Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy
Abstract
1. Introduction
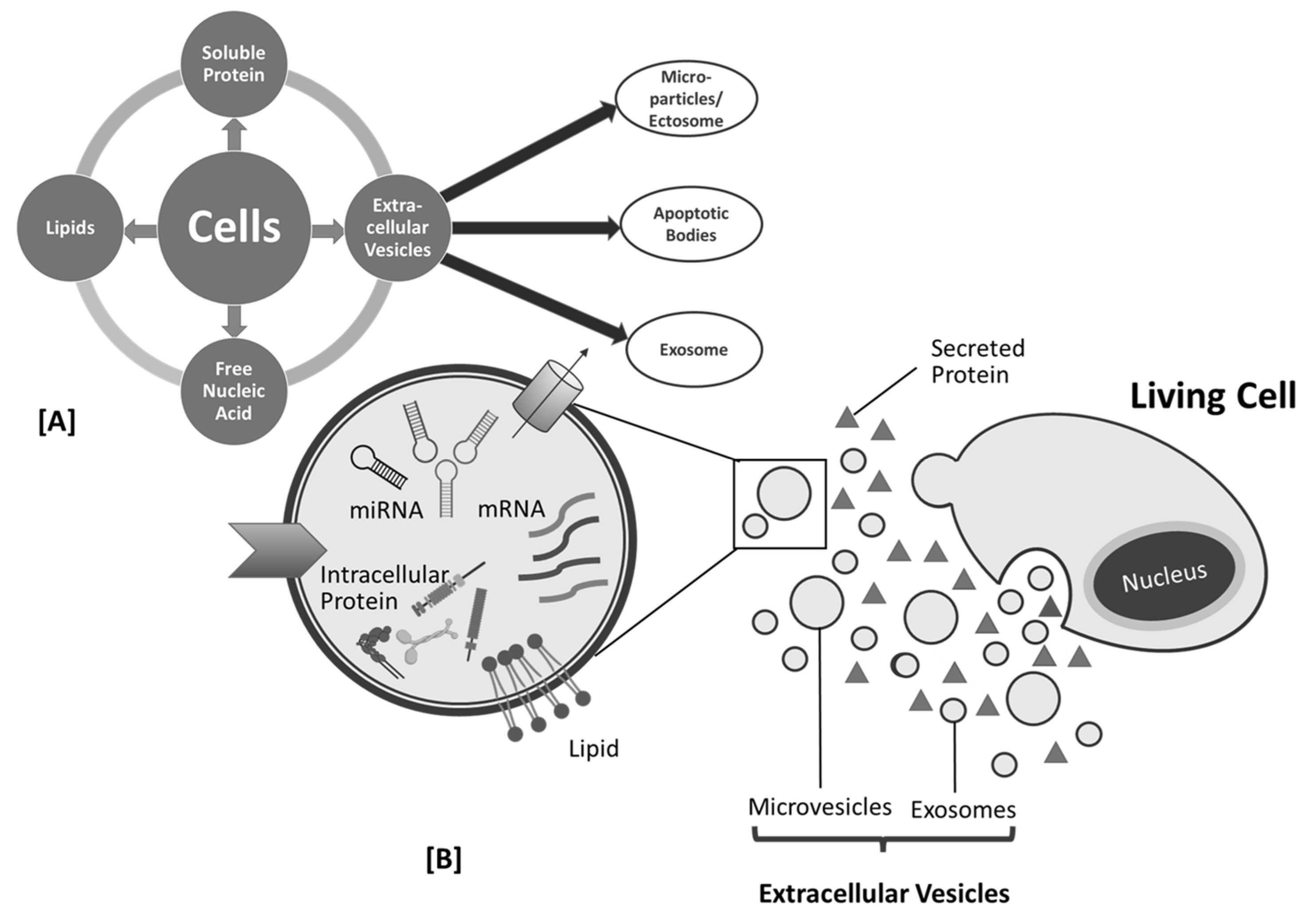
2. Plant-Derived Exosome-like Nanoparticle as Biomolecules
3. Biomedical Applications of PDENs
| PDENs Source | Type of Study | Findings | Refs. |
|---|---|---|---|
| Phellinus linteus | In vitro, in vivo, and clinical trial | PDENs stimulate anti-aging effects through the promotion of COL1A2 and inhibition of MMP-1, ROS, MDA, and SA-β-Gal in UV-induced aging skin cells | [188] |
| Coffee | In vitro | PDENs promote proliferation of LX2 and HEP40 cell lines in a concentration-dependent manner exerted by their miRNA contents | [189] |
| Momordica charantia | In vivo and in vivo | PDENs inhibit MMP-9 and apoptosis, and activate AKT and GSK3β phosphorylation, thus preserving the blood brain barrier to act as a neuroprotective agent | [190] |
| Beta vulgaris | In vitro | PDENs show antioxidant activities, angiogenesis, and inhibition of fibroblast migration to prevent scar formation and exert anti-aging effects | [191] |
| Rice | In vitro and in vivo | PDENs and their miRNA contents enhanced cell proliferation and GLUT1 expression to regulate blood glucose and metabolism | [192] |
| Hawaiian ginger roots | In vitro and in vivo | PDENs inhibit NF-κB-mediated inflammation and apoptosis, and limit SARS-CoV-2 S and Nsp12 expression through their miRNA contents | [185] |
| Blueberry | In vitro and in vivo | PDENs inhibit oxidative stress by attenuating ROS, Bcl-2, and HO-1, and accelerating Nrf2 translocation, thus ameliorating insulin resistance and liver dysfunction | [193] |
| Asparagus cochinchinensis | In vitro and in vivo | PDENs are internalized through phagocytosis and inhibition of cancer cells growth. PEGylated PDENs show promoted blood retention and anti-tumor capacity than bare PDENs | [194] |
| Strawberry | In vitro | PDENs accumulate in the cytoplasm, promote cell viability, and exert antioxidant activity through their vitamin C constituents | [88] |
| Blueberry | In vitro | PDENs inhibit TNF-α-induced cytotoxicity and oxidative stress, as well as mRNA expression of IL1R1, IL-6, MAPK1, ICAM1, TLR8, TNF, HMOX1, and NFR1 post-TNF-α administration | [195] |
| Shiitake mushroom | In vitro and in vivo | PDENs attenuate NLRP3 activation through the inhibited Casp1 p10 level, and inhibit IL-1β protein assembly through the significant reduction in pro-IL-1β, leading to liver protection | [196] |
| Ginger | In vitro and in vivo | PDENs are internalized by P. gingivalis and inhibition of bacterial growth through interaction with HBP35 and membrane depolarization thus inhibiting, bacterial attachment to oral epithelial cells and periodontal bone loss | [186] |
| Ginger | In vitro, in vivo, and clinical trial | PDENs internalization by gut microbiome regulated by its lipid composition, whereas its RNAs affect bacterial metabolisms and alter pro-inflammatory cytokines profile | [185] |
| Blueberry, coconut, ginger, grapefruit, Hami melon, kiwifruit, orange, pea, pear, soybean, and tomato | In vitro | PDENs comprised of miRNAs that could directly interact with mammalian mRNAs of inflammatory mediators | [197] |
| Citrus limon | In vitro and in vivo | PDENs inhibited tumor cell viability, but provided no inhibitory effects on normal cells. PDEN promotes Bad and Bax genes, and reduces Survivin and Bcl-xL in tumor cells, thus inducing TRAIL-induced cell death and dampening tumor growth | [182] |
| Aucklandia lapp, Rhodiola crenulata and Taraxacum mongolicum | In vitro and in vivo | PDENs have anti-fibrotic and anti-inflammatory effects through their sRNA, thus ameliorating lung fibrosis and inflammation | [198] |
| Ginger roots | In vitro | PDENs modification with arrowtail RNA was able to deliver and target specific cells with significant reduction in survivin expression and tumor growth | [199] |
| Grapes, grapefruit, ginger, and carrot | In vitro and in vivo | PDENs internalized by macrophages and stem cells promote HO-1, IL-6, and IL-10 expression, Nrf2 translocation, and activate TCF4 transcription through Wnt activation in the gut tissue | [200] |
| Citrus sinensis | In vitro | PDENs protect their miRNA contents from human salivary degrading enzymes to act as a delivery agent | [201] |
| Ginger | In vitro | PDENs significantly reduced TNF-α, IL-8, and IL-1β mRNA level by inhibiting NF-κB in intestinal cells through their regulatory miRNAs | [202] |
