Abstract
Musculoskeletal diseases continue to rise on a global scale, causing significant socioeconomic impact and decreased quality of life. The most common disorders affecting musculoskeletal structures are osteoarthritis and tendinopathies, complicated orthopedic conditions responsible for major pain and debilitation. Intra-articular hyaluronic acid (HA) has been a safe, effective, and minimally invasive therapeutic tool for treating these diseases. Several studies from bedside to clinical practice reveal the multiple benefits of HA such as lubrication, anti-inflammation, and stimulation of cellular activity associated with proliferation, differentiation, migration, and secretion of additional molecules. Collectively, these effects have demonstrated positive outcomes that assist in the regeneration of chondral and tendinous tissues which are otherwise destroyed by the predominant catabolic and inflammatory conditions seen in tissue injury. The literature describes the physicochemical, mechanical, and biological properties of HA, their commercial product types, and clinical applications individually, while their interfaces are seldom reported. Our review addresses the frontiers of basic sciences, products, and clinical approaches. It provides physicians with a better understanding of the boundaries between the processes that lead to diseases, the molecular mechanisms that contribute to tissue repair, and the benefits of the HA types for a conscientious choice. In addition, it points out the current needs for the treatments.
1. Introduction
Hyaluronic acid (HA), commonly referred to as hyaluronan, is a natural biological compound present in many tissues and fluids [1]. HA was first isolated as glycosaminoglycan (GAG) in 1934 by Meyer and Palmer from bovine vitreous humor. The term “hyaluronic acid” is broken down into hyaloid, which means vitreous, and uronic acid [1]. Posteriorly, HA was identified in other organs and tissue types, such as skin, joints, and the human umbilical cord, to name a few. Researchers discovered that this product could also be synthesized by many bacterial species such as Escherichia coli, Bacillus subtilis, and Streptococcus zooepidemicus via fermentation [2]. Conveniently, the chemical structure and properties of HA are identical in both vertebrates and bacteria [1]. More importantly, it was found to be produced by various cell types in the body during different stages of the cell cycle, being a major component of the extracellular matrix (ECM) [3]. In humans, HA has been considered for the treatment of painful musculoskeletal conditions such as tendinopathies and degenerative disorders like osteoarthritis (OA) [4,5]. HA has been utilized for the management of several health conditions since the 1970s due to its unique physicochemical properties and biological functions, as this molecule establishes strong interactions with cells and ECM [3,6]. The objective of this review is to discuss the biological functions of hyaluronic acid and its regenerative medicine potential in the treatment of osteoarthritis and tendinopathies.
2. Etiopathogenesis of Osteoarthritis
Osteoarthritis (OA), one of the most common degenerative and progressive joint diseases, is a major cause of pain and disability in adult individuals, affecting approximately 7% of the global population [7]. The numbers have been increasing significantly in the past few decades [8], and this is likely attributed to factors such as the aging of the population and the incidence of poor health status, especially metabolic syndrome [9,10,11]. OA is influenced by the complex interplay between local, systemic, and external factors, which consequently dictate the progression outcome and the patient’s response to treatment [12]. The most notable features include progressive loss of articular cartilage, osteophyte formation, subchondral bone thickening, escalated synovial inflammation, ligament and meniscal deterioration, and overall joint hypertrophy [10].
OA has been observed to arise from a confluence of diverse elements, encompassing genetic predisposition, adiposity, trauma, senescence, and even the co-occurrence of additional systemic maladies [13]. This condition harms the complete joint complex, and earlier studies have indicated that the degenerative process takes place in two discernible stages. Firstly, in the anabolic phase, chondrocytes make multiple efforts to mend injured ECM. Subsequently, in the catabolic phase, increased activity of enzymes that break down molecules leads to ECM digestion and hindrance of fresh ECM creation [14]. Prolonged biomechanical and biochemical strain induces secondary modifications, culminating in a preponderant shift towards catabolic reactions. These physiological mechanisms are accountable for the wearing away of cartilage and damage to the subchondral bone and surrounding structures, exacerbating physical discomfort and disability [15].
To elaborate, synoviocytes and osteoarthritic chondrocytes generate high amounts of MMPs (matrix metalloproteinase) 1, 3, 9, and 13, at least in vitro [16]. Synoviocytes secrete proteolytic enzymes and pro-inflammatory cytokines such as IL-(interleukin) 1β, IL-6, and tumor necrosis factor-alpha (TNF-α), which additionally impact OA progression and the perception of noxious stimuli associated with the disease [17]. Other molecules, including resistin and osteopontin, are linked to the severity of the disease and exhibit a notable increase in expression within the osteoarthritic synovial tissue [18,19,20]. In addition, it has been reported that the synovium alone is capable of producing certain chemokines and metalloproteinases that contribute to the degeneration of cartilage, even though cartilage itself also generates most of the catabolic molecules through autocrine and paracrine signaling mechanisms [21]. As a result, the remnants generated from cartilage degradation, whether through mechanical or enzymatic disintegration, may elicit the discharge of collagenase and other hydrolytic enzymes from synovial cells. This sequence of events instigates vascular hyperplasia in synovial membranes affected by osteoarthritis [22].
Within the joints of healthy adult individuals, the articular cartilage is mainly composed of chondrocytes and ECM, which comprises various substances, including water, chondroitin sulfate, type II collagen, proteoglycans, HA, as well as other proteins such as fibronectin and laminin. Additionally, the fibrous components of the ECM contain elastin and collagen, which consist of several types of fibrillar collagens such as types I, II, III, V, and XI, as well as non-fibrillar collagens including FACIT types IX, XII, and XIV, short chain types VIII and X, and basement membrane type IV [23,24]. The rate of collagen turnover is rather slow whereas that of the proteoglycan is relatively faster [15]. This process is controlled by chondrocytes, which are responsible for the synthesis of molecular components including proteolytic enzymes that regulate their breakdown [15]. These cells are also exposed to multiple sources of stimuli, including polypeptides, cytokines, biomechanical signals, and even fragmented components of the ECM itself [15].
Osteoarthritis occurs due to dystrophic damage to the articular cartilage in response to an imbalance between anabolic and catabolic reactions in the chondral and subchondral bone compartments [15,25]. Factors such as metabolic syndrome, physical trauma, microfractures, and inflammation contribute to a slight increase in enzymatic activity, resulting in the formation of “wear” particles, hence, the so-called “wear-and-tear” process [11,26]. Molecularly, the dysregulated proteoglycan metabolism destabilizes collagen fibers, leading to dehydration and disorganization of cartilage. Increased degradation of glycosaminoglycans, especially chondroitin sulphate and hyaluronic acid, leads to a decrease in matrix resistance to biomechanical stress [15]. This also increases the sensitivity of the cartilage surface to damage. Excessive synthesis and local release of MMPs by chondrocytes gradually delay cartilage repair. Collectively, these reactions contribute to softening, fibrillation, ulceration, and ultimately, the destruction of articular cartilage [15].
