Biological Role of Zinc in Liver Cirrhosis: An Updated Review
Abstract
1. Introduction
2. Biological Functions of Zinc
3. Clinical Implications of Zinc Deficiency
Mechanism of Zinc Activity and Effects of Deficiency
4. Metabolism of Zinc in the Liver
5. Deficiency of Zinc in Liver Diseases
Zinc Deficiency in Liver Cirrhosis Complications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almaeen, A.H.; Alduraywish, A.A.; Mobasher, M.A.; Almadhi, O.I.M.; Nafeh, H.M.; El-Metwally, T.H. Oxidative stress, immunological and cellular hypoxia biomarkers in hepatitis C treatment-naïve and cirrhotic patients. Arch. Med. Sci. 2021, 17, 368–375. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Ghabril, M.; Chalasani, N.; Juluri, R.; Bell, L.N.; Kamendulis, L.; Klaunig, J.E.; Saxena, R.; Agarwal, D.; Johnson, M.S. Oxidative stress in chronic liver disease: Relationship between peripheral and hepatic measurements. Am. J. Med. Sci. 2011, 342, 314–317. [Google Scholar] [CrossRef]
- Amodio, P.; Bemeur, C.; Butterworth, R.; Cordoba, J.; Kato, A.; Montagnese, S.; Uribe, M.; Vilstrup, H.; Morgan, M.Y. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology 2013, 58, 325–336. [Google Scholar] [CrossRef]
- Conde de la Rosa, L.; Goicoechea, L.; Torres, S.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Role of Oxidative Stress in Liver Disorders. Livers 2022, 2, 283–314. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease, Professional Edition E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Himoto, T.; Masaki, T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients 2018, 10, 88. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Zago, M.P.; Oteiza, P.I. The antioxidant properties of zinc: Interactions with iron and antioxidants. Free Radic. Biol. Med. 2001, 31, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Schechter, P.; Giroux, E.; Schlienger, J.; Hoenig, V.; Sjoerdsma, A. Distribution of serum zinc between albumin and alpha2-macroglobulin in patients with decompensated hepatic cirrhosis. Eur. J. Clin. Investig. 1976, 6, 147–150. [Google Scholar] [CrossRef]
- Karayalcin, S.; Arcasoy, A.; Uzunalimoglu, O. Zinc plasma levels after oral zinc tolerance test in nonalcoholic cirrhosis. Dig. Dis. Sci. 1988, 33, 1096–1102. [Google Scholar] [CrossRef]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef]