| Mulberry bark | In vitro and in vivo | PDENs enhanced AhR-mediated pathway mediated by HSP70, leading to COPS8 induction and inhibition of bacterial mRNAs in gut tissue, thus reducing the level of IL-6 and IL-1β | [187] |
4. Roles of PDENs in Regenerative Therapy
5. Strategies for PDENs Application in Tissue Engineering
6. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farley, A.; McLafferty, E.; Hendry, C. Cells, Tissues, Organs and Systems. Nurs. Stand. 2012, 26, 40–45. [Google Scholar] [CrossRef]
- Caplan, A.I. Design Parameters For Functional Tissue Engineering. In Functional Tissue Engineering; Guilak, F., Butler, D.L., Goldstein, S.A., Mooney, D.J., Eds.; Springer: New York, NY, USA, 2003; Volume 1. [Google Scholar]
- Farach-Carson, M.C.; Wagner, R.C.; Kiick, K.L. Extracellular Matrix: Structure, Function, and Appliations to Tissue Engineering. In Tissue Engineering; Fisher, J.P., Mikos, A.G., Bronzino, J.D., Eds.; CRC Press: Boca Raton, FL, USA, 2007; Volume 1, pp. 1–17. [Google Scholar]
- Badylak, S.; Gilbert, T.; Myers-Irvin, J. The Extracellular Matrix as a Biologic Scaffold for Tissue Engineering. In Tissue Engineering; Bronzino, J., Thomsen, P., Lindahl, A., Hubbel, J., Williams, D., Cancedda, R., de Brujin, J., Sohier, J., Eds.; Academic Press: San Diego, CA, USA, 2008; Volume 1, pp. 121–143. [Google Scholar]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Study of Intravenous Adult Human Mesenchymal Stem Cells (Prochymal) After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Beyth, S.; Schroeder, J.; Liebergall, M. Stem Cells in Bone Diseases: Current Clinical Practice. Br. Med. Bull. 2011, 99, 199–210. [Google Scholar] [CrossRef]
- Casteilla, L.; Planat-Benard, V.; Laharrague, P.; Cousin, B. Adipose-Derived Stromal Cells: Their Identity and Uses in Clinical Trials, an Update. World. J. Stem. Cells 2011, 3, 25–33. [Google Scholar] [CrossRef]
- Mingliang, R.; Bo, Z.; Zhengguo, W. Stem Cells for Cardiac Repair: Status, Mechanisms, and New Strategies. Stem. Cells Int. 2011, 2011, 310928. [Google Scholar] [CrossRef]
- Yokoo, T.; Matsumoto, K.; Yokote, S. Potential Use of Stem Cells for Kidney Regeneration. Int. J. Nephrol. 2011, 2011, 591731. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem. Cell Res. 2019, 10, 68. [Google Scholar] [CrossRef]
- Kunter, U.; Rong, S.; Boor, P.; Eitner, F.; Müller-Newen, G.; Djuric, Z.; van Roeyen, C.R.; Konieczny, A.; Ostendorf, T.; Villa, L.; et al. Mesenchymal Stem Cells Prevent Progressive Experimental Renal Failure but Maldifferentiate into Glomerular Adipocytes. J. Am. Soc. Nephrol. 2007, 18, 1754–1764. [Google Scholar] [CrossRef]
- Michalopoulos, G.K. Liver Regeneration. J. Cell Physiol. 2007, 213, 286–300. [Google Scholar] [CrossRef]
- Rubina, K.; Kalinina, N.; Efimenko, A.; Lopatina, T.; Melikhova, V.; Tsokolaeva, Z.; Sysoeva, V.; Tkachuk, V.; Parfyonova, Y. Adipose Stromal Cells Stimulate Angiogenesis via Promoting Progenitor Cell Differentiation, Secretion of Angiogenic Factors, and Enhancing Vessel Maturation. Tissue. Eng. Part A 2009, 15, 2039–2048. [Google Scholar] [CrossRef]
- Tögel, F.E.; Westenfelder, C. The Role of Multipotent Marrow Stromal Cells (MSCs) in Tissue Regeneration. Organogenesis 2011, 7, 96–100. [Google Scholar] [CrossRef]
- Mahanani, E.S.; Bachtiar, I.; Ana, I.D. Human Mesenchymal Stem Cells Behavior on Synthetic Coral Scaffold. Key. Eng. Mater. 2016, 696, 205–211. [Google Scholar] [CrossRef]
- Breitbach, M.; Bostani, T.; Roell, W.; Xia, Y.; Dewald, O.; Nygren, J.M.; Fries, J.W.U.; Tiemann, K.; Bohlen, H.; Hescheler, J.; et al. Potential Risks of Bone Marrow Cell Transplantation into Infarcted Hearts. Blood 2007, 110, 1362–1369. [Google Scholar] [CrossRef]
- Wang, J.J.; Ye, F.; Cheng, L.J.; Shi, Y.J.; Bao, J.; Sun, H.Q.; Wang, W.; Zhang, P.; Bu, H. Osteogenic Differentiation of Mesenchymal Stem Cells Promoted by Overexpression of Connective Tissue Growth Factor. J. Zhejiang Univ. Sci. B 2009, 10, 355–367. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; Rizvanov, A.A. Current Trends in Regenerative Medicine: From Cell to Cell-Free Therapy. Bionanoscience 2017, 7, 240–245. [Google Scholar] [CrossRef]
- Ana, I.D.; Barlian, A.; Hidajah, A.C.; Wijaya, C.H.; Notobroto, H.B.; Kencana Wungu, T.D. Challenges and Strategy in Treatment with Exosomes for Cell-Free-Based Tissue Engineering in Dentistry. Future Sci. OA 2021, 7, 1–21. [Google Scholar] [CrossRef]