At the cellular level, macrophages may very well phagocytize microparticles and cellular debris, eventually. However, the overproduction of these particles causes significant cell stress, making it harder to dispose of them. Eventually, they assume the role of mediators of inflammation, eliciting chondrocytes to secrete elevated quantities of catabolic enzymes [26]. Molecules arising from the breakdown of collagen and proteoglycan are additionally subjected to processing by synovial macrophages, prompting the release of TNFα, IL-1, and IL-6. Subsequently, these molecules bind to chondrocyte receptors, stimulating further MMP discharge and the hindrance of collagen type 2 synthesis. This sequence of events aggravates cartilage degeneration, favoring a more debilitated microenvironment [27]. In summary, disturbance of homeostasis leads to an elevation in water content and a reduction in proteoglycan content within the ECM. This undermines the integrity of the collagen framework due to decreased synthesis of collagen type 2 and the amplified degradation of pre-existing collagen. Ultimately, this culminates in an increased rate of chondrocyte apoptosis [28].
3. Etiopathogenesis of Tendinopathy
Tendinopathies are a common type of pathology amongst the general population and are even more frequent in sportsmen. Tendon disorders range from traumatic injuries to chronic disease processes [29]. They are one of the most frequent orthopedic diagnoses and account for approximately 30% of musculoskeletal cases [30]. Every year, at least 30 million medical procedures involving tendons take place worldwide, generating a major socioeconomic impact on affected individuals [31]. It is estimated that at least 50% of sports-related injuries involving tendons are attributed to overuse conditions causing significant physical stress on these tissues [32]. The most frequently affected anatomical sites include the long head of the brachial biceps, extensors, and flexors of the wrist, rotator cuff, patellar tendon, tibial tendon, thigh adductors, and the Achilles tendon [29,32].
In addition to sports-related injuries, other intrinsic and extrinsic risk factors such as aging, biological sex, and even poor workplace ergonomics can contribute to the development of specific tendinopathies [33,34]. Prolonged use of certain pharmacological substances can be quite detrimental to tendon tissue biology and render individuals more susceptible to this disorder. Intrinsic mechanisms can also play a role in the progression of tendon pathology, especially in terms of metabolic dysregulation [35]. In patients with hypercholesterolemia, for instance, cholesterol deposition in tendons can mark the establishment of chronic low-grade inflammation and degeneration of tendons over time. Similarly, the glycation end-products in diabetic patients also deteriorate the biological properties and mechanical function of not only tendons but all musculoskeletal tissues in general [11,36,37]. Another notorious hallmark of metabolic syndrome is the accumulation of visceral fat and body weight. Elevated mechanical effort applies more biomechanical stress to weight-bearing joints, leading to the deterioration of musculoskeletal structures [38].
On the molecular level, tendons are organized according to a hierarchical structure. Collagen protein subunits make up the smallest building blocks of the tendon, which combine to form tropocollagen helixes [39]. Tendons contain types I, II, and III collagen, elastin, water, and proteoglycans, such as decorin [40]. Type I collagen forms stiff structures that convey mechanical durability and strength, whereas type II collagen has comparably smaller fibrils. Generally associated with scar tissue and injury, type III collagen gives rise to thinner fibers which are mostly concentrated in the skin, blood vessels, and other tissues which contain high amounts of elastic fibers [40].
These structures also have a cellular component and an ECM as inherent parts of their structure. Tenoblasts are immature spindle-shaped cells that ultimately differentiate into tenocytes upon reaching maturation. These two cells are the most abundant cell types present in tendons and play a key role in producing ECM, which must contain proteoglycan, collagen, and fibronectin, the essential proteins for tendon homeostasis and regeneration [41]. A small percentage of different cell types also populate tendinous structures, including capillary endothelial cells, arteriolar smooth muscle cells, synovial cells, and chondrocytes [42]. All of these cells are surrounded and supported by the ECM, which is a complex organic structure containing mostly collagen, elastin, fibrillin, fibronectin, and proteoglycans [43].
Both the cellular and molecular components in tendon health are in constant interplay. The production of various biomechanical and biochemical signals elicits a wide set of responses that may be either beneficial or detrimental depending on the patient’s overall health status [11,44]. The activation of certain cell signaling pathways may or may not change the ECM function and composition. The Scx and Mkx pathways, for example, interact with Smad3. This molecule is a key transcriptional mediator of TGF-β signaling to control ECM synthesis in tendinous tissues [45]. In addition to regulating ECM production, the Mkx pathway also controls tendon maturation. Therefore it may partially contribute to the maintenance of tenocytes by impeding their ability to undergo differentiation into other cell fates such as myogenic or skeletogenic phenotypes [45]. It is also worth noting that signaling pathways activated by mechanical loading can increase the expression of Scx, Mkx, and Smad3, stimulating the production of more ECM [45].
The maintenance of tendon homeostasis is primarily governed by mechanical loading, which is subsequently modulated by cellular activity under the influence of neuronal and cellular mediators. Mediators may be released locally or remotely and then carried away through either blood circulation or nerve supply [46,47]. Considering these facts, impaired biomechanics and dysregulated cellular processes may be recognized as the major culprits in tendon pathology. Proper loading of tendons is what stimulates anabolic responses, especially the upregulation of collagen type I gene expression and increased synthesis of these proteins [48,49]. Type I collagen synthesis peaks approximately 24 h following physical modulation and its levels are kept high for up to 80 h. Nevertheless, an excess of physical impact can trigger the deterioration of collagen proteins and consequently provoke a prevailing catabolic response. However, the occurrence of the catabolic peak precedes the anabolic response in terms of timing. This results in a net reduction of collagen within the initial 24 to 36 h after exercise, followed by a subsequent net gain [50]. This implies that sufficient rest periods between physical activity sessions are crucial for maintaining a healthy tendon homeostasis. Should these limitations not be met due to excessive or repetitive loading, tenocytes are then forced to produce inflammatory molecules. This in turn fragilizes collagen fibrils and increases the risk of microdamage [51].
Tendons subjected to repetitive mechanical loading have been found to exhibit an exacerbated production of inflammatory cytokines, including prostaglandin E2 (PGE2) [52]. An animal study shows that injections of PGE2 in leporine cause degenerative alterations in the tendon proper [53], whilst peritendinous injections of PGE1 lead to tendinopathy in murines [54]. The inflammatory response in tendons is prominently characterized by pro-inflammatory biomarkers, including but not limited to, IL-18, IL-15, IL-6, IL-1β, and TNF-α [55,56], which are associated with the activation of the prototypical pro-inflammatory signaling pathway, nuclear factor kappa B (NF-κB) [57]. Moreover, in models of Achilles tendinopathy, it is also common to observe granulation changes in capillary vessels as well as the infiltration of inflammatory cells such as macrophages, mast cells, and B and T lymphocytes [58], illustrating the regulatory role of the innate immune system in the early onset of the disease. It is worth noting that the role of macrophages during inflammation and tissue repair is vital. Signaling pathways can induce the polarization of macrophages into either M1 subtype (pro-inflammatory) or M2 subtype (anti-inflammatory) [59,60]. The differentiation of monocytes and polarization of macrophages are regulated by various inflammatory mediators, including interferons, NF-κB, and glucocorticoid receptor activation pathways [60]. Therefore, inflammatory pathways in tendon disorders control macrophage polarization, leading to failed, fibrotic healing responses [61].