- The Global Health Observatory. Global Health Estimates: Leading Causes of Death. 2023. WHO. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 20 February 2023).
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Reinhold, D. Liver cirrhosis and “liver” diabetes mellitus are linked by zinc deficiency. Med. Hypotheses. 2005, 64, 316–317. [Google Scholar] [CrossRef]
- Riggio, O.; Merli, M.; Capocaccia, L.; Caschera, M.; Zullo, A.; Pinto, G.; Gaudio, E.; Franchitto, A.; Spagnoli, R.; D’Aquilino, E.; et al. Zinc supplementation reduces blood ammonia and increases liver ornithine transcarbamylase activity in experimental cirrhosis. Hepatology 1992, 16, 785–789. [Google Scholar] [CrossRef]
- Williams, R.; Alexander, G.; Aspinall, R.; Batterham, R.; Bhala, N.; Bosanquet, N.; Severi, K.; Burton, A.; Burton, R.; Cramp, M.E. Gathering momentum for the way ahead: Fifth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet 2018, 392, 2398–2412. [Google Scholar] [CrossRef]
- Vega, S.; Neira, J.L.; Marcuello, C.; Lostao, A.; Abian, O.; Velazquez-Campoy, A. NS3 Protease from Hepatitis C Virus: Biophysical Studies on an Intrinsically Disordered Protein Domain. Int. J. Mol. Sci. 2013, 14, 13282–13306. [Google Scholar] [CrossRef]
- Williams, R.; Aspinall, R.; Bellis, M.; Camps-Walsh, G.; Cramp, M.; Dhawan, A.; Ferguson, J.; Forton, D.; Foster, G.; Gilmore, I. Addressing liver disease in the UK: A blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014, 384, 1953–1997. [Google Scholar] [CrossRef] [PubMed]
- Grüngreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016, 15, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Higashi, T.; Nouso, K.; Nakatsukasa, H.; Nakamura, S.-I.; Watanabe, A.; Tsuji, T. Effects of zinc deficiency/zinc supplementation on ammonia metabolism in patients with decompensated liver cirrhosis. Acta Med. Okayama 2001, 55, 349–355. [Google Scholar] [PubMed]
- McClain, C.J.; Antonow, D.R.; Cohen, D.A.; Shedlofsky, S.I. Zinc metabolism in alcoholic liver disease. Alcohol. Clin. Exp. Res. 1986, 10, 582–589. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Presser, H.; Franke, D.; Lössner, B.; Abicht, K.; Kleine, F. Correlations between zinc, amino acids and ammonia in liver cirrhosis. Z. Gastroenterol. 1989, 27, 731–735. [Google Scholar]
