 or IDH1/2 mutations and improved outcome. Am. J. Hematol. 2018, 93, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.P.; Brodersen, L.E.; Alonzo, T.A.; Gerbing, R.B.; Menssen, A.J.; Wilson, E.R.; Kahwash, S.; Raimondi, S.C.; Hirsch, B.A.; Gamis, A.S.; et al. Phenotype in combination with genotype improves outcome prediction in acute myeloid leukemia: A report from children’s oncology group protocol AAML0531. Haematologica 2017, 102, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Craddock, C.F.; Houlton, A.E.; Quek, L.S.; Ferguson, P.; Gbandi, E.; Roberts, C.; Metzner, M.; Garcia-Martin, N.; Kennedy, A.; Hamblin, A.; et al. Outcome of azacitidine therapy in acute myeloid leukemia is not improved by concurrent vorinostat therapy but is predicted by a diagnostic molecular signature. Clin. Cancer Res. 2017, 23, 6430–6440. [Google Scholar] [CrossRef] [PubMed]
- Kontro, M.; Kumar, A.; Majumder, M.M.; Eldfors, S.; Parsons, A.; Pemovska, T.; Saarela, J.; Yadav, B.; Malani, D.; Fløisand, Y.; et al. HOX gene expression predicts response to BCL-2 inhibition in acute myeloid leukemia. Leukemia 2017, 31, 301–309. [Google Scholar] [CrossRef]
- Pang, C.S.; Pettenati, M.J.; Pardee, T.S. Clinicopathological analysis of near-tetraploidy/tetraploidy acute myeloid leukaemia. J. Clin. Pathol. 2015, 68, 236–240. [Google Scholar] [CrossRef]
- Döhner, K.; Schlenk, R.F.; Habdank, M.; Scholl, C.; Rücker, F.G.; Corbacioglu, A.; Bullinger, L.; Fröhling, S.; Döhner, H. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: Interaction with other gene mutations. Blood 2005, 106, 3740–3746. [Google Scholar] [CrossRef]
- Liu, Y.R.; Zhu, H.H.; Ruan, G.R.; Qin, Y.Z.; Shi, H.X.; Lai, Y.Y.; Chang, Y.; Wang, Y.Z.; Lu, D.; Hao, L.; et al. NPM1-mutated acute myeloid leukemia of monocytic or myeloid origin exhibit distinct immunophenotypes. Leuk. Res. 2013, 37, 737–741. [Google Scholar] [CrossRef]
- Zhou, Y.; Moon, A.; Hoyle, E.; Fromm, J.R.; Chen, X.; Soma, L.; Salipante, S.J.; Wood, B.L.; Wu, D. Pattern associated leukemia immunophenotypes and measurable disease detection in acute myeloid leukemia or myelodysplastic syndrome with mutated NPM1. Cytom. Part B-Clin. Cytom. 2019, 96, 67–72. [Google Scholar] [CrossRef]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef]
- Senapati, J.; Kadia, T.M. Which FLT3 Inhibitor for Treatment of AML? Curr. Treat. Options Oncol. 2022, 23, 359–380. [Google Scholar] [CrossRef]
- Travaglini, S.; Angelini, D.F.; Alfonso, V.; Guerrera, G.; Lavorgna, S.; Divona, M.; Nardozza, A.M.; Consalvo, M.I.; Fabiani, E.; De Bardi, M.; et al. Characterization of FLT3-ITDmut acute myeloid leukemia: Molecular profiling of leukemic precursor cells. Blood Cancer J. 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Vaikari, V.P.; Du, Y.; Wu, S.; Zhang, T.; Metzeler, K.; Batcha, A.M.N.; Herold, T.; Hiddemann, W.; Akhtari, M.; Alachkar, H. Clinical and preclinical characterization of CD99 isoforms in acute myeloid leukemia. Haematologica 2020, 105, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.J.; Kalaycio, M.; Weiner, G.; Frankel, S.; Schulman, P.; Schwartzberg, L.; Jurcic, J.; Velez-Garcia, E.; Seiter, K.; Scheinberg, D.; et al. Treatment of relapsed or refractory acute myeloid leukemia with humanized anti-CD33 monoclonal antibody HuM195. Leukemia 2003, 17, 314–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walter, R.B.; Appelbaum, F.R.; Estey, E.H.; Bernstein, I.D. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood 2012, 119, 6198–6208. [Google Scholar] [CrossRef] [PubMed]
- Notopuro, P.B.; Nugraha, J.; Utomo, B.; Notopuro, H. The association of FLT3-ITD gene mutation with bone marrow blast cell count, cd34, cyclin d1, bcl-xl and hent1 expression in acute myeloid leukemia patients. Iran. J. Pathol. 2020, 15, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Kiyoi, H.; Nakao, M.; Iwai, T.; Misawa, T.; Okuda, T.; Sonoda, Y.; Abe, T.; Kahsima, K.; Matsuo, Y.; et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia 1997, 11, 1605–1609. [Google Scholar] [CrossRef]
- Brunetti, L.; Gundry, M.C.; Goodell, M.A. DNMT3A in Leukemia. Cold Spring Harb. Perspect. Med. 2017, 7, a030320. [Google Scholar] [CrossRef]
- Khrabrova, D.A.; Yakubovskaya, M.G.; Gromova, E.S. AML-Associated Mutations in DNA Methyltransferase DNMT3A. Biochemistry 2021, 86, 307–318. [Google Scholar] [CrossRef]