Repetitive mechanical stress can cause tenocytes and fibroblasts to bind transforming growth factor β (TGF-β) and pro-inflammatory cytokines, leading to their differentiation into myofibroblasts [62]. Once the healing process is completed, the mechanical stress on myofibroblasts is removed, and these cells undergo apoptosis. The problem begins when this mechanism fails, as myofibroblasts then trigger a hyperproliferative process that culminates in fibrosis, a major histological feature of tendinopathy [63].
Angiogenesis is a critical event in the healing process and is regulated mainly by the vascular endothelial growth factor (VEGF), which stimulates endothelial cell migration via chemotaxis and vasodilation. However, in tendinopathy, neovascularization can lead to the deterioration of mechanical properties and even ruptures. Furthermore, the sprouting and ingrowth of sensory nerve fibers following the neoangiogenic process into the tendon can trigger nociception and pain in patients with tendinopathy [58]. The uncontrolled and aberrant sprouting of sensory nerve fibers into the tendon during tendinopathy is indicative of a failed healing response, which can lead to increased pain signaling. This process also plays a role in the hyperproliferative changes observed in tendinosis [63]. Many interventional therapies have been proposed for both OA and tendinopathies. Conservative methods such as the administration of pharmacological agents only bring temporary alleviation of pain but do not address the root of the pathology [64,65]. Physicians may prescribe a combination of drugs at different stages of OA, aiming to block inflammatory nociceptive pain. Non-steroidal anti-inflammatory drugs (NSAIDs), other analgesics, and corticosteroids, for example, are commonly indicated for the management of pain. However, prolonged administration of NSAIDs is known to cause secondary health problems. Although they may effectively target pain, their prolonged use can lead to serious side effects such as peptic ulcer disease, acute renal failure, and myocardial infarction [66]. Non-pharmacological alternatives such as physical therapy, low-impact exercise, weight loss, physical aids, and nerve ablation are often recommended to minimize the risks associated with NSAIDs. Even so, in severe cases such as grade 4 OA or complete tendon rupture, invasive procedures may be inevitable, causing a significant impact on the quality of life [12,64].
4. Physicochemical and Biological Properties of Hyaluronic Acid
The physicochemical properties of HA are given by molecular mass and spatial conformation, classifying it as high (HMW), medium (MMW), or low molecular weight (LMW) [67]. The molecular weight of HA seems to be a crucial factor regarding the biological functions it elicits in human tissues. Typically, LMW HA falls in the range of 500,000 to 730,000 Daltons (Da), MMW in between 800,000 and 2,000,000, and HMW with an average of 6,000,000 [68]. In the joints of healthy individuals, HA has a mass of about 5 to 7 million Da, whereas in osteoarthritic joints, its mass falls to approximately 1 million Da. The crosslinking of HMW HA molecules forms a solution with high viscosity, serving as a shock absorber and lubricant. Additionally, this type of HA has properties that support the growth of cells [69]. HA is the primary non-protein component of synovial fluid, and it surrounds cells as a layer. Whether natural or artificial, it interacts with pro-inflammatory mediators and binds to cellular receptors, regulating cell proliferation, migration, and gene expression [70]. HA is also a potent collagen stimulator (especially type I collagen), and is thus capable of promoting tissue recovery and maintenance of cellular integrity [71]. These properties make HA a particularly useful orthobiologic tool for the healing of tendon and chondral tissues in many circumstances.
The IA injection of HA is considered a minimally invasive intervention method that has not been associated with any significant systemic adverse events, unlike other types of IA injections, such as corticosteroids, or even oral administration of NSAIDs [72]. Studies have shown that this alternative approach has demonstrated positive effects in vitro. The ECM has been found to have an impact on cell metabolism, particularly on osteoblasts in subchondral bone affected by osteoarthritis, and HA has been shown to reverse abnormal homeostatic activity [73]. As Figure 1 suggests, IA-HA has been demonstrated to have the potential to reduce chondrocyte apoptosis, as well as increase chondrocyte proliferation [74]. In humans, it is important to use formulations with medium to high MWs to mimic the conditions and biological properties of HA naturally produced in the body. In addition, it is important to use HA derived from biological synthesis, to avoid undesired side effects [67].
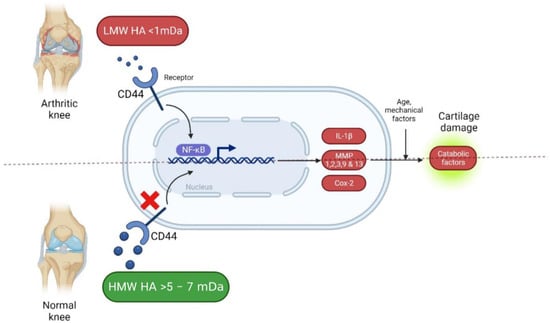
Figure 1.
Hyaluronic acid in arthritis knees (Created with Biorender.com). [https://app.biorender.com/, accessed on 20 December 2022].
Administration of LMW HA results in weak binding and therefore weak HA biosynthesis. With MMW HA, there is stronger binding and a higher number of HA receptors being stimulated, which enhances endogenous HA production. On the other hand, it must be emphasized that extremely large molecules present in HMW HA products may not always be convenient as the large domains of these molecules can limit the number of free binding sites on the cell surface, which logically implies a relatively weak stimulation of HA biosynthesis [68]. Despite these physiological hurdles, practitioners must not forget that MW is not the only limiting factor because HA concentration in IA approaches is also another variable with its fair share of influence on clinical outcomes.
The balance in HA turnover has a key role in determining its concentrations, MW, and, consequently, the properties it will display in disease processes. Formulations with higher molecular weights usually elicit anti-inflammatory effects, as it regulates the recruitment of immune cells. Conversely, HA with lower molecular weights is known to promote angiogenesis and tissue remodeling in wound healing but it may also exhibit a more pro-inflammatory activity in specific cell types such as chondrocytes [75,76]. Endogenously, HA synthesis by synovial fibroblasts is influenced by MW and HA concentration in the extracellular environment [68].
The protective effects of this modality are attributed to the ability of HA to bind to CD44 receptors. This inhibits the expression of IL-1β, which dampens the production of MMPs 1, 2, 3, 9, and 13 [77,78,79], impeding catabolic enzyme activity within musculoskeletal tissues [80]. Upon interacting with surface receptors, HA activates intracellular signaling systems involved in the proliferation, differentiation, migration, and degradation of HA itself as shown in Figure 2 [81]. CD44 is the most widely studied HA receptor as it is expressed in nearly all human cell types. The affinity of CD44 for HA is what determines the potential of HA as a signaling molecule. However, this depends both on the concentration and molecular weight of HA, by glycosylation of extracellular domains and phosphorylation of serine [82]. CD44 is clustered by HMW HA polymers and can interact with other ligands, including growth factors, ECM proteins, MMPs, and cytokines [83]. Another important HA surface receptor is RHAMM, commonly referred to as CD168. It is expressed in many different tissues and regulates cell migration by interacting with skeletal proteins, especially in the healing cascade [82]. The interaction between HA and CD168 plays a major role in the activation of signaling pathways that involve Src and other kinase protein complexes of focal adhesions [84].