- Miatto, O.; Casaril, M.; Gabrielli, G.B.; Nicoli, N.; Bellisola, G.; Corrocher, R. Diagnostic and prognostic value of serum copper and plasma fibrinogen in hepatic carcinoma. Cancer 1985, 55, 774–778. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Ullah, M.I.; Alsrhani, A.; Atif, M.; Shaukat, I.; Hussain, S.; Ejaz, H. Estimation of serum iron, serum lipids and serum liver enzymes in celiac disease patients of Saudi Arabia. Pak. J. Med. Sci. 2022, 38, 2101–2106. [Google Scholar] [PubMed]
- McClain, C.J.; Marsano, L.; Burk, R.F.; Bacon, B. Trace metals in liver disease. Semin. Liver Dis. 1991, 11, 321–339. [Google Scholar] [CrossRef]
- Sengupta, S.; Wroblewski, K.; Aronsohn, A.; Reau, N.; Reddy, K.G.; Jensen, D.; Te, H. Screening for Zinc Deficiency in Patients with Cirrhosis: When Should We Start? Dig. Dis. Sci. 2015, 60, 3130–3135. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.; Rosato, A.; Perozzi, G.; Murgia, C. Zinc proteome interaction network as a model to identify nutrient-affected pathways in human pathologies. Genes. Nutr. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Wessells, K.; King, J.; Brown, K. Development of a plasma zinc concentration cutoff to identify individuals with severe zinc deficiency based on results from adults undergoing experimental severe dietary zinc restriction and individuals with acrodermatitis enteropathica. J. Nutr. 2014, 144, 1204–1210. [Google Scholar] [CrossRef]
- Maret, W. Zinc and sulfur: A critical biological partnership. Biochemistry 2004, 43, 3301–3309. [Google Scholar] [CrossRef]
- Stamoulis, I.; Kouraklis, G.; Theocharis, S. Zinc and the liver: An active interaction. Dig. Dis. Sci. 2007, 52, 1595–1612. [Google Scholar] [CrossRef]
- Maret, W.; Krezel, A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol. Med. 2007, 13, 371–375. [Google Scholar] [CrossRef]
- Faure, P.; Roussel, A.; Richard, M.; Foulon, T.; Groslambert, P.; Hadjian, A.; Favier, A. Effect of an acute zinc depletion on rat lipoprotein distribution and peroxidation. Biol. Trace Elem. Res. 1991, 28, 135–146. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency and studies in an experimental human model. Am. J. Clin. Nutr. 1991, 53, 403–412. [Google Scholar] [CrossRef]