- Kuželová, K.; Brodská, B.; Marková, J.; Petráčková, M.; Schetelig, J.; Ransdorfová, Š.; Gašová, Z.; Šálek, C. NPM1 and DNMT3A mutations are associated with distinct blast immunophenotype in acute myeloid leukemia. Oncoimmunology 2022, 11, 2073050. [Google Scholar] [CrossRef]
- Kumar, D.; Mehta, A.; Panigrahi, M.K.; Nath, S.; Saikia, K.K. DNMT3A (R882) mutation features and prognostic effect in acute myeloid leukemia in Coexistent with NPM1 and FLT3 mutations. Hematol. Oncol. Stem Cell Ther. 2018, 11, 82–89. [Google Scholar] [CrossRef]
- Farhi, D.C.; Al, W.E.T. CD117/CD34 Expression in Leukemic Blasts. Am. J. Clin. Pathol. 1996, 106, 192–195. [Google Scholar]
- Elrhman, H.A.E.A.; El-Meligui, Y.M.; Elalawi, S.M. Prognostic Impact of Concurrent DNMT3A, FLT3 and NPM1 Gene Mutations in Acute Myeloid Leukemia Patients. Clin. Lymphoma Myeloma Leuk. 2021, 21, e960–e969. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Riccioni, R.; Militi, S.; Coccia, E.; Stellacci, E.; Samoggia, P.; Latagliata, R.; Mariani, G.; Rossini, A.; Battistini, A.; et al. Elevated expression of IL-3Rα in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity, and poor prognosis. Blood 2002, 100, 2980–2988. [Google Scholar] [CrossRef]
- Ihle, J.N. Interleukin-3 and hematopoiesis. Chem. Immunol. 1992, 51, 65–106. [Google Scholar] [CrossRef]
- Tolba, F.M.; Foda, M.E.; Kamal, H.M.; Elshabrawy, D.A. Expression of CD133 in acute leukemia. Med. Oncol. 2013, 30, 527. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, S.; Rotiroti, M.C.; Bardelli, M.; Simonelli, L.; Magnani, C.F.; Biondi, A.; Biagi, E.; Tettamanti, S.; Varani, L. Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia. Mol. Ther. 2017, 25, 1933–1945. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.M.; Elsharkawy, N.M.; Kandeel, E.Z.; Hanafi, M.; Samra, M.; Osman, R.A. Leukemia Stem Cell Frequency at Diagnosis Correlates With Measurable/Minimal Residual Disease and Impacts Survival in Adult Acute Myeloid Leukemia. Front. Oncol. 2022, 12, 867684. [Google Scholar] [CrossRef]
- Guglielmi, C.; Martelli, M.P.; Diverio, D.; Fenu, S.; Vegna, M.L.; Cantù-Rajnoldi, A.; Biondi, A.; Cocito, M.G.; Del Vecchio, L.; Tabilio, A.; et al. Immunophenotype of adult and childhood acute promyelocytic leukaemia: Correlation with morphology, type of PML gene breakpoint and clinical outcome. A cooperative Italian study on 196 cases. Br. J. Haematol. 1998, 102, 1035–1041. [Google Scholar] [CrossRef]
- Takenokuchi, M.; Kawano, S.; Nakamachi, Y.; Sakota, Y.; Syampurnawati, M.; Saigo, K.; Tatsumi, E.; Kumagai, S. FLT3/ITD associated with an immature immunophenotype in PML-RARα leukemia. Hematol. Rep. 2012, 4, 80–85. [Google Scholar] [CrossRef]
- Gorczyca, W. Acute promyelocytic leukemia: Four distinct patterns by flow cytometry immunophenotyping. Pol. J. Pathol. 2012, 63, 8–17. [Google Scholar]
- Ouyang, J.; Goswami, M.; Peng, J.; Zuo, Z.; Daver, N.; Borthakur, G.; Tang, G.; Medeiros, L.J.; Jorgensen, J.L.; Ravandi, F.; et al. Comparison of Multiparameter Flow Cytometry Immunophenotypic Analysis and Quantitative RT-PCR for the Detection of Minimal Residual Disease of Core Binding Factor Acute Myeloid Leukemia. Am. J. Clin. Pathol. 2016, 145, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Bae, Y.K.; Cho, S.Y.; Koo, H.; Kim, H.J.; Nam, D.H.; Kim, S.H.; Chun, S.; Joo, K.M.; Park, W.Y. Elucidation of novel therapeutic targets for acute myeloid leukemias with RUNX1-RUNX1T1 fusion. Int. J. Mol. Sci. 2019, 20, 1717. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.A.; Schlette, E.J.; Xu, J.; Jorgensen, J.L.; Cameron Yin, C.; Li, S.; Jeffrey Medeiros, L.; Tang, G. Myeloid neoplasms with t(16;21)(q24;q22)/RUNX1-RUNX1T3 mimics acute myeloid leukemia with RUNX1-RUNX1T1. Ann. Hematol. 2018, 97, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, S.; Bacher, U.; Haferlach, C.; Kern, W.; Haferlach, T. Rare CBFB-MYH11 fusion transcripts in AML with inv(16)/t(16;16) are associated with therapy-related AML M4eo, atypical cytomorphology, atypical immunophenotype, atypical additional chromosomal rearrangements and low white blood cell count: A study on 162 patients. Leukemia 2007, 21, 725–731. [Google Scholar] [CrossRef]
- Cao, T.T.; Zhou, M.H.; Yuan, L.; Wang, Q.; Dou, L.P.; Xu, Y.Y.W.N.; Wang, L.-L.; Wang, S. Clinical analysis of acute myeloid leukemia with CBFB-MYH11-positive. Eur. PMC Funders Gr. Author Manuscr. 2013, 21, 305–310. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).