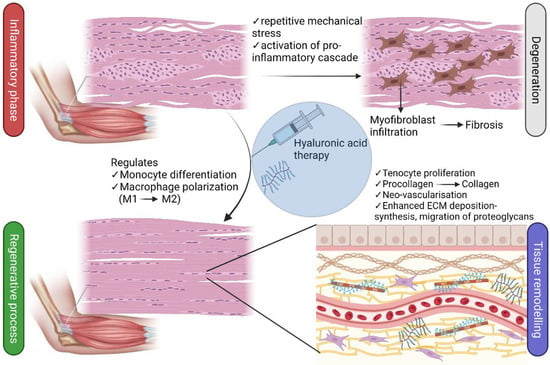
Figure 2.
Hyaluronic acid in tendinopathies (Created with Biorender.com). Hyaluronic acid in orthopedic conditions. [https://app.biorender.com/, accessed on 20 December 2022].
The application and benefits of HA for several orthopedic conditions have been well documented in the literature, from basic laboratory studies to robust clinical trials and systematic reviews. Viscosupplementation with HA and HA-derived biomaterials has been propelling major advances in the treatment of OA since it was initially proposed in the 1970s by Endre A. Balazs [82]. Posteriorly, in the 1980s, new HA derivatives emerged as suitable IA injection strategies envisaging the restoration of joint homeostasis and protection against mechanical damage [85].
The most widely used HA products in clinical trials were Synvisc and Hyalgan due to their safety, efficacy, and long-lasting effects despite the need for IA injections [82,86]. Hyalgan, in particular, has been shown to enhance the survival and proliferation of human chondrocytes which are exposed to reactive oxygen species (ROS) [87]. Since then, many additional HA derivatives with different MWs were made available as depicted in Table 1, which was created based on the research developed by Migliore et al. [88]. More recently, an alternative product consisting of the mixture of HA and lactose-modified chitosan (Chitlac®) has shown promising results in improving the anti-inflammatory effect and therapeutic value of HA in OA. In vitro and in vivo studies have shown an expressive increase in cartilage regeneration after administration of this derivative in experimentally-induced OA [89,90]. Its effects have been further revealed in a recent study published in 2021. The HA-Chitlac® mixture significantly attenuates triamcinolone acetonide-hydroxypropyl-β-cyclodextrin (TA-CD) drug cytotoxicity in human chondrocyte cultures and sustains anti-inflammatory effects, reinforcing yet again the chondroprotective role of the HA for OA [91]. In more advanced and severe stages, HA alone may not suffice, requiring additional interventions such as autologous chondrocyte implantation. Three-dimensional biodegradable and biocompatible HA-based scaffold polymers such as Hyaff-11® have been successfully utilized in the past for human chondrocyte cultures [92]. Once chondrocytes are implanted, the regenerated tissue undergoes a process of maturation and forms hyaline tissue instead of fibrous cartilage [92].

Table 1.
Typical hyaluronic acid formulations in orthopedics.
A randomized controlled trial [93] compared the clinical effects of platelet-rich plasma (PRP) and HA individually and synergistically for mild to moderate degrees of knee OA in 105 patients. The patients were randomly allocated to HA, PRP, or HA+PRP groups and received 3 intra-articular knee injections of their assigned substance with 2-week intervals between each infiltration. Clinical outcomes were evaluated according to Western Ontario and McMaster Universities Arthritis Index (WOMAC) and the Visual Analogue Scale (VAS) questionnaires at baseline and after 1, 3, 6, and 12 months. In this study, the association of HA and leukocyte-rich PRP was found to be statistically significant in terms of clinical outcome as physical function and pain were dramatically improved in the first 30 days after treatment. These observations may be attributed to the fact that HA provides a functional matrix with supportive scaffold properties enhancing cartilage biomechanics and tissue repair [94].
The exact mechanisms of action regarding exogenous HA administration are complex. However, current hypotheses suggest that HA can relay its known protective effects in 2 distinct stages. The first stage is known as the mechanical stage, where synovial fluid is substituted by higher concentrations of HA to improve viscosity [70]. Additionally, this allows the restoration and improvement of lubrication, and shock-absorbing properties of synovial fluid and establishes a layer around nociceptors that reduces pain signaling [95]. The second and final stage is often referred to as the pharmacological stage, where biosynthesis of endogenous HA and ECM components takes place [96]. This reduces the scarcity of proteoglycans in cartilage and prevents chondrocyte apoptosis [97]. Moreover, it also dampens inflammatory cell activity, reducing HA degradation and production of nociceptive mediators [70].
Similarly, the viscoelastic properties of HA have also promoted beneficial effects in numerous in vitro studies of tendinous tissue without significant adverse effects [98,99,100,101,102,103,104,105,106,107]. Most of the early in vitro studies [98,99,100,101] aimed at investigating the effects of various HA coating solutions on the gliding resistance of tendons. Collectively, the findings show that HA coating of tendons does not significantly improve gliding resistance compared to saline controls, although their surface becomes significantly smoother, in contrast. In the in vitro study led by Taguchi et al. [100], the researchers attempted to replicate these experiments in human tendons. Much like the animal models, similar observations were reported. Saline solutions promoted significantly higher gliding resistance in comparison to HA treatment, thus suggesting that HA may limit tendon adhesion to some extent.
In regard to tendon healing, however, the results were relatively more optimistic. In a few models of tendon injury [102,103,104], HA did not significantly increase the expression of procollagen alpha 1 but was still able to bind to its receptors and induce cell proliferation in dose-dependent manners, likely by HA sensitivity. Furthermore, one study on human tendons derived from rotator cuff tears found that HA increases cell viability and proliferation at 24 h compared to controls, regardless of molecular weight [108]. In contrast to the previous studies, the synthesis of collagen type I was stimulated at 14 days, being significantly higher in the HMW HA group. Speaking of which, another investigation [109] found that an HMW HA formulation with a weight greater than 1.2 megadaltons inhibits activation of the NF-kB inflammatory pathway caused by advanced glycation end products. Conversely, LMW HA does not convey benefits and is associated with pro-inflammatory status [75,76].
A parallel study led by Tanimoto et al. [106] evaluated the effects of pro-inflammatory cytokines TNF-α and IL-1β on rabbit HA-synthetase (HAS) mRNA expression. Under inflammatory conditions typically seen in wound healing, these cytokines increase HAS mRNA expression and may therefore contribute to the fragmentation and accumulation of HA. Similarly, Smith and colleagues [107] demonstrated that the exogenous addition of HA to synovial fibroblast cultures stimulates HA synthesis according to increasing concentrations and MW.
In regards to cost-effectiveness and comparison with other known treatments for orthopedic conditions, a recently published study has compared IA administration of HA versus PRP for the treatment of symptomatic knee osteoarthritis [110]. Samuelson and colleagues found that the cost per quality-adjusted life-year (QALY) of a series of PRP injections was USD 8635.23/QALY, whereas HA injections were USD 5331.75/QALY; however, PRP was significantly more effective at 1 year.
Rosen et al. [111] aimed to compare IA-HA with conservative treatments (physical therapy, orthosis, NSAIDs, and analgesics) for early to moderate-stage knee osteoarthritis. The authors revealed that HMW HA was superior to LMW HA and physical therapy, being less expensive yet providing greater benefits. HMW HA was cost-effective in comparison to orthosis and NSAIDs/analgesics.