- Danscher, G.; Stoltenberg, M. Zinc-specific autometallographic in vivo selenium methods: Tracing of zinc-enriched (ZEN) terminals, ZEN pathways, and pools of zinc ions in a multitude of other ZEN cells. J. Histochem. Cytochem. 2005, 53, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Muhamed, P.K.; Vadstrup, S. Zinc is the most important trace element. Ugeskr. Laeger 2014, 176, V11120654. (In Danish) [Google Scholar]
- Huber, K.L.; Hardy, J.A. Mechanism of zinc-mediated inhibition of caspase-9. Protein Sci. 2012, 21, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- Maret, W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid. Redox. Signal. 2006, 8, 1419–1441. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Temple, V.J.; Masta, A. Zinc in human health. Papua New Guin. Med. J. 2004, 47, 146–158. [Google Scholar]
- Liuzzi, J.P.; Cousins, R.J. Mammalian zinc transporters. Annu. Rev. Nutr. 2004, 24, 151. [Google Scholar] [CrossRef] [PubMed]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Hancock, D.; Petocz, P.; Samman, S. Zinc transporter genes are coordinately expressed in men and women independently of dietary or plasma zinc. J. Nutr. 2011, 141, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Simons, T. The mechanism of zinc uptake by cultured rat liver cells. J. Physiol. 1994, 474, 55–64. [Google Scholar] [CrossRef]
- Cousins, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef]
- Peng, H.; Wisse, E.; Tian, Z. Liver natural killer cells: Subsets and roles in liver immunity. Cell. Mol. Immunol. 2016, 13, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Tom Dieck, H.; Döring, F.; Roth, H.-P.; Daniel, H. Changes in rat hepatic gene expression in response to zinc deficiency as assessed by DNA arrays. J. Nutr. 2003, 133, 1004–1010. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Levy, B.A.; Zou, J.; Hanna, N.; Desouki, M.M.; Bagasra, O.; Johnson, L.A.; Costello, L.C. ZIP14 zinc transporter downregulation and zinc depletion in the development and progression of hepatocellular cancer. J. Gastrointest. Cancer 2012, 43, 249–257. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Islam, M.R.; Virag, J.; Oyen, M.L. Micromechanical poroelastic and viscoelastic properties of ex-vivo soft tissues. J. Biomech. 2020, 113, 110090. [Google Scholar] [CrossRef]