- Amsar, R.M.; Wijaya, C.H.; Ana, I.D.; Hidajah, A.C.; Notobroto, H.B.; Kencana Wungu, T.D.; Barlian, A. Extracellular Vesicles: A Promising Cell-Free Therapy for Cartilage Repair. Future Sci. OA 2022, 8, FSO774. [Google Scholar] [CrossRef]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-Derived Exosome-like Nanoparticles: A Concise Review on Its Extraction Methods, Content, Bioactivities, and Potential as Functional Food Ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef]
- Ratnadewi, D.; Widjaja, C.H.; Barlian, A.; Amsar, R.M.; Ana, I.D.; Hidajah, A.C.; Notobroto, H.B.; Wungu, T.D.K. Isolation of Native Plant-Derived Exosome-like Nanoparticles and Their Uptake by Human Cells. Hayati 2023, 30, 182–192. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, N.J.; Heine, U. Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Johnstone, R.M. Revisiting the Road to the Discovery of Exosomes. Blood Cells Mol. Dis. 2005, 34, 214–219. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted Vesicles and Intercellular Communications. F1000 Biol. Rep. 2011, 3, 1–8. [Google Scholar] [CrossRef]
- el Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and Exosomes: Shedding the Confusion between Extracellular Vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Pluchino, S.; Smith, J.A. Explicating Exosomes: Reclassifying the Rising Stars of Intercellular Communication. Cell 2019, 177, 225–227. [Google Scholar] [CrossRef]
- Weaver, J.W.; Zhang, J.; Rojas, J.; Musich, P.R.; Yao, Z.; Jiang, Y. The Application of Exosomes in the Treatment of Triple-Negative Breast Cancer. Front. Mol. Biosci. 2022, 9, 1022725. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Lopez-Verrilli, M.A.; Caviedes, A.; Cabrera, A.; Sandoval, S.; Wyneken, U.; Khoury, M. Mesenchymal Stem Cell-Derived Exosomes from Different Sources Selectively Promote Neuritic Outgrowth. Neurosci 2016, 320, 129–139. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; el Andaloussi, S.; et al. Cells Release Subpopulations of Exosomes with Distinct Molecular and Biological Properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Yang, X.-X.; Sun, C.; Wang, L.; Guo, X.-L. New Insight into Isolation, Identification Techniques and Medical Applications of Exosomes. J. Control. Release 2019, 308, 119–129. [Google Scholar] [CrossRef]
- Harry Heijnen, B.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived From Exocytosis of Multivesicular Bodies and-Granules. Blood 1999, 94, 3791–3799. [Google Scholar] [CrossRef]
- Stremersch, S.; de Smedt, S.C.; Raemdonck, K. Therapeutic and Diagnostic Applications of Extracellular Vesicles. JCR 2016, 244, 167–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Cassarà, D.; Ginestra, A.; Dolo, V.; Miele, M.; Caruso, G.; Lucania, G.; Vittorelli, M.L. Modulation of Vesicle Shedding in 8701 BC Human Breast Carcinoma Cells. J. Submicrosc. Cytol. Pathol. 1998, 30, 45–53. [Google Scholar]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Stein, J.M.; Luzio, J.P. Ectocytosis Caused by Sublytic Autologous Complement Attack on Human Neutrophils. The Sorting of Endogenous Plasma-Membrane Proteins and Lipids into Shed Vesicles. Biochem. J. 1991, 274 Pt 2, 381–386. [Google Scholar] [CrossRef]
- Marote, A.; Teixeira, F.G.; Mendes-Pinheiro, B.; Salgado, A.J. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front. Pharm. 2016, 7, 231. [Google Scholar] [CrossRef]
- McGough, I.J.; Vincent, J.-P. Exosomes in Developmental Signalling. Development 2016, 143, 2482–2493. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes and Tumor-Mediated Immune Suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Schorey, J.S.; Bhatnagar, S. Exosome Function: From Tumor Immunology to Pathogen Biology. Traffic 2008, 9, 871–881. [Google Scholar] [CrossRef]
- Bang, C.; Thum, T. Exosomes: New Players in Cell-Cell Communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064. [Google Scholar] [CrossRef]
- Eitan, E.; Suire, C.; Zhang, S.; Mattson, M.P. Impact of Lysosome Status on Extracellular Vesicle Content and Release. Ageing Res. Rev. 2016, 32, 65–74. [Google Scholar] [CrossRef]
- Kalluri, R. The Biology and Function of Exosomes in Cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Sarko, D.K.; McKinney, C.E. Exosomes: Origins and Therapeutic Potential for Neurodegenerative Disease. Front. Neurosci. 2017, 11, 82. [Google Scholar] [CrossRef]