5. Conclusions
Hyaluronic acid is an essential compound that can be naturally found in many organs and tissues. This molecule plays a vital role in musculoskeletal health, especially in painful conditions such as osteoarthritis and tendinopathies. Intra-articular administration of hyaluronic acid as an orthobiologic tool is a minimally invasive procedure with demonstrated efficacy and safety. This therapeutic alternative offers multiple benefits associated with attenuated inflammation, lubrication, improved biomechanics, cell proliferation, differentiation, migration, and enhanced protein biosynthesis and secretion. Despite the growing number of new HA derivatives for the treatment of orthopedic diseases, future investigations are still needed to further comprehend the factors that contribute to musculoskeletal tissue repair.
Author Contributions
Conceptualization, F.R.C. and G.S.S.; writing—original draft preparation, M.J., A.N., G.S.S., R.A.M. and M.d.S.S.; writing—review and editing, M.R.C.M. and V.C.C.; supervision, M.J. and J.F.S.D.L.; project administration, M.J., J.V.B.L. and M.H.A.S. and J.F.S.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial Production of Hyaluronic Acid: Current State, Challenges, and Perspectives. Microb. Cell Factories 2011, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Hermans, J.; Bierma-Zeinstra, S.M.A.; Bos, P.K.; Niesten, D.D.; Verhaar, J.A.N.; Reijman, M. The Effectiveness of High Molecular Weight Hyaluronic Acid for Knee Osteoarthritis in Patients in the Working Age: A Randomised Controlled Trial. BMC Musculoskelet. Disord. 2019, 20, 196. [Google Scholar] [CrossRef]
- Muneta, T.; Koga, H.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Hyaluronan Injection Therapy for Athletic Patients with Patellar Tendinopathy. J. Orthop. Sci. 2012, 17, 425–431. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, D.; Noble, P.W. Hyaluronan as a Therapeutic Target in Human Diseases. Adv. Drug Deliv. Rev. 2016, 97, 186–203. [Google Scholar] [CrossRef]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results; Global Burden of Disease Collaborative Network: Seattle, WA, USA, 2019. [Google Scholar]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Azzini, G.O.M.; Santos, G.S.; Visoni, S.B.C.; Azzini, V.O.M.; dos Santos, R.G.; Huber, S.C.; Lana, J.F. Metabolic Syndrome and Subchondral Bone Alterations: The Rise of Osteoarthritis—A Review. J. Clin. Orthop. Trauma 2020, 11, S849–S855. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-Rich PRP for Knee Osteoarthritis: Current Concepts. J. Clin. Orthop. Trauma 2019, 10, S179–S182. [Google Scholar] [CrossRef]
- Man, G.S.; Mologhianu, G. Osteoarthritis Pathogenesis—A Complex Process That Involves the Entire Joint. J. Med. Life 2014, 7, 37–41. [Google Scholar]
- Yuan, G.-H.; Tanaka, M.; Masuko-Hongo, K.; Shibakawa, A.; Kato, T.; Nishioka, K.; Nakamura, H. Characterization of Cells from Pannus-like Tissue over Articular Cartilage of Advanced Osteoarthritis. Osteoarthr. Cartil. 2004, 12, 38–45. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Lee, J.H.; Ort, T.; Ma, K.; Picha, K.; Carton, J.; Marsters, P.A.; Lohmander, L.S.; Baribaud, F.; Song, X.-Y.R.; Blake, S. Resistin Is Elevated Following Traumatic Joint Injury and Causes Matrix Degradation and Release of Inflammatory Cytokines from Articular Cartilage in Vitro. Osteoarthr. Cartil. 2009, 17, 613–620. [Google Scholar] [CrossRef]
- Presle, N.; Pottie, P.; Dumond, H.; Guillaume, C.; Lapicque, F.; Pallu, S.; Mainard, D.; Netter, P.; Terlain, B. Differential Distribution of Adipokines between Serum and Synovial Fluid in Patients with Osteoarthritis. Contribution of Joint Tissues to Their Articular Production. Osteoarthr. Cartil. 2006, 14, 690–695. [Google Scholar] [CrossRef]
- Hasegawa, M.; Segawa, T.; Maeda, M.; Yoshida, T.; Sudo, A. Thrombin-Cleaved Osteopontin Levels in Synovial Fluid Correlate with Disease Severity of Knee Osteoarthritis. J. Rheumatol. 2011, 38, 129–134. [Google Scholar] [CrossRef]
- Krasnokutsky, S.; Attur, M.; Palmer, G.; Samuels, J.; Abramson, S.B. Current Concepts in the Pathogenesis of Osteoarthritis. Osteoarthr. Cartil. 2008, 16, S1–S3. [Google Scholar] [CrossRef]
- Ashraf, S.; Walsh, D.A. Angiogenesis in Osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage Homeostasis in Health and Rheumatic Diseases. Arthritis Res. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-Cell Interaction of Cartilage Extracellular Matrix on Chondrocytes. BioMed Res. Int. 2014, 2014, e648459. [Google Scholar] [CrossRef] [PubMed]
- Heijink, A.; Gomoll, A.H.; Madry, H.; Drobnič, M.; Filardo, G.; Espregueira-Mendes, J.; Van Dijk, C.N. Biomechanical Considerations in the Pathogenesis of Osteoarthritis of the Knee. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 423–435. [Google Scholar] [CrossRef]
- Wang, M.; Peng, Z.; Vasilev, K.; Ketheesan, N. Investigation of Wear Particles Generated in Human Knee Joints Using Atomic Force Microscopy. Tribol. Lett. 2013, 51, 161–170. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating Levels of IL-6 and TNF-α Are Associated with Knee Radiographic Osteoarthritis and Knee Cartilage Loss in Older Adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular Cartilage and Osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar]
- Loiacono, C.; Palermi, S.; Massa, B.; Belviso, I.; Romano, V.; Di Gregorio, A.; Sirico, F.; Sacco, A.M. Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina 2019, 55, 447. [Google Scholar] [CrossRef]
- Andarawis-Puri, N.; Flatow, E.L.; Soslowsky, L.J. Tendon Basic Science: Development, Repair, Regeneration, and Healing: Tendon Development, Injury, and Repair. J. Orthop. Res. 2015, 33, 780–784. [Google Scholar] [CrossRef]
- Abbah, S.A.; Spanoudes, K.; O’Brien, T.; Pandit, A.; Zeugolis, D.I. Assessment of Stem Cell Carriers for Tendon Tissue Engineering in Pre-Clinical Models. Stem. Cell Res. 2014, 5, 38. [Google Scholar] [CrossRef]
- Maffulli, N.; Wong, J.; Almekinders, L.C. Types and Epidemiology of Tendinopathy. Clin. Sport. Med. 2003, 22, 675–692. [Google Scholar] [CrossRef]