- Tognato, R.; Jones, P.H. Ray Optics Model for Optical Trapping of Biconcave Red Blood Cells. Micromachines 2022, 14, 83. [Google Scholar] [CrossRef]
- Ullah, M.I.; Alzahrani, B.; Alsrhani, A.; Atif, M.; Alameen, A.A.M.; Ejaz, H. Determination of serum tumor necrosis factor-alpha (TNF-α) levels in metabolic syndrome patients from Saudi population. Pak. J. Med. Sci. 2021, 37, 700–705. [Google Scholar] [CrossRef]
- Hameed, T.; Khan, Z.; Imran, M.; Ali, S.; Albegali, A.A.; Ullah, M.I.; Ejaz, H. Associations of transcription factor 7-Like 2 (TCF7L2) gene polymorphism in patients of type 2 diabetes mellitus from Khyber Pakhtunkhwa population of Pakistan. Afr. Health Sci. 2021, 21, 15–22. [Google Scholar] [CrossRef]
- Hara, K.; Yonezawa, K.; Weng, Q.P.; Kozlowski, M.T.; Belham, C.; Avruch, J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.P.; Fell, G.S.; Smith, C.M.; Tilstone, W.J. Metabolism after injury. I. Effects of severity, nutrition, and environmental temperatue on protein potassium, zinc, and creatine. Br. J. Surg. 1972, 59, 926–931. [Google Scholar]
- Sattar, A.; Khan, J.; Kakar, N.H.; Butt, M.U.A.; Yousaf, H.; Ullah, M.I. Serum zinc levels and the prevalence of zinc deficiency in patients with liver cirrhosis. Rawal. Med. J. 2021, 46, 37–40. [Google Scholar]
- Barry, M.; Keeling, P.W.; Feely, J. Tissue zinc status and drug elimination in patients with chronic liver disease. Clin. Sci. 1990, 78, 547–549. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Mackenzie, G.G. Zinc, oxidant-triggered cell signaling, and human health. Mol. Aspects Med. 2005, 26, 245–255. [Google Scholar] [CrossRef]
- DiSilvestro, R.A. Zinc in relation to diabetes and oxidative disease. J. Nutr. 2000, 130 (Suppl. S5), 1509s–1511s. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.E.; DiSilvestro, R.A. Effects of mild zinc deficiency, plus or minus an acute-phase response, on galactosamine-induced hepatitis in rats. Br. J. Nutr. 1994, 72, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; McClain, C.J.; Talwalkar, R.T.; Shedlofsky, S.I. Effects of endotoxin on zinc metabolism in human volunteers. Am. J. Physiol. 1997, 272 Pt 1, E952–E956. [Google Scholar] [CrossRef] [PubMed]