- Properzi, F.; Logozzi, M.; Fais, S. Exosomes: The Future of Biomarkers in Medicine. Biomark. Med. 2013, 7, 769–778. [Google Scholar] [CrossRef]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.-M.; Xu, Y.-M.; Huang, L.-F.; Wang, X.-Z. Exosomes: Novel Biomarkers for Clinical Diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Wu, S.; Cui, M.; Xu, T. Focus on Mesenchymal Stem Cell-Derived Exosomes: Opportunities and Challenges in Cell-Free Therapy. Stem. Cells Int. 2017, 2017, 6305295. [Google Scholar] [CrossRef]
- He, C.; Hua, W.; Liu, J.; Fan, L.; Hua, W.; Sun, G. Exosomes Derived from Endoplasmic Reticulum-Stressed Liver Cancer Cells Enhance the Expression of Cytokines in Macrophages via the STAT3 Signaling Pathway. Oncol. Lett. 2020, 20, 589–600. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Jin, X.; Hu, D.; Xia, C.; Xu, H.; Hu, J. NK Cell-Derived Exosomes Carry MiR-207 and Alleviate Depression-like Symptoms in Mice. J. Neuroinflammation 2020, 17, 126. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA Combined with Sustained Release of HUVECs Derived Exosomes for Promoting Cutaneous Wound Healing and Facilitating Skin Regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-Derived Exosomes for Oral Delivery of Paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Srivastava, A.; Moxley, K.; Ruskin, R.; Dhanasekaran, D.N.; Zhao, Y.D.; Ramesh, R. A Non-Invasive Liquid Biopsy Screening of Urine-Derived Exosomes for MiRNAs as Biomarkers in Endometrial Cancer Patients. AAPS J. 2018, 20, 82. [Google Scholar] [CrossRef]
- Shaimardanova, A.A.; Solovyeva, V.V.; Chulpanova, D.S.; James, V.; Kitaeva, K.V.; Rizvanov, A.A. Extracellular Vesicles in the Diagnosis and Treatment of Central Nervous System Diseases. Neural. Regen. Res. 2020, 15, 586–596. [Google Scholar] [CrossRef]
- Zhuo, C.J.; Hou, W.H.; Jiang, D.G.; Tian, H.J.; Wang, L.N.; Jia, F.; Zhou, C.H.; Zhu, J.J. Circular RNAs in Early Brain Development and Their Influence and Clinical Significance in Neuropsychiatric Disorders. Neural. Regen. Res. 2020, 15, 817–823. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Pathan, M.; Fonseka, P.; Chitti, S.; Kang, T.; Sanwlani, R.; van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A Compendium of RNA, Proteins, Lipids and Metabolites in Extracellular Vesicles. Nucleic. Acids. Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular Fractions of Sunflower Apoplastic Fluids Are Associated with Potential Exosome Marker Proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Baldini, N.; Torreggiani, E.; Roncuzzi, L.; Perut, F.; Zini, N.; Avnet, S. Exosome-like Nanovesicles Isolated from Citrus Limon L. Exert Antioxidative Effect. Curr. Pharm. Biotechnol. 2018, 19, 877–885. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Li, D.; Yao, X.; Yue, J.; Fang, Y.; Cao, G.; Midgley, A.C.; Nishinari, K.; Yang, Y. Advances in Bioactivity of MicroRNAs of Plant-Derived Exosome-Like Nanoparticles and Milk-Derived Extracellular Vesicles. J. Agric. Food Chem. 2022, 70, 6285–6299. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Sun, J.; Zhu, Z.; Cribbs, A.P.; Xiao, B. Edible Plant-Derived Nanotherapeutics and Nanocarriers: Recent Progress and Future Directions. Expert. Opin. Drug Deliv. 2022, 19, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; di Raimo, R.; Mizzoni, D.; Fais, S. The Potentiality of Plant-Derived Nanovesicles in Human Health-A Comparison with Human Exosomes and Artificial Nanoparticles. Int. J. Mol. Sci. 2022, 23, 4919. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int. J. Mol. Sci. 2022, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, L.; Zhang, Y.; Lu, R. Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics 2022, 14, 822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant Derived Edible Nanoparticles as a New Therapeutic Approach against Diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Q.; Kim, K.-H. Emergence of Edible Plant-Derived Nanovesicles as Functional Food Components and Nanocarriers for Therapeutics Delivery: Potentials in Human Health and Disease. Cells 2022, 11, 2232. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Wang, J.; Stierhof, Y.-D.; Robinson, D.G.; Jiang, L. Unconventional Protein Secretion. Trends. Plant Sci. 2012, 17, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; Elizalde, M.; de la Canal, L. Apoplastic Exosome-like Vesicles: A New Way of Protein Secretion in Plants? Plant Signal Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef]
- Canitano, A.; Venturi, G.; Borghi, M.; Ammendolia, M.G.; Fais, S. Exosomes Released in Vitro from Epstein-Barr Virus (EBV)-Infected Cells Contain EBV-Encoded Latent Phase MRNAs. Cancer Lett. 2013, 337, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Cossetti, C.; Lugini, L.; Astrologo, L.; Saggio, I.; Fais, S.; Spadafora, C. Soma-to-Germline Transmission of RNA in Mice Xenografted with Human Tumour Cells: Possible Transport by Exosomes. PLoS ONE 2014, 9, e101629. [Google Scholar] [CrossRef]
- Federici, C.; Petrucci, F.; Caimi, S.; Cesolini, A.; Logozzi, M.; Borghi, M.; D’Ilio, S.; Lugini, L.; Violante, N.; Azzarito, T.; et al. Exosome Release and Low PH Belong to a Framework of Resistance of Human Melanoma Cells to Cisplatin. PLoS ONE 2014, 9, e88193. [Google Scholar] [CrossRef]
- Properzi, F.; Logozzi, M.; Abdel-Haq, H.; Federici, C.; Lugini, L.; Azzarito, T.; Cristofaro, I.; Sevo, D.; Ferroni, E.; Cardone, F.; et al. Detection of Exosomal Prions in Blood by Immunochemistry Techniques. J. Gen. Virol. 2015, 96, 1969–1974. [Google Scholar] [CrossRef]
- Lugini, L.; Valtieri, M.; Federici, C.; Cecchetti, S.; Meschini, S.; Condello, M.; Signore, M.; Fais, S. Exosomes from Human Colorectal Cancer Induce a Tumor-like Behavior in Colonic Mesenchymal Stromal Cells. Oncotarget 2016, 7, 50086–50098. [Google Scholar] [CrossRef]
- Iessi, E.; Logozzi, M.; Lugini, L.; Azzarito, T.; Federici, C.; Spugnini, E.P.; Mizzoni, D.; di Raimo, R.; Angelini, D.F.; Battistini, L.; et al. Acridine Orange/Exosomes Increase the Delivery and the Effectiveness of Acridine Orange in Human Melanoma Cells: A New Prototype for Theranostics of Tumors. J. Enzym. Inhib. Med. Chem. 2017, 32, 648–657. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Bocca, B.; di Raimo, R.; Petrucci, F.; Caimi, S.; Alimonti, A.; Falchi, M.; Cappello, F.; Campanella, C.; et al. Human Primary Macrophages Scavenge AuNPs and Eliminate It through Exosomes. A Natural Shuttling for Nanomaterials. Eur. J. Pharm. Biopharm. 2019, 137, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bermúdez, P.; Blesa, J.; Soriano, J.M.; Marcilla, A. Extracellular Vesicles in Food: Experimental Evidence of Their Secretion in Grape Fruits. Eur. J. Pharm. Sci. 2017, 98, 40–50. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-Derived Extracellular Vesicles: A Novel Nanomedicine Approach with Advantages and Challenges. Cell Commun. Signal 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Crescitelli, R.; Lässer, C.; Jang, S.C.; Cvjetkovic, A.; Malmhäll, C.; Karimi, N.; Höög, J.L.; Johansson, I.; Fuchs, J.; Thorsell, A.; et al. Subpopulations of Extracellular Vesicles from Human Metastatic Melanoma Tissue Identified by Quantitative Proteomics after Optimized Isolation. J. Extracell. Vesicles 2020, 9, 1722433. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, S.; Hashimoto, K.; Santana, O.; Aguirre, J.; Kuchitsu, K.; Cárdenas, L. Emerging Roles of Tetraspanins in Plant Inter-Cellular and Inter-Kingdom Communication. Plant Signal Behav. 2019, 14, 1581559. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Özkan, İ.; Koçak, P.; Yıldırım, M.; Ünsal, N.; Yılmaz, H.; Telci, D.; Şahin, F. Garlic (Allium Sativum)-Derived SEVs Inhibit Cancer Cell Proliferation and Induce Caspase Mediated Apoptosis. Sci. Rep. 2021, 11, 14773. [Google Scholar] [CrossRef] [PubMed]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-Derived Extracellular Vesicles as a Promising Cell-Free Therapeutic Tool for Wound Healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein Biocargo of Citrus Fruit-Derived Vesicles Reveals Heterogeneous Transport and Extracellular Vesicle Populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef]
- Sarvarian, P.; Samadi, P.; Gholipour, E.; Shams Asenjan, K.; Hojjat-Farsangi, M.; Motavalli, R.; Motavalli Khiavi, F.; Yousefi, M. Application of Emerging Plant-Derived Nanoparticles as a Novel Approach for Nano-Drug Delivery Systems. Immunol. Investig. 2022, 51, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.-B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral Administration of Turmeric-Derived Exosome-like Nanovesicles with Anti-Inflammatory and pro-Resolving Bioactions for Murine Colitis Therapy. J. Nanobiotechnology 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.B.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J.P.; et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host. Microbe. 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering MiRNAs’ Action through MiRNA Editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Liao, H.; Fu, H.; Yang, X.; Xiang, Q.; Zhang, S. Plant Exosome-like Nanoparticles as Biological Shuttles for Transdermal Drug Delivery. Bioengineering 2023, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Cai, Q.; Qiao, L.; Huang, C.-Y.; Wang, S.; Miao, W.; Ha, T.; Wang, Y.; Jin, H. RNA-Binding Proteins Contribute to Small RNA Loading in Plant Extracellular Vesicles. Nat. Plants 2021, 7, 342–352. [Google Scholar] [CrossRef]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular Vesicles: Emerging Players in Plant Defense Against Pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef] [PubMed]
- Stotz, H.U.; Brotherton, D.; Inal, J. Communication Is Key: Extracellular Vesicles as Mediators of Infection and Defence during Host-Microbe Interactions in Animals and Plants. FEMS Microbiol. Rev. 2022, 46, fuab044. [Google Scholar] [CrossRef]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 722. [Google Scholar] [CrossRef]
- Lei, C.; Teng, Y.; He, L.; Sayed, M.; Mu, J.; Xu, F.; Zhang, X.; Kumar, A.; Sundaram, K.; Sriwastva, M.K.; et al. Lemon Exosome-like Nanoparticles Enhance Stress Survival of Gut Bacteria by RNase P-Mediated Specific TRNA Decay. iScience. 2021, 24, 102511. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural Exosome-like Nanovesicles from Edible Tea Flowers Suppress Metastatic Breast Cancer via ROS Generation and Microbiota Modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef]
- Man, F.; Meng, C.; Liu, Y.; Wang, Y.; Zhou, Y.; Ma, J.; Lu, R. The Study of Ginger-Derived Extracellular Vesicles as a Natural Nanoscale Drug Carrier and Their Intestinal Absorption in Rats. AAPS PharmSciTech. 2021, 22, 206. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Hadizadeh, N.; Bagheri, D.; Shamsara, M.; Hamblin, M.R.; Farmany, A.; Xu, M.; Liang, Z.; Razi, F.; Hashemi, E. Extracellular Vesicles Biogenesis, Isolation, Manipulation and Genetic Engineering for Potential in Vitro and in Vivo Therapeutics: An Overview. Front. Bioeng. Biotechnol. 2022, 10, 1019821. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Bosque, A.; Dietz, L.; Gallego-Lleyda, A.; Sanclemente, M.; Iturralde, M.; Naval, J.; Alava, M.A.; Martínez-Lostao, L.; Thierse, H.-J.; Anel, A. Comparative Proteomics of Exosomes Secreted by Tumoral Jurkat T Cells and Normal Human T Cell Blasts Unravels a Potential Tumorigenic Role for Valosin-Containing Protein. Oncotarget 2016, 7, 29287–29305. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 466. [Google Scholar] [CrossRef]
- Livshts, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.v.; Govorun, V.M. Isolation of Exosomes by Differential Centrifugation: Theoretical Analysis of a Commonly Used Protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, P.D.; Bihari, C.; Sarin, S.K.; Baweja, S. Emerging Role of Edible Exosomes-Like Nanoparticles (ELNs) as Hepatoprotective Agents. Nanotheranostics 2022, 6, 365–375. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-Speed Centrifugation Induces Aggregation of Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Barran, P.E.; Mackay, C.L.; Weidt, S.; Balmforth, C.; Walsh, T.S.; Chalmers, R.T.A.; Webb, D.J.; Dear, J.W. Identification and Proteomic Profiling of Exosomes in Human Cerebrospinal Fluid. J. Transl. Med. 2012, 10, 5. [Google Scholar] [CrossRef]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of Extracellular Vesicles: Determining the Correct Approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef]
- Yu, L.L.; Zhu, J.; Liu, J.X.; Jiang, F.; Ni, W.K.; Qu, L.S.; Ni, R.Z.; Lu, C.H.; Xiao, M.B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed. Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef]
- Ebert, B.; Rai, A.J. Isolation and Characterization of Amniotic Fluid-Derived Extracellular Vesicles for Biomarker Discovery. In Methods in Molecular Biology; Humana Press Inc.: Totova, NJ, USA, 2019; Volume 1885, pp. 287–294. [Google Scholar] [CrossRef]
- Alexander, R.P.; Chiou, N.-T.; Ansel, K.M. Improved Exosome Isolation by Sucrose Gradient Fractionation of Ultracentrifuged Crude Exosome Pellets. Protoc. Exch. 2016, 1–4. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of Ultracentrifugation, Density Gradient Separation, and Immunoaffinity Capture Methods for Isolating Human Colon Cancer Cell Line LIM1863-Derived Exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized Exosome Isolation Protocol for Cell Culture Supernatant and Human Plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.v.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; Bertier, L.; et al. EV-TRACK: Transparent Reporting and Centralizing Knowledge in Extracellular Vesicle Research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-Mediated SiRNA Delivery to Suppress Postoperative Breast Cancer Metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Vergauwen, G.; Dhondt, B.; van Deun, J.; de Smedt, E.; Berx, G.; Timmerman, E.; Gevaert, K.; Miinalainen, I.; Cocquyt, V.; Braems, G.; et al. Confounding Factors of Ultrafiltration and Protein Analysis in Extracellular Vesicle Research. Sci. Rep. 2017, 7, 2704. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-Step Isolation of Extracellular Vesicles by Size-Exclusion Chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-Based Isolation Minimally Alters Extracellular Vesicles’ Characteristics Compared to Precipitating Agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, D.; Wang, Y.; Chen, Z. Exosomes as Novel Delivery Systems for Application in Traditional Chinese Medicine. Molecules 2022, 27, 7789. [Google Scholar] [CrossRef]
- Gercel-Taylor, C.; Atay, S.; Tullis, R.H.; Kesimer, M.; Taylor, D.D. Nanoparticle Analysis of Circulating Cell-Derived Vesicles in Ovarian Cancer Patients. Anal. Biochem. 2012, 428, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lyden, D. Asymmetric-Flow Field-Flow Fractionation Technology for Exomere and Small Extracellular Vesicle Separation and Characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Metcalf, T.G. Polyethylene Glycol Precipitation for Recovery of Pathogenic Viruses, Including Hepatitis A Virus and Human Rotavirus, from Oyster, Water, and Sediment Samples. Appl. Env. Microbiol. 1988, 54, 1983–1988. [Google Scholar] [CrossRef]
- Yamamoto, K.R.; Alberts, B.M.; Benzinger, R.; Lawhorne, L.; Treiber, G. Rapid Bacteriophage Sedimentation in the Presence of Polyethylene Glycol and Its Application to Large-Scale Virus Purification. Virology 1970, 40, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.K.; Hyun, C.K.; Kim, J.H.; Park, Y.H. Production of Penicillin in a Fluidized-Bed Bioreactor: Control of Cell Growth and Penicillin Production by Phosphate Limitation. Biotechnol. Bioeng. 1988, 32, 569–573. [Google Scholar] [CrossRef]
- Tian, J.; Casella, G.; Zhang, Y.; Rostami, A.; Li, X. Potential Roles of Extracellular Vesicles in the Pathophysiology, Diagnosis, and Treatment of Autoimmune Diseases. Int. J. Biol. Sci. 2020, 16, 620–632. [Google Scholar] [CrossRef]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A Cost-Effective Polyethylene Glycol-Based Method for the Isolation of Functional Edible Nanoparticles from Ginger Rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar] [CrossRef]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated Isolation and Quantitative Analysis of Exosome Shuttled Proteins and Nucleic Acids Using Immunocapture Approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef]
- Koliha, N.; Wiencek, Y.; Heider, U.; Jüngst, C.; Kladt, N.; Krauthäuser, S.; Johnston, I.C.D.; Bosio, A.; Schauss, A.; Wild, S. A Novel Multiplex Bead-Based Platform Highlights the Diversity of Extracellular Vesicles. J. Extracell. Vesicles 2016, 5, 29975. [Google Scholar] [CrossRef]
- Boriachek, K.; Masud, M.K.; Palma, C.; Phan, H.P.; Yamauchi, Y.; Hossain, M.S.A.; Nguyen, N.T.; Salomon, C.; Shiddiky, M.J.A. Avoiding Pre-Isolation Step in Exosome Analysis: Direct Isolation and Sensitive Detection of Exosomes Using Gold-Loaded Nanoporous Ferric Oxide Nanozymes. Anal. Chem. 2019, 91, 3827–3834. [Google Scholar] [CrossRef]
- Ghosh, A.; Davey, M.; Chute, I.C.; Griffiths, S.G.; Lewis, S.; Chacko, S.; Barnett, D.; Crapoulet, N.; Fournier, S.; Joy, A.; et al. Rapid Isolation of Extracellular Vesicles from Cell Culture and Biological Fluids Using a Synthetic Peptide with Specific Affinity for Heat Shock Proteins. PLoS ONE 2014, 9, 110443. [Google Scholar] [CrossRef]
- Balaj, L.; Atai, N.A.; Chen, W.; Mu, D.; Tannous, B.A.; Breakefield, X.O.; Skog, J.; Maguire, C.A. Heparin Affinity Purification of Extracellular Vesicles. Sci. Rep. 2015, 5, 10266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.G.; Mohamadi, R.M.; Poudineh, M.; Kermanshah, L.; Ahmed, S.; Safaei, T.S.; Stojcic, J.; Nam, R.K.; Sargent, E.H.; Kelley, S.O. Interrogating Circulating Microsomes and Exosomes Using Metal Nanoparticles. Small 2016, 12, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef]
- Théry, C.; Clayton, A.; Amigorena, S.; Raposo, G. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, H.J.; Fine, D.; Schmulen, J.; Hu, Y.; Godin, B.; Zhang, J.X.J.; Liu, X. Ciliated Micropillars for the Microfluidic-Based Isolation of Nanoscale Lipid Vesicles. Lab. Chip. 2013, 13, 2879–2882. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of Exosomes from Whole Blood by Integrating Acoustics and Microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, 0170628. [Google Scholar] [CrossRef]
- Martins, T.S.; Catita, J.; Rosa, I.M.; da Cruz e Silva, O.A.B.; Henriques, A.G. Exosome Isolation from Distinct Biofluids Using Precipitation and Column-Based Approaches. PLoS ONE 2018, 13, 198820. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, K.; Gao, L.; Zhang, Z.; Li, F.; Zhou, F.; Zhang, L. Methods and Technologies for Exosome Isolation and Characterization. Small Methods 2018, 2, 21. [Google Scholar] [CrossRef]
- van der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; van Leeuwen, T.G.; Nieuwland, R. Optical and Non-Optical Methods for Detection and Characterization of Microparticles and Exosomes. J. Thromb. Haemost. 2010, 8, 2596–2607. [Google Scholar] [CrossRef]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and Proteomic Profiling of Exosomes in Human Urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, W.; Klinke, D.J. Exosomes: Improved Methods to Characterize Their Morphology, RNA Content, and Surface Protein Biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Maas, S.L.N.; de Vrij, J.; van der Vlist, E.J.; Geragousian, B.; van Bloois, L.; Mastrobattista, E.; Schiffelers, R.M.; Wauben, M.H.M.; Broekman, M.L.D.; Nolte-’T Hoen, E.N.M. Possibilities and Limitations of Current Technologies for Quantification of Biological Extracellular Vesicles and Synthetic Mimics. J. Control. Release 2015, 200, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; de Vrij, J.; Broekman, M.L.D. Quantification and Size-Profiling of Extracellular Vesicles Using Tunable Resistive Pulse Sensing. J. Vis. Exp 2014, 92, 51623. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T.; et al. Effects of Storage Temperature on Airway Exosome Integrity for Diagnostic and Functional Analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef] [PubMed]
- Madison, M.N.; Jones, P.H.; Okeoma, C.M. Exosomes in Human Semen Restrict HIV-1 Transmission by Vaginal Cells and Block Intravaginal Replication of LP-BM5 Murine AIDS Virus Complex. Virology 2015, 482, 189–201. [Google Scholar] [CrossRef]
- Welch, J.L.; Madison, M.N.; Margolick, J.B.; Galvin, S.; Gupta, P.; Martínez-Maza, O.; Dash, C.; Okeoma, C.M. Effect of Prolonged Freezing of Semen on Exosome Recovery and Biologic Activity. Sci. Rep. 2017, 7, 45034. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Böing, A.N.; Grootemaat, A.E.; van der Pol, E.; Hau, C.M.; Cizmar, P.; Buhr, E.; Sturk, A.; Nieuwland, R. Handling and Storage of Human Body Fluids for Analysis of Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 29260. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyzen, W.; Sime, N.E.L.; Ivy, J.R.; Turtle, E.J.; Street, J.M.; Pound, J.; Bath, L.E.; Webb, D.J.; Gregory, C.D.; Bailey, M.A.; et al. Quantification of Human Urinary Exosomes by Nanoparticle Tracking Analysis. J. Physiol. 2013, 591, 5833–5842. [Google Scholar] [CrossRef]
- Sivanantham, A.; Jin, Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697. [Google Scholar] [CrossRef]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; El-Tookhy, O.S. Preservation Techniques of Stem Cells Extracellular Vesicles: A Gate for Manufacturing of Clinical Grade Therapeutic Extracellular Vesicles and Long-Term Clinical Trials. Int. J. Vet. Sci. Med. 2020, 8, 1704992. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.V.; Frith, J.E.; Lim, R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharm. 2018, 9, 1199. [Google Scholar] [CrossRef]
- Budgude, P.; Kale, V.; Vaidya, A. Cryopreservation of Mesenchymal Stromal Cell-Derived Extracellular Vesicles Using Trehalose Maintains Their Ability to Expand Hematopoