Profiling the Spectrum of Headache Disorders on 440 Breast Cancer Patients: Highlights on Clinical and Pathological Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
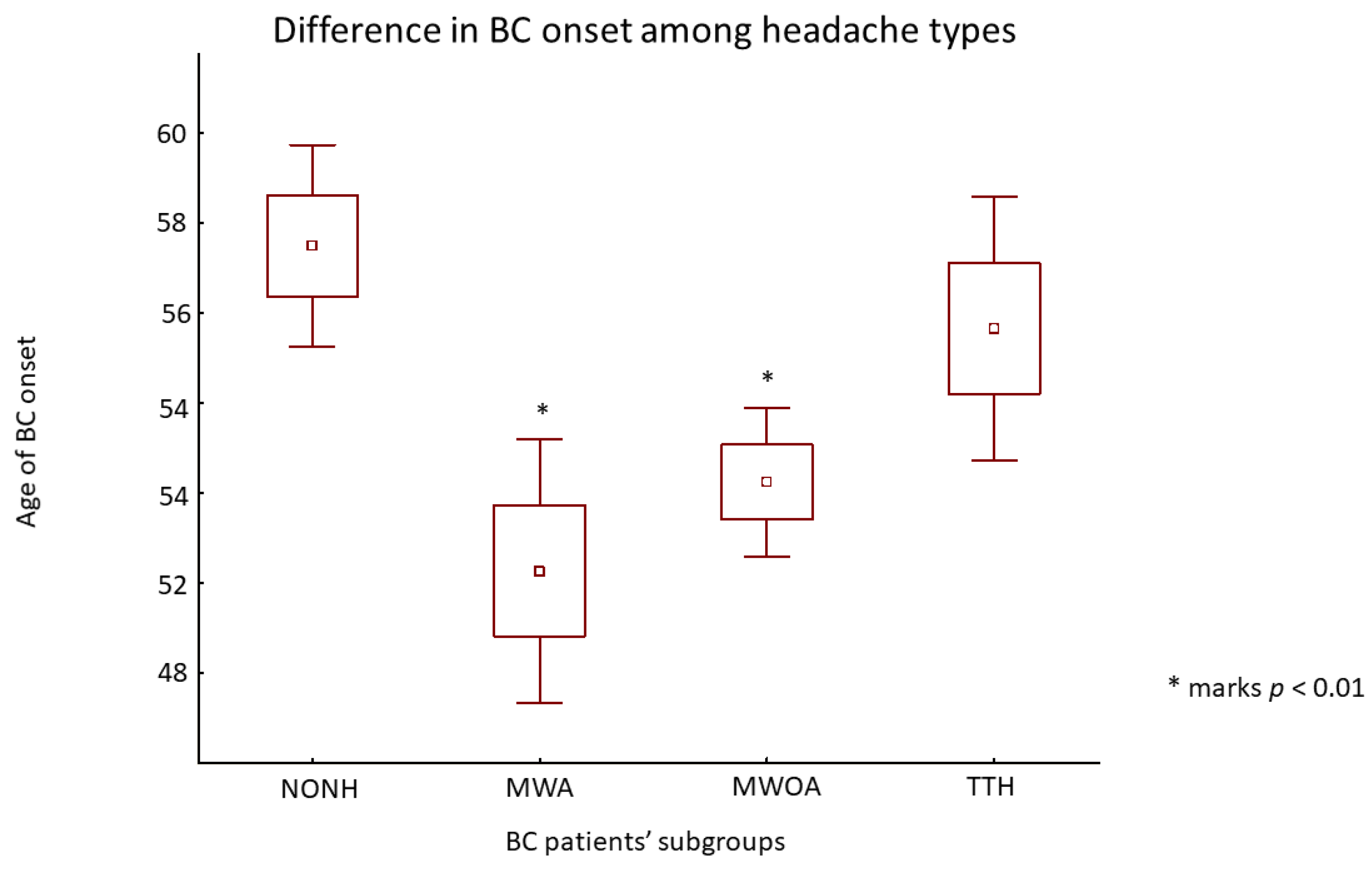
3. Results
3.1. BC Patients’ Characteristics
3.2. Headache Profiles
3.3. Effects of BC Therapies on Headache
3.4. Comorbidities Interplay and Adverse Event Rate
3.5. Relationship between BC and M
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Broeders, M.; Moss, S.; Nyström, L.; Njor, S.; Jonsson, H.; Paap, E.; Massat, N.; Duffy, S.; Lynge, E.; Paci, E.; et al. The impact of mammographic screening on breast cancer mortality in Europe: A review of observational studies. J. Med. Screen. 2012, 19, 14–25. [Google Scholar] [CrossRef]
- Mathes, R.W.; Malone, K.E.; Daling, J.R.; Davis, S.; Lucas, S.M.; Porter, P.L.; Li, C.I. Migraine in postmenopausal women and the risk of invasive breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3116–3122. [Google Scholar] [CrossRef]
- Li, C.I.; Mathes, R.W.; Malone, K.E.; Daling, J.R.; Bernstein, L.; Marchbanks, P.A.; Strom, B.L.; Simon, M.S.; Press, M.F.; Deapen, D.; et al. Relationship between migraine history and breast cancer risk among premenopausal and postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2030–2034. [Google Scholar] [CrossRef]
- Ghorbani, A.; Moradi, A.; Gookizadeh, A.; Jokar, S.; Sonbolestan, S.A. Evaluation of relationship between breast cancer and migraine. Adv. Biomed. Res. 2015, 4, 14. [Google Scholar] [CrossRef]
- Li, C.I.; Mathes, R.W.; Bluhm, E.C.; Caan, B.; Cavanagh, M.F.; Chlebowski, R.T.; Michael, Y.; O′Sullivan, M.J.; Stefanick, M.L.; Prentice, R. Migraine history and breast cancer risk among postmenopausal women. J. Clin. Oncol. 2010, 28, 1005–1010. [Google Scholar] [CrossRef]
- Lowry, S.J.; Malone, K.E.; Cushing-Haugen, K.L.; Li, C.I. The risk of breast cancer associated with specific patterns of migraine history. Cancer Causes Control 2014, 25, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.C.; Rice, M.S.; Fortner, R.T.; Eliassen, A.H.; Kurth, T.; Tamimi, R.M. Migraine and breast cancer risk: A prospective cohort study and meta-analysis. J. Natl. Cancer Inst. 2014, 107, 381. [Google Scholar] [CrossRef]
- Shi, M.; DeRoo, L.A.; Sandler, D.P.; Weinberg, C.R. Migraine and possible etiologic heterogeneity for hormone-receptor-negative breast cancer. Sci. Rep. 2015, 5, 14943. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, S.; Veisani, Y.; Ghorbani, M.; Delpisheh, A.; Abbastabar, H. Migraine History and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Adv. Breast Cancer Res. 2015, 4, 63–70. [Google Scholar] [CrossRef]
- Wu, X.; Wang, M.; Li, S.; Zhang, Y. Migraine and breast cancer risk: A meta-analysis of observational studies based on MOOSE compliant. Medicine 2016, 95, e4031. [Google Scholar] [CrossRef] [PubMed]
- Hesari, E.; Ahmadinezhad, M.; Arshadi, M.; Azizi, H.; Khodamoradi, F. The association between migraine and breast cancer risk: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263628. [Google Scholar] [CrossRef]
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- European Network of Cancer Registries. Available online: https://www.encr.eu (accessed on 17 November 2022).
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Ashina, S.; Mitsikostas, D.D.; Lee, M.J.; Yamani, N.; Wang, S.-J.; Messina, R.; Ashina, H.; Buse, D.C.; Pozo-Rosich, P.; Jensen, R.H.; et al. Tension-type headache. Nat. Rev. Dis. Prim. 2021, 7, 24. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.M.J.M.; Dodick, D.W. Migraine. Nat. Rev. Dis. Prim. 2022, 8, 2. [Google Scholar] [CrossRef]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef]
- Olesen, J.; Bendtsen, L.; Goadsby, P. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Winter, A.C.; Rexrode, K.M.; Lee, I.M.; Buring, J.E.; Tamimi, R.M.; Kurth, T. Migraine and subsequent risk of breast cancer: A prospective cohort study. Cancer Causes Control 2013, 24, 81–89. [Google Scholar] [CrossRef]
- De Sanctis, R.; Agostinetto, E.; Masci, G.; Ferraro, E.; Losurdo, A.; Viganò, A.; Antunovic, L.; Zuradelli, M.; Torrisi, R.M.C.; Santoro, A. Predictive Factors of Eribulin Activity in Metastatic Breast Cancer Patients. Oncology 2018, 94, 19–28. [Google Scholar] [CrossRef]
- Viganò, A.; Savastano, E.; Petolicchio, B.; Toscano, M.; De Sanctis, R.; Maestrini, I.; Di Piero, V. A Study of Clinical Features and Risk Factors of Self-Referring Emergency Department Headache Patients: A Comparison with Headache Center Outpatients. Eur. Neurol. 2020, 83, 34–40. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, R.; Viganò, A.; Giuliani, A.; Gronchi, A.; De Paoli, A.; Navarria, P.; Quagliuolo, V.; Santoro, A.; Colosimo, A. Unsupervised versus Supervised Identification of Prognostic Factors in Patients with Localized Retroperitoneal Sarcoma: A Data Clustering and Mahalanobis Distance Approach. Biomed. Res. Int. 2018, 2018, 2786163. [Google Scholar] [CrossRef] [PubMed]
- Alessiani, M.; Petolicchio, B.; De Sanctis, R.; Squitieri, M.; Di Giambattista, R.; Puma, M.; Franzese, C.; Toscano, M.; Derchi, C.C.; Gilliéron, E.; et al. A Propensity Score Matching Study on the Effect of OnabotulinumtoxinA Alone versus Short-Term Psychodynamic Psychotherapy Plus Drug-of-Choice as Preventive Therapy in Chronic Migraine: Effects and Predictive Factors. Eur. Neurol. 2022, 85, 453–459. [Google Scholar] [CrossRef]
- Tiberio, P.; Antunovic, L.; Gaudio, M.; Viganò, A.; Pastore, M.; Miggiano, C.; Jacobs, F.; Benvenuti, C.; Farina, E.; Chiti, A.; et al. The Relationship among Bowel [18]F-FDG PET Uptake, Pathological Complete Response, and Eating Habits in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Nutrients 2023, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Hagen, K.; Linde, M.; Steiner, T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 2022, 23, 34. [Google Scholar] [CrossRef]