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat. Immunol. 2013, 14, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43 (Suppl. 1), S54–S62. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Bémeur, C.; Desjardins, P.; Butterworth, R.F. Role of nutrition in the management of hepatic encephalopathy in end-stage liver failure. J. Nutr. Metab. 2010, 2010, 489823. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.; Williams, R. Nutrition in end-stage liver disease: Principles and practice. Gastroenterology 2008, 134, 1729–1740. [Google Scholar] [CrossRef]
- Grüngreiff, K. Branched Amino Acids and Zinc in the Nutrition of Liver Cirrhosis. J. Clin. Exp. Hepatol. 2018, 8, 480–483. [Google Scholar] [CrossRef]
- Buchman, A. L Total parenteral nutrition: Challenges and practice in the cirrhotic patient. Transpl. Proc. 2006, 38, 1659–1663. [Google Scholar] [CrossRef]
- Bianchi, G.; Marzocchi, R.; Lorusso, C.; Ridolfi, V.; Marchesini, G. Nutritional treatment of chronic liver failure. Hepatol. Res. 2008, 38 (Suppl. 1), S93–S101. [Google Scholar] [CrossRef]
- Hayashi, M.; Ikezawa, K.; Ono, A.; Okabayashi, S.; Hayashi, Y.; Shimizu, S.; Mizuno, T.; Maeda, K.; Akasaka, T.; Naito, M.; et al. Evaluation of the effects of combination therapy with branched-chain amino acid and zinc supplements on nitrogen metabolism in liver cirrhosis. Hepatol. Res. 2007, 37, 615–619. [Google Scholar] [CrossRef]
- Itou, M.; Kawaguchi, T.; Taniguchi, E.; Oku, Y.; Fukushima, N.; Ando, E.; Oriishi, T.; Uchida, Y.; Otsuka, M.; Tanaka, S.; et al. Branched-chain amino acid supplements reduced ascites and increased the quality of life in a patient with liver cirrhosis: A case repor. Mol. Med. Rep. 2009, 2, 977–981. [Google Scholar] [CrossRef]
- Garcia-Martinez, R.; Caraceni, P.; Bernardi, M.; Gines, P.; Arroyo, V.; Jalan, R. Albumin: Pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology 2013, 58, 1836–1846. [Google Scholar] [CrossRef]
- Bal, W.; Sokołowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. Albumin as a zinc carrier: Properties of its high-affinity zinc-binding site. Biochem. Soc. Trans. 2008, 36 Pt 6, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. World. J. Gastroenterol. 2013, 19, 7620–7629. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Branched-chain amino acids and ammonia metabolism in liver disease: Therapeutic implications. Nutrition 2013, 29, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Takuma, Y.; Nouso, K.; Makino, Y.; Hayashi, M.; Takahashi, H. Clinical trial: Oral zinc in hepatic encephalopathy. Aliment. Pharmacol. Ther. 2010, 32, 1080–1090. [Google Scholar] [CrossRef]