- Albers, I.S.; Zwerver, J.; Diercks, R.L.; Dekker, J.H.; Van den Akker-Scheek, I. Incidence and Prevalence of Lower Extremity Tendinopathy in a Dutch General Practice Population: A Cross Sectional Study. BMC Musculoskelet. Disord 2016, 17, 16. [Google Scholar] [CrossRef]
- Morton, S.; Williams, S.; Valle, X.; Diaz-Cueli, D.; Malliaras, P.; Morrissey, D. Patellar Tendinopathy and Potential Risk Factors: An International Database of Cases and Controls. Clin. J. Sport Med. 2017, 27, 468–474. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V.; Andia, I. Occurrence of Tendon Pathologies in Metabolic Disorders. Rheumatology 2013, 52, 599–608. [Google Scholar] [CrossRef]
- Li, Y.; Fessel, G.; Georgiadis, M.; Snedeker, J.G. Advanced Glycation End-Products Diminish Tendon Collagen Fiber Sliding. Matrix Biol. 2013, 32, 169–177. [Google Scholar] [CrossRef]
- Couppé, C.; Svensson, R.B.; Kongsgaard, M.; Kovanen, V.; Grosset, J.-F.; Snorgaard, O.; Bencke, J.; Larsen, J.O.; Bandholm, T.; Christensen, T.M.; et al. Human Achilles Tendon Glycation and Function in Diabetes. J. Appl. Physiol. 2016, 120, 130–137. [Google Scholar] [CrossRef]
- Abate, M.; Gravare Silbernagel, K.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of Tendinopathies: Inflammation or Degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef]
- Thorpe, C.T.; Screen, H.R.C. Tendon Structure and Composition. In Metabolic Influences on Risk for Tendon Disorders; Advances in Experimental Medicine and, Biology; Ackermann, P.W., Hart, D.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 920, pp. 3–10. ISBN 978-3-319-33941-2. [Google Scholar]
- Buckley, M.R.; Evans, E.; Satchel, L.N.; Matuszewski, P.E.; Chen, Y.-L.; Elliott, D.M.; Soslowsky, L.J.; Dodge, G.R. Distributions of Types I, II and III Collagen by Region in the Human Supraspinatus Tendon. Connect. Tissue Res. 2013, 54, 374–379. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Tendon Injury and Tendinopathy: Healing and Repair. J. Bone Jt. Surg. 2005, 87, 187–202. [Google Scholar] [CrossRef]
- Screen, H.R.C.; Berk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon Functional Extracellular Matrix. J. Orthop. Res. 2015, 33, 793–799. [Google Scholar] [CrossRef]
- Bosman, F.T.; Stamenkovic, I. Functional Structure and Composition of the Extracellular Matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Schilling, T.F. Tendon Development and Musculoskeletal Assembly: Emerging Roles for the Extracellular Matrix. Development 2015, 142, 4191–4204. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, P.W. Neuronal Regulation of Tendon Homoeostasis. Int. J. Exp. Pathol. 2013, 94, 271–286. [Google Scholar] [CrossRef]
- Killian, M.L.; Cavinatto, L.; Galatz, L.M.; Thomopoulos, S. The Role of Mechanobiology in Tendon Healing. J. Shoulder Elb. Surg. 2012, 21, 228–237. [Google Scholar] [CrossRef]
- Guzzoni, V.; Selistre-de-Araújo, H.; de Cássia Marqueti, R. Tendon Remodeling in Response to Resistance Training, Anabolic Androgenic Steroids and Aging. Cells 2018, 7, 251. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Kjaer, M. The Impact of Loading, Unloading, Ageing and Injury on the Human Tendon. J. Physiol. 2019, 597, 1283–1298. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Langberg, H.; Kjaer, M. The Pathogenesis of Tendinopathy: Balancing the Response to Loading. Nat. Rev. Rheumatol. 2010, 6, 262–268. [Google Scholar] [CrossRef]
- Snedeker, J.G.; Foolen, J. Tendon Injury and Repair—A Perspective on the Basic Mechanisms of Tendon Disease and Future Clinical Therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef]
- Yang, G.; Im, H.J.; Wang, J.H.C. Repetitive Mechanical Stretching Modulates IL-1β Induced COX-2, MMP-1 Expression, and PGE2 Production in Human Patellar Tendon Fibroblasts. Gene 2005, 363, 166–172. [Google Scholar] [CrossRef]
- Khan, M.H.; Li, Z.; Wang, J.H.C. Repeated Exposure of Tendon to Prostaglandin-E2 Leads to Localized Tendon Degeneration. Clin. J. Sport Med. 2005, 15, 27–33. [Google Scholar] [CrossRef]
- Sullo, A.; Maffulli, N.; Capasso, G.; Testa, V. The Effects of Prolonged Peritendinous Administration of PGE1 to the Rat Achilles Tendon: A Possible Animal Model of Chronic Achilles Tendinopathy. J. Orthop. Sci. 2001, 6, 349–357. [Google Scholar] [CrossRef]
- Morita, W.; Dakin, S.G.; Snelling, S.J.B.; Carr, A.J. Cytokines in Tendon Disease: A Systematic Review. Bone Jt. Res. 2017, 6, 656–664. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Huang, J.; Zhao, K.; Chen, X.; Yin, Z.; Heng, B.C.; Chen, W.; Shen, W. The Roles of Inflammatory Mediators and Immunocytes in Tendinopathy. J. Orthop. Transl. 2018, 14, 23–33. [Google Scholar] [CrossRef]
- Abraham, A.C.; Shah, S.A.; Golman, M.; Song, L.; Li, X.; Kurtaliaj, I.; Akbar, M.; Millar, N.L.; Abu-Amer, Y.; Galatz, L.M.; et al. Targeting the NF-KB Signaling Pathway in Chronic Tendon Disease. Sci. Transl. Med. 2019, 11, eaav4319. [Google Scholar] [CrossRef]
- Schubert, T.E.O.; Weidler, C.; Lerch, K.; Hofstädter, F.; Straub, R.H. Achilles Tendinosis Is Associated with Sprouting of Substance P Positive Nerve Fibres. Ann. Rheum. Dis. 2005, 64, 1083–1086. [Google Scholar] [CrossRef]
- Lana, J.F.J.F.; Huber, S.C.S.C.; Purita, J.; Tambeli, C.H.C.H.; Santos, G.S.G.S.; Paulus, C.; Annichino-Bizzacchi, J.M.J.M. Leukocyte-Rich PRP versus Leukocyte-Poor PRP—The Role of Monocyte/Macrophage Function in the Healing Cascade. J. Clin. Orthop. Trauma 2019, 10, S7–S12. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Zhang, D.; Chistiakov, D.A. Monocyte Differentiation and Macrophage Polarization. Vessel Plus 2019, 2019, 10. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef]
- Kaji, D.A.; Howell, K.L.; Balic, Z.; Hubmacher, D.; Huang, A.H. Tgfβ Signaling Is Required for Tenocyte Recruitment and Functional Neonatal Tendon Regeneration. eLife 2020, 9, e51779. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Renström, P. Tendinopathy in Sport. Sports Health 2012, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The Protective Role of Glutathione in Osteoarthritis. J. Clin. Orthop. Trauma 2020, 15, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, K.; McKay, J.; Li, J.; Lana, J.F.; Macedo, A.; Santos, G.S.; Murrell, W.D. Nutritional, Metabolic and Genetic Considerations to Optimise Regenerative Medicine Outcome for Knee Osteoarthritis. J. Clin. Orthop. Trauma 2019, 10, 2–8. [Google Scholar] [CrossRef]
- Marcum, Z.A.; Hanlon, J.T. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann. Long-Term Care 2010, 18, 24–27. [Google Scholar]
- Kruel, A.V.S.; Ribeiro, L.L.; Gusmão, P.D.; Huber, S.C.; Lana, J.F.S.D. Orthobiologics in the Treatment of Hip Disorders. World J. Stem. Cells 2021, 13, 304–316. [Google Scholar] [CrossRef]
- Maheu, E.; Rannou, F.; Reginster, J.Y. Efficacy and Safety of Hyaluronic Acid in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Semin. Arthritis Rheum. 2016, 45, S28–S33. [Google Scholar] [CrossRef]
- Prieto, J.G.; Pulido, M.M.; Zapico, J.; Molina, A.J.; Gimeno, M.; Coronel, P.; Alvarez, A.I. Comparative Study of Hyaluronic Derivatives: Rheological Behaviour, Mechanical and Chemical Degradation. Int. J. Biol. Macromol. 2005, 35, 63–69. [Google Scholar] [CrossRef]
- Ghosh, P.; Guidolin, D. Potential Mechanism of Action of Intra-Articular Hyaluronan Therapy in Osteoarthritis: Are the Effects Molecular Weight Dependent? Semin. Arthritis Rheum. 2002, 32, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.D.; Ronchetti, I.P.; Lini, E.; Guerra, D.; Frizziero, L. Morphological Analysis of Articular Cartilage Biopsies from a Randomized, Clinical Study Comparing the Effects of 500–730 KDa Sodium Hyaluronate (Hyalgan®) and Methylprednisolone Acetate on Primary Osteoarthritis of the Knee. Osteoarthr. Cartil. 2001, 9, 371–381. [Google Scholar] [CrossRef]
- Bruyère, O.; Cooper, C.; Pelletier, J.P.; Branco, J.; Luisa Brandi, M.; Guillemin, F.; Hochberg, M.C.; Kanis, J.A.; Kvien, T.K.; Martel-Pelletier, J.; et al. An Algorithm Recommendation for the Management of Knee Osteoarthritis in Europe and Internationally: A Report from a Task Force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 2014, 44, 253–263. [Google Scholar] [CrossRef]
- Lajeunesse, D.; Delalandre, A.; Martel-Pelletier, J.; Pelletier, J.P. Hyaluronic Acid Reverses the Abnormal Synthetic Activity of Human Osteoarthritic Subchondral Bone Osteoblasts. Bone 2003, 33, 703–710. [Google Scholar] [CrossRef]
- Brun, P.; Zavan, B.; Vindigni, V.; Schiavinato, A.; Pozzuoli, A.; Iacobellis, C.; Abatangelo, G. In Vitro Response of Osteoarthritic Chondrocytes and Fibroblast-like Synoviocytes to a 500-730 KDa Hyaluronan Amide Derivative. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2073–2081. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Rugolo, C.A.; Calatroni, A. Differential Effect of Molecular Mass Hyaluronan on Lipopolysaccharide-Induced Damage in Chondrocytes. Innate Immun 2010, 16, 48–63. [Google Scholar] [CrossRef]
- Day, A.J.; de la Motte, C.A. Hyaluronan Cross-Linking: A Protective Mechanism in Inflammation? Trends Immunol. 2005, 26, 637–643. [Google Scholar] [CrossRef]
- Julovi, S.M.; Yasuda, T.; Shimizu, M.; Hiramitsu, T.; Nakamura, T. Inhibition of Interleukin-1β-Stimulated Production of Matrix Metalloproteinases by Hyaluronan via CD44 in Human Articular Cartilage. Arthritis Rheum. 2004, 50, 516–525. [Google Scholar] [CrossRef]
- Kalaci, A.; Yilmaz, R.H.; Aslan, B.; Söğüt, S.; Yanat, A.N.; Uz, E. Effects of Hyaluronan on Nitric Oxide Levels and Superoxide Dismutase Activities in Synovial Fluid in Knee Osteoarthritis. Clin. Rheumatol. 2007, 26, 1306–1311. [Google Scholar] [CrossRef]
- Karna, E.; Miltyk, W.; Surażyński, A.; Pałka, J.A. Protective Effect of Hyaluronic Acid on Interleukin-1-Induced Deregulation of Βeta 1 -Integrin and Insulin-like Growth Factor-I Receptor Signaling and Collagen Biosynthesis in Cultured Human Chondrocytes. Mol. Cell. Biochem. 2008, 308, 57–64. [Google Scholar] [CrossRef]
- Abate, M.; Pelotti, P.; De Amicis, D.; Di Iorio, A.; Galletti, S.; Salini, V. Viscosupplementation with Hyaluronic Acid in Hip Osteoarthritis (a Review). Upsala J. Med. Sci. 2008, 113, 261–278. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and Signaling. Biochim. Biophys. Acta 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Kouvidi, K.; Berdiaki, A.; Nikitovic, D.; Katonis, P.; Afratis, N.; Hascall, V.C.; Karamanos, N.K.; Tzanakakis, G.N. Role of Receptor for Hyaluronic Acid-Mediated Motility (RHAMM) in Low Molecular Weight Hyaluronan (LMWHA)-Mediated Fibrosarcoma Cell Adhesion. J. Biol. Chem. 2011, 286, 38509–38520. [Google Scholar] [CrossRef]
- McCain, J.P.; Balazs, E.A.; de la Rua, H. Preliminary Studies on the Use of a Viscoelastic Solution in Arthroscopic Surgery of the Temporomandibular Joint. J. Oral Maxillofac. Surg. 1989, 47, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Moskowitz, R. Intraarticular Sodium Hyaluronate (Hyalgan) in the Treatment of Patients with Osteoarthritis of the Knee: A Randomized Clinical Trial. Hyalgan Study Group. J. Rheumatol. 1998, 25, 2203–2212. [Google Scholar] [PubMed]
- Kolarz, G.; Kotz, R.; Hochmayer, I. Long-Term Benefits and Repeated Treatment Cycles of Intra-Articular Sodium Hyaluronate (Hyalgan) in Patients with Osteoarthritis of the Knee. Semin. Arthritis Rheum. 2003, 32, 310–319. [Google Scholar] [CrossRef]
- Migliore, A.; Bizzi, E.; De Lucia, O.; Delle Sedie, A.; Bentivegna, M.; Mahmoud, A.; Foti, C. Differences among Branded Hyaluronic Acids in Italy, Part 1: Data from In Vitro and Animal Studies and Instructions for Use. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 89–101. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Parrilli, A.; Martini, L.; Nicoli Aldini, N.; Abatangelo, G.; Frizziero, A.; Fini, M. Effects of Intra-Articular Hyaluronic Acid Associated to Chitlac (Arty-Duo®) in a Rat Knee Osteoarthritis Model. J. Orthop. Res. 2019, 37, 867–876. [Google Scholar] [CrossRef]
- Tarricone, E.; Mattiuzzo, E.; Belluzzi, E.; Elia, R.; Benetti, A.; Venerando, R.; Vindigni, V.; Ruggieri, P.; Brun, P. Anti-Inflammatory Performance of Lactose-Modified Chitosan and Hyaluronic Acid Mixtures in an In Vitro Macrophage-Mediated Inflammation Osteoarthritis Model. Cells 2020, 9, E1328. [Google Scholar] [CrossRef]
- Tarricone, E.; Elia, R.; Mattiuzzo, E.; Faggian, A.; Pozzuoli, A.; Ruggieri, P.; Brun, P. The Viability and Anti-Inflammatory Effects of Hyaluronic Acid-Chitlac-Tracimolone Acetonide- β-Cyclodextrin Complex on Human Chondrocytes. Cartilage 2021, 13, 920S–924S. [Google Scholar] [CrossRef]
- Hollander, A.P.; Dickinson, S.C.; Sims, T.J.; Brun, P.; Cortivo, R.; Kon, E.; Marcacci, M.; Zanasi, S.; Borrione, A.; De Luca, C.; et al. Maturation of Tissue Engineered Cartilage Implanted in Injured and Osteoarthritic Human Knees. Tissue Eng. 2006, 12, 1787–1798. [Google Scholar] [CrossRef]
- Lana, J.F.S.D.; Weglein, A.; Sampson, S.E.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.K.; et al. Randomized Controlled Trial Comparing Hyaluronic Acid, Platelet-Rich Plasma and the Combination of Both in the Treatment of Mild and Moderate Osteoarthritis of the Knee. J. Stem. Cells Regen Med. 2016, 12, 69–78. [Google Scholar]
- Levett, P.A.; Hutmacher, D.W.; Malda, J.; Klein, T.J. Hyaluronic Acid Enhances the Mechanical Properties of Tissue-Engineered Cartilage Constructs. PLoS ONE 2014, 9, e113216. [Google Scholar] [CrossRef]
- Gomis, A.; Miralles, A.; Schmidt, R.F.; Belmonte, C. Intra-Articular Injections of Hyaluronan Solutions of Different Elastoviscosity Reduce Nociceptive Nerve Activity in a Model of Osteoarthritic Knee Joint of the Guinea Pig. Osteoarthr. Cartil. 2009, 17, 798–804. [Google Scholar] [CrossRef]
- Kuroki, K.; Cook, J.L.; Kreeger, J.M. Mechanisms of Action and Potential Uses of Hyaluronan in Dogs with Osteoarthritis. J. Am. Vet. Med. Assoc. 2002, 221, 944–950. [Google Scholar] [CrossRef]
- Díaz-Gallego, L.; Prieto, J.G.; Coronel, P.; Gamazo, L.E.; Gimeno, M.; Alvarez, A.I. Apoptosis and Nitric Oxide in an Experimental Model of Osteoarthritis in Rabbit after Hyaluronic Acid Treatment. J. Orthop. Res. 2005, 23, 1370–1376. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Yang, C.; Amadio, P.C.; Zhao, C.; Zobitz, M.E.; An, K.-N. Reducing Friction by Chemically Modifying the Surface of Extrasynovial Tendon Grafts. J. Orthop. Res. 2004, 22, 984–989. [Google Scholar] [CrossRef]
- Nishida, J.; Araki, S.; Akasaka, T.; Toba, T.; Shimamura, T.; Amadio, P.C.; An, K.N. Effect of Hyaluronic Acid on the Excursion Resistance of Tendon Grafts. A Biomechanical Study in a Canine Model in Vitro. J. Bone Jt. Surg. Br. 2004, 86, 918–924. [Google Scholar] [CrossRef]
- Taguchi, M.; Zhao, C.; Sun, Y.-L.; Jay, G.D.; An, K.-N.; Amadio, P.C. The Effect of Surface Treatment Using Hyaluronic Acid and Lubricin on the Gliding Resistance of Human Extrasynovial Tendons in Vitro. J. Hand. Surg. Am. 2009, 34, 1276–1281. [Google Scholar] [CrossRef]
- Akasaka, T.; Nishida, J.; Imaeda, T.; Shimamura, T.; Amadio, P.C.; An, K.-N. Effect of Hyaluronic Acid on the Excursion Resistance of Tendon Graft: A Biomechanical in Vitro Study in a Modified Human Model. Clin. Biomech. (Bristol Avon) 2006, 21, 810–815. [Google Scholar] [CrossRef]
- Yamada, T.; Gotoh, M.; Nakama, K.; Mitsui, Y.; Higuchi, F.; Nagata, K. Effects of Hyaluronan on Cell Proliferation and MRNA Expression of Procollagens Alpha 1 (I) and Alpha 1 (III) in Tendon-Derived Fibroblasts from Patients with Rotator Cuff Disease: An in Vitro Study. Am. J. Sport. Med. 2007, 35, 1870–1876. [Google Scholar] [CrossRef]
- Shimpuku, E.; Hamada, K.; Handa, A.; Kobayashi, K.; Nakajima, T.; Yamazaki, H.; Nakamura, M.; Fukuda, H.; Mochida, J. Molecular Effects of Sodium Hyaluronate on the Healing of Avian Supracoracoid Tendon Tear: According to in Situ Hybridization and Real-Time Polymerase Chain Reaction. J. Orthop. Res. 2007, 25, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Wiig, M.; Abrahamsson, S.O. Hyaluronic Acid Modulates Cell Proliferation Unequally in Intrasynovial and Extrasynovial Rabbit Tendons in Vitro. J. Hand Surg. Br. 2000, 25, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Gotoh, M.; Kanazawa, T.; Ohta, K.; Nakamura, K.; Honda, H.; Ohzono, H.; Shimokobe, H.; Mitsui, Y.; Shirachi, I.; et al. Effects of Corticosteroids and Hyaluronic Acid on Torn Rotator Cuff Tendons in Vitro and in Rats. J. Orthop. Res. 2015, 33, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, K.; Ohno, S.; Fujimoto, K.; Honda, K.; Ijuin, C.; Tanaka, N.; Doi, T.; Nakahara, M.; Tanne, K. Proinflammatory Cytokines Regulate the Gene Expression of Hyaluronic Acid Synthetase in Cultured Rabbit Synovial Membrane Cells. Connect Tissue Res. 2001, 42, 187–195. [Google Scholar] [CrossRef]
- Smith, M.M.; Ghosh, P. The Synthesis of Hyaluronic Acid by Human Synovial Fibroblasts Is Influenced by the Nature of the Hyaluronate in the Extracellular Environment. Rheumatol. Int. 1987, 7, 113–122. [Google Scholar] [CrossRef]
- Osti, L.; Berardocco, M.; di Giacomo, V.; Di Bernardo, G.; Oliva, F.; Berardi, A.C. Hyaluronic Acid Increases Tendon Derived Cell Viability and Collagen Type I Expression in Vitro: Comparative Study of Four Different Hyaluronic Acid Preparations by Molecular Weight. BMC Musculoskelet. Disord. 2015, 16, 284. [Google Scholar] [CrossRef]
- Neumann, A.; Schinzel, R.; Palm, D.; Riederer, P.; Münch, G. High Molecular Weight Hyaluronic Acid Inhibits Advanced Glycation Endproduct-Induced NF-KappaB Activation and Cytokine Expression. FEBS Lett. 1999, 453, 283–287. [Google Scholar] [CrossRef]
- Samuelson, E.M.; Ebel, J.A.; Reynolds, S.B.; Arnold, R.M.; Brown, D.E. The Cost-Effectiveness of Platelet-Rich Plasma Compared With Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 3072–3078. [Google Scholar] [CrossRef]
- Rosen, J.; Niazi, F.; Dysart, S. Cost-Effectiveness of Treating Early to Moderate Stage Knee Osteoarthritis with Intra-Articular Hyaluronic Acid Compared to Conservative Interventions. Adv. Ther. 2020, 37, 344–352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).