- Lipton, R.B.; Scher, A.I.; Kolodner, K.; Liberman, J.; Steiner, T.J.; Stewart, W.F. Migraine in the United States: Epidemiology and patterns of health care use. Neurology 2002, 58, 885–894. [Google Scholar] [CrossRef]
- De Sanctis, R.; Viganò, A.; Pindilli, S.; Torrisi, R.; Santoro, A. A pilot analysis of headache disorders in breast cancer patients. Neurol. Sci. 2022, 43, 3313–3320. [Google Scholar] [CrossRef]
- Fan, C.Y.; Lin, C.S.; Huang, W.Y.; Lin, K.T.; Chao, H.L.; Tsao, C.C.; Liu, M.Y.; Tsai, I.J.; Kao, C.H. Association Between Migraine and Breast Cancer Risk: A Population-Based Cohort Study and Literature Review. J. Womens Health 2018, 27, 1499–1507. [Google Scholar] [CrossRef]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007, 27, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Ouchchane, L.; Guy, N.; Bayle, D.J.; Dallel, R.; Clavelou, P. Migraine headaches and pain with neuropathic characteristics: Comorbid conditions in patients with multiple sclerosis. Pain 2013, 154, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Biondi, D.M. Is migraine a neuropathic pain syndrome? Curr. Pain Headache Rep. 2006, 10, 167–178. [Google Scholar] [CrossRef]
- Ghosh, J.; Joshi, G.; Pradhan, S.; Mittal, B. Potential role of aromatase over estrogen receptor gene polymorphisms in migraine susceptibility: A case control study from North India. PLoS ONE 2012, 7, e34828. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Dong, Z.; Wang, X.; Yu, S. Impact of ESR1 Gene Polymorphisms on Migraine Susceptibility: A Meta-Analysis. Medicine 2015, 94, e0976. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kilinc, E.; Camsari, C.; Ogun, M.N. Effects of estrogen and progesterone on the neurogenic inflammatory neuropeptides: Implications for gender differences in migraine. Exp. Brain Res. 2020, 238, 2625–2639. [Google Scholar] [CrossRef]
- Coppola, G.; Pierelli, F.; Schoenen, J. Habituation and migraine. Neurobiol. Learn. Mem. 2009, 92, 249–259. [Google Scholar] [CrossRef]
- Viganò, A.; Torrieri, M.C.; Toscano, M.; Puledda, F.; Petolicchio, B.; Sasso D’Elia, T.; Verzina, A.; Ruggiero, S.; Altieri, M.; Vicenzini, E.; et al. Neurophysiological correlates of clinical improvement after greater occipital nerve (GON) block in chronic migraine: Relevance for chronic migraine pathophysiology. J. Headache Pain 2018, 19, 73. [Google Scholar] [CrossRef]
- Kopparapu, P.K.; Tinzl, M.; Anagnostaki, L.; Persson, J.L.; Dizeyi, N. Expression and localization of serotonin receptors in human breast cancer. Anticancer Res. 2013, 33, 363–370. [Google Scholar] [PubMed]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Warfvinge, K.; Krause, D.N.; Maddahi, A.; Edvinsson, J.C.A.; Edvinsson, L.; Haanes, K.A. Estrogen receptors α, β and GPER in the CNS and trigeminal system—Molecular and functional aspects. J. Headache Pain 2020, 21, 131. [Google Scholar] [CrossRef]
- Giugliano, F.; Curigliano, G.; Tarantino, P. HER2-low expression in breast oncology: Treatment implications in the smart chemotherapy era. Eur. J. Cancer Prev. 2023, 32, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Colombo, B.M.; Puppo, F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern. Emerg. Med. 2011, 6, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Fidan, I.; Yüksel, S.; Ymir, T.; Irkeç, C.; Aksakal, F.N. The importance of cytokines, chemokines and nitric oxide in pathophysiology of migraine. J. Neuroimmunol. 2006, 171, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Djalali, M.; Abdolahi, M.; Hosseini, R.; Miraghajani, M.; Mohammadi, H.; Djalali, M. The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2020, 41, 101256. [Google Scholar] [CrossRef]
- Seif, F.; Torki, Z.; Zalpoor, H.; Habibi, M.; Pornour, M. Breast cancer tumor microenvironment affects Treg/IL-17-producing Treg/Th17 cell axis: Molecular and therapeutic perspectives. Mol. Ther. Oncolytics 2023, 28, 132–157. [Google Scholar] [CrossRef]
- Lipton, R.B.; Nicholson, R.A.; Reed, M.L.; Araujo, A.B.; Jaffe, D.H.; Faries, D.E.; Buse, D.C.; Shapiro, R.E.; Ashina, S.; Cambron-Mellott, M.J.; et al. Diagnosis, consultation, treatment, and impact of migraine in the US: Results of the OVERCOME (US) study. Headache 2022, 62, 122–140. [Google Scholar] [CrossRef]

| Patients (n = 440) | ||
|---|---|---|
| Mean | SD | |
| Age | 53.8 | 12.1 |
| BMI | 24.7 | 4.9 |
| ECOG-PS, median (range) | 0 | (0–3) |
| n | % | |
| Menopausal status | ||
| Premenopause | 161 | 36.6 |
| Postmenopause | 242 | 55.0 |
| Perimenopause | 32 | 7.3 |
| NA | 5 | 1.1 |
| Comorbidities | ||
| Cardiovascular | 49 | 11.1 |
| Metabolic | 32 | 7.3 |
| Cardiovascular + metabolic | 14 | 3.2 |
| Cardiovascular + other | 34 | 7.7 |
| Metabolic + other | 30 | 6.8 |
| Cardiovascular + metabolic + other | 36 | 8.2 |
| Other comorbidities | 84 | 19.1 |
| No comorbidity | 161 | 36.6 |
| Histologic subtype | ||
| NST | 347 | 78.9 |
| Lobular | 52 | 11.8 |
| Other | 17 | 3.9 |
| Mixed | 20 | 4.5 |
| NA | 4 | 0.9 |
| Nottingham score | ||
| G1 | 26 | 5.9 |
| G2 | 271 | 61.6 |
| G3 | 132 | 30.0 |
| NA | 11 | 2.5 |
| Clinical stage | ||
| I | 204 | 46.4 |
| II | 191 | 43.4 |
| III | 45 | 10.2 |
| Molecular Subtype | ||
| Luminal A | 187 | 42.5 |
| Luminal B HER2 negative | 127 | 28.9 |
| Luminal B HER2 positive | 60 | 13.6 |
| HER2 positive | 29 | 6.6 |
| TNBC | 36 | 8.2 |
| HR (HER2 NA) | 1 | 0.2 |
| n | % | |
|---|---|---|
| Chemotherapy (n = 207) | ||
| AC | 40 | 19.3 |
| Taxanes | 14 | 6.8 |
| AC + Taxanes | 143 | 69.1 |
| Other | 10 | 4.8 |
| Hormonal therapy (n = 365) | ||
| Tamoxifen | 131 | 35.9 |
| AI | 173 | 47.4 |
| Switch | 57 | 15.6 |
| Other | 4 | 1.1 |
| LH-RH analog | ||
| yes | 122 | 27.7 |
| no | 318 | 72.3 |
| Radiotherapy | ||
| yes | 324 | 73.6 |
| no | 116 | 26.4 |
| Headache Patients (n = 319) | |||
|---|---|---|---|
| MWA | MWOA | TTH | |
| Number of patients (%) | 59 (18.5) | 188 (58.9) | 72 (22.6) |
| Frequency days/month (SD) | 4.2 (±7.7) | 2.7 (±5.2) | 1.7 (±4.1) |
| Age at onset (SD) | 24.8 (±14.1) | 23.7 (±12.5) | 25.1 (±14.0) |
| % Menstrually related (% on total headache patients) | 50.8 (9.4) | 59.6 (35.0) | 25.0 (5.8) |
| % Ongoing preventive therapy (% on total headache patients) | 6.8 (1.3) | 2.1 (1.3) | 2.8 (0.6) |
| MWA% | MWOA% | TTH% | |
|---|---|---|---|
| Menopause | |||
| Improved | 43.9 | 41.1 | 26.1 |
| Worsened | 17.5 | 8.1 | 5.8 |
| Started | 0.0 | 1.1 | 0.0 |
| No change | 33.3 | 39.5 | 66.7 |
| Premenopause | 5.3 | 10.3 | 1.4 |
| BC diagnosis | |||
| Improved | 10.5 | 3.8 | 1.4 |
| Worsened | 8.8 | 4.8 | 15.5 |
| Started | 0.0 | 0.5 | 0.0 |
| No change | 80.7 | 90.4 | 83.1 |
| NA | 0.0 | 0.5 | 0.0 |
| Chemotherapy | |||
| Improved | 5.3 | 3.8 | 2.9 |
| Worsened | 14.0 | 5.9 | 10.1 |
| Started | 0.0 | 0.0 | 0.0 |
| No change | 26.3 | 39.5 | 34.8 |
| Therapy not carried out | 54.4 | 50.8 | 52.2 |
| Hormonal therapy | |||
| Improved | 8.8 | 10.9 | 1.4 |
| Worsened | 19.3 | 12.0 | 11.4 |
| Started | 0.0 | 0.0 | 0.0 |
| No change | 57.9 | 62.0 | 60.0 |
| NA | 0.0 | 0.5 | 0.0 |
| Therapy not carried out | 14.0 | 14.7 | 27.1 |
| Radiotherapy | |||
| Improved | 3.5 | 0.0 | 0.0 |
| Worsened | 7.0 | 2.2 | 4.4 |
| Started | 0.0 | 0.0 | 0.0 |
| No change | 52.6 | 74.5 | 66.2 |
| Therapy not carried out | 36.8 | 23.4 | 29.4 |
| BC Patients (n = 435) | ||||
|---|---|---|---|---|
| NONH | MWA | MWOA | TTH | |
| Stage I | 57 | 17 | 93 | 36 |
| Stage II | 49 | 36 | 75 | 27 |
| Stage III | 10 | 6 | 20 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieva, M.B.; Tiberio, P.; Torrisi, R.; Lanzone, J.; Di Piero, V.; Santoro, A.; Viganò, A.; De Sanctis, R. Profiling the Spectrum of Headache Disorders on 440 Breast Cancer Patients: Highlights on Clinical and Pathological Mechanisms. Biomedicines 2023, 11, 1059. https://doi.org/10.3390/biomedicines11041059
Ilieva MB, Tiberio P, Torrisi R, Lanzone J, Di Piero V, Santoro A, Viganò A, De Sanctis R. Profiling the Spectrum of Headache Disorders on 440 Breast Cancer Patients: Highlights on Clinical and Pathological Mechanisms. Biomedicines. 2023; 11(4):1059. https://doi.org/10.3390/biomedicines11041059
Chicago/Turabian StyleIlieva, Mariya Boyanova, Paola Tiberio, Rosalba Torrisi, Jacopo Lanzone, Vittorio Di Piero, Armando Santoro, Alessandro Viganò, and Rita De Sanctis. 2023. "Profiling the Spectrum of Headache Disorders on 440 Breast Cancer Patients: Highlights on Clinical and Pathological Mechanisms" Biomedicines 11, no. 4: 1059. https://doi.org/10.3390/biomedicines11041059
APA StyleIlieva, M. B., Tiberio, P., Torrisi, R., Lanzone, J., Di Piero, V., Santoro, A., Viganò, A., & De Sanctis, R. (2023). Profiling the Spectrum of Headache Disorders on 440 Breast Cancer Patients: Highlights on Clinical and Pathological Mechanisms. Biomedicines, 11(4), 1059. https://doi.org/10.3390/biomedicines11041059