- Padula, G.; González, H.F.; Varea, A.; Seoane, A.I. Protein energy-malnutrition: Does the in vitro zinc sulfate supplementation improve chromosomal damage repair? Biol. Trace. Elem. Res. 2014, 162, 64–71. [Google Scholar] [CrossRef]
- Riggio, O.; Ridola, L.; Pasquale, C. Hepatic encephalopathy therapy: An overview. World J. Gastrointest. Pharmacol. Ther. 2010, 1, 54–63. [Google Scholar] [CrossRef]
- Schliess, F.; Görg, B.; Häussinger, D. RNA oxidation and zinc in hepatic encephalopathy and hyperammonemia. Metab. Brain Dis. 2009, 24, 119–134. [Google Scholar] [CrossRef]
- Shawcross, D.L.; Shabbir, S.S.; Taylor, N.J.; Hughes, R.D. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology 2010, 51, 1062–1069. [Google Scholar] [CrossRef]
- Marchesini, G.; Fabbri, A.; Bianchi, G.; Brizi, M.; Zoli, M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology 1996, 23, 1084–1092. [Google Scholar] [CrossRef]
- Reding, P.; Duchateau, J.; Bataille, C. Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial. Lancet 1984, 2, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Van der Rijt, C.C.; Schalm, S.W.; Schat, H.; Foeken, K.; De Jong, G. Overt hepatic encephalopathy precipitated by zinc deficiency. Gastroenterology 1991, 100, 1114–1118. [Google Scholar] [CrossRef]
- Riggio, O.; Ariosto, F.; Merli, M.; Caschera, M.; Zullo, A.; Balducci, G.; Ziparo, V.; Pedretti, G.; Fiaccadori, F.; Bottari, E.; et al. Short-term oral zinc supplementation does not improve chronic hepatic encephalopathy. Results of a double-blind crossover trial. Dig. Dis. Sci. 1991, 36, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Kugelmas, M. Preliminary observation: Oral zinc sulfate replacement is effective in treating muscle cramps in cirrhotic patients. J. Am. Coll. Nutr. 2000, 19, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; El-Serag, H.B.; Sada, Y.; Mittal, S.; Ying, J.; Duan, Z.; Richardson, P.; Davila, J.A.; Kanwal, F. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment. Pharmacol. Ther. 2016, 43, 621–630. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Hebell, T.; Gutensohn, K.; Reinhold, A.; Reinhold, D. Plasma concentrations of zinc, copper, interleukin-6 and interferon-γ, and plasma dipeptidyl peptidase IV activity in chronic hepatitis C. Mol. Med. Rep. 2009, 2, 63–68. [Google Scholar] [CrossRef]
- Ebara, T.; Ohno, T.; Nakano, T. Quantitative medical cost-effectiveness analysis of molecular-targeting cancer drugs in Japan. Daru. 2013, 21, 40. [Google Scholar] [CrossRef]
- Nishito, Y.; Tsuji, N.; Fujishiro, H.; Takeda, T.A.; Yamazaki, T.; Teranishi, F.; Okazaki, F.; Matsunaga, A.; Tuschl, K.; Rao, R.; et al. Direct Comparison of Manganese Detoxification/Efflux Proteins and Molecular Characterization of ZnT10 Protein as a Manganese Transporter. J. Biol. Chem. 2016, 291, 14773–14787. [Google Scholar] [CrossRef]
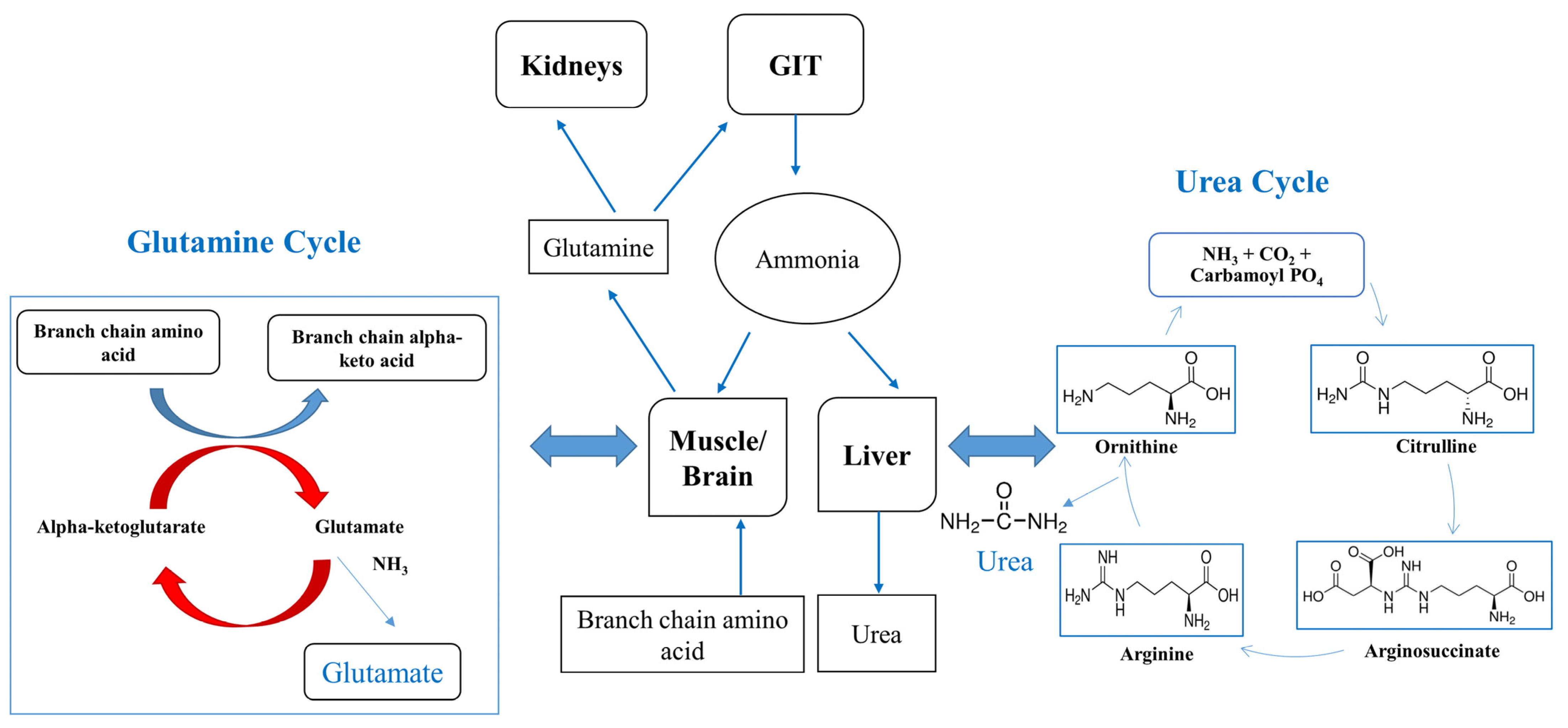
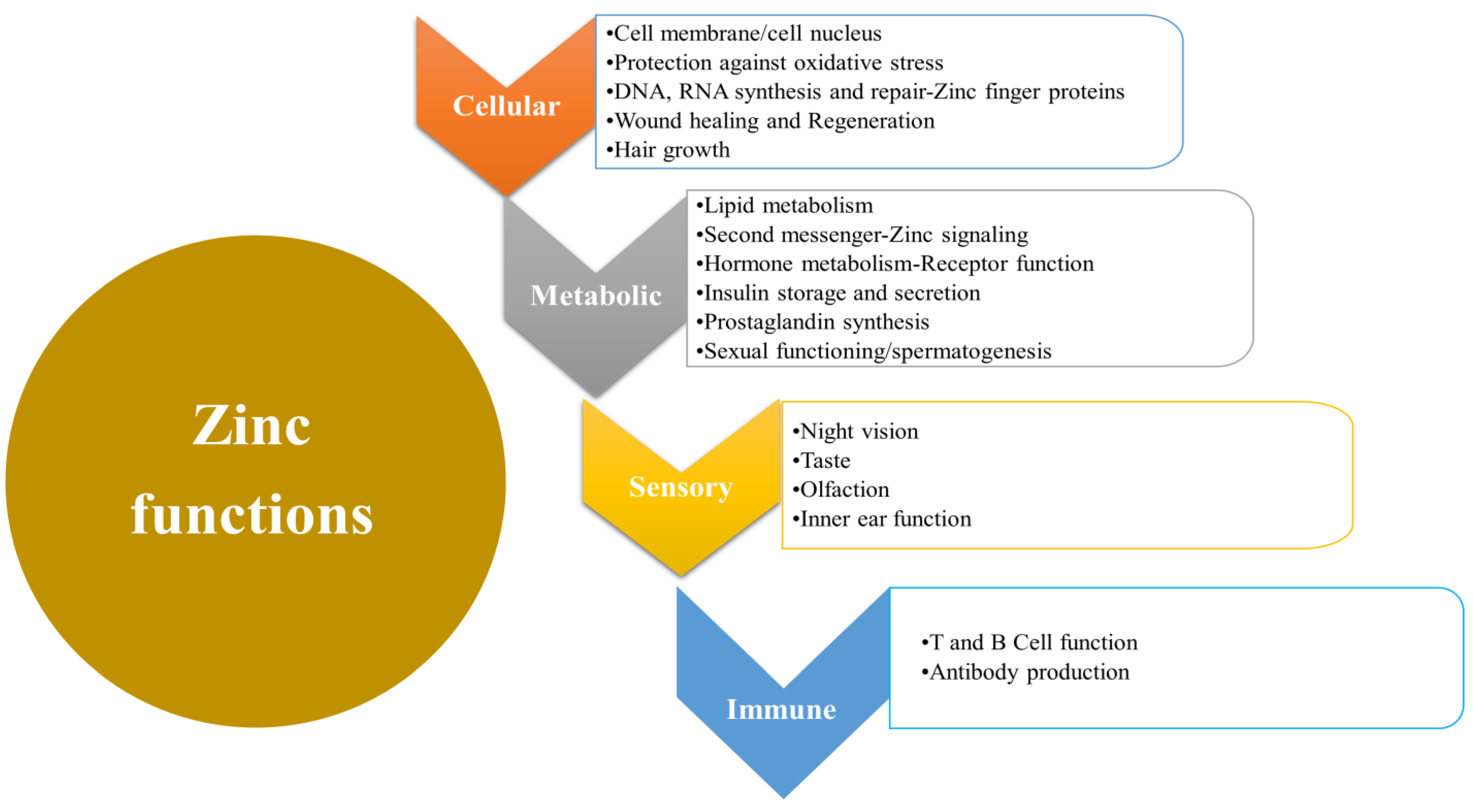
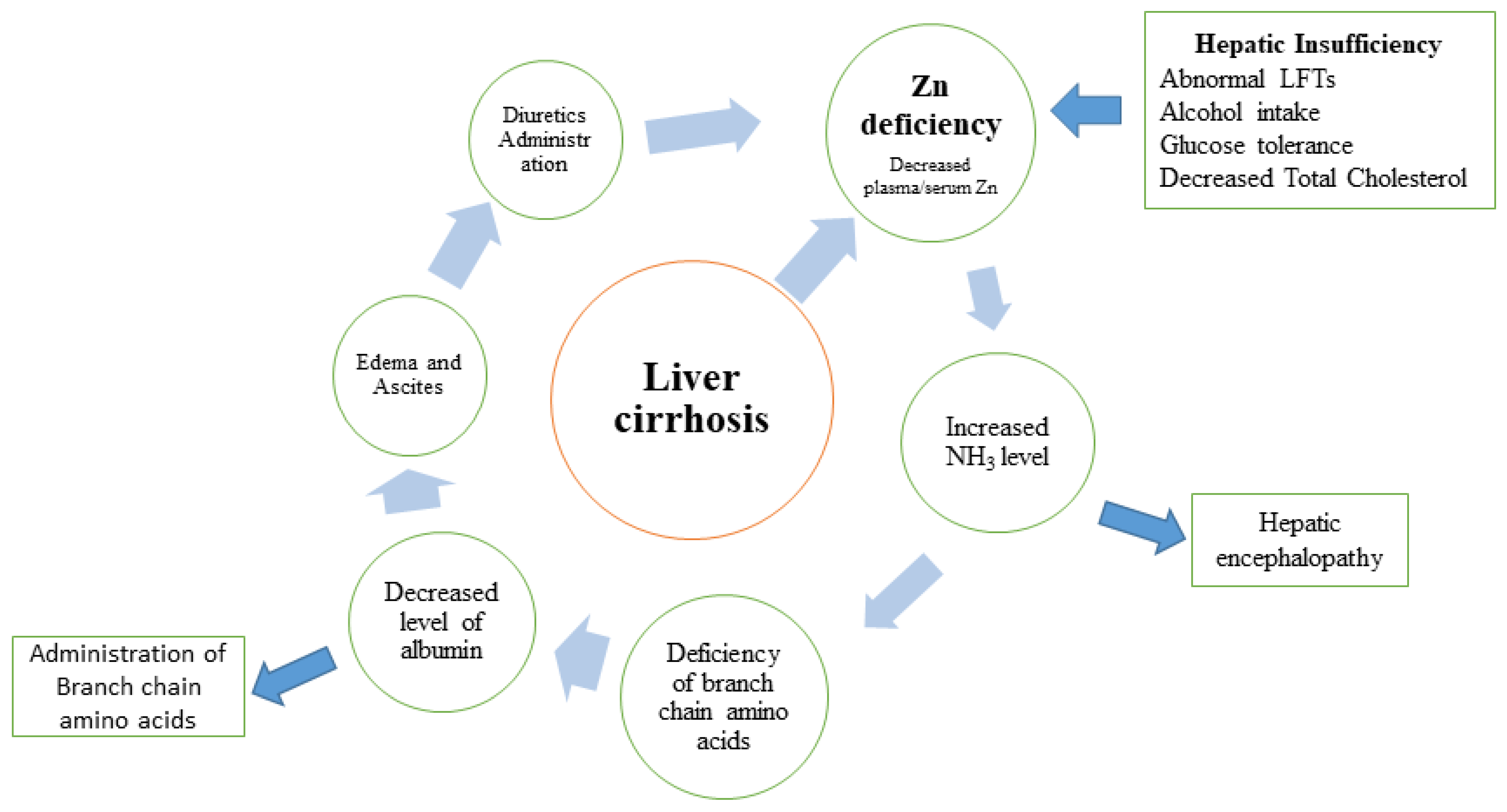
| Causes of Liver Disease | Mechanism |
|---|---|
| Insufficient nutritional consumption | Protein and amino acids metabolism variabilities |
| Contracted hepatic abstraction | Porto-systemic shunts |
| Alcohol-induced defective absorption | Production of cytokines, primarily interleukin-6 (IL-6) Production of Endotoxins by Biological pathogens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, M.I.; Alameen, A.A.M.; Al-Oanzi, Z.H.; Eltayeb, L.B.; Atif, M.; Munir, M.U.; Ejaz, H. Biological Role of Zinc in Liver Cirrhosis: An Updated Review. Biomedicines 2023, 11, 1094. https://doi.org/10.3390/biomedicines11041094
Ullah MI, Alameen AAM, Al-Oanzi ZH, Eltayeb LB, Atif M, Munir MU, Ejaz H. Biological Role of Zinc in Liver Cirrhosis: An Updated Review. Biomedicines. 2023; 11(4):1094. https://doi.org/10.3390/biomedicines11041094
Chicago/Turabian StyleUllah, Muhammad Ikram, Ayman Ali Mohammed Alameen, Ziad H. Al-Oanzi, Lienda Bashier Eltayeb, Muhammad Atif, Muhammad Usman Munir, and Hasan Ejaz. 2023. "Biological Role of Zinc in Liver Cirrhosis: An Updated Review" Biomedicines 11, no. 4: 1094. https://doi.org/10.3390/biomedicines11041094
APA StyleUllah, M. I., Alameen, A. A. M., Al-Oanzi, Z. H., Eltayeb, L. B., Atif, M., Munir, M. U., & Ejaz, H. (2023). Biological Role of Zinc in Liver Cirrhosis: An Updated Review. Biomedicines, 11(4), 1094. https://doi.org/10.3390/biomedicines11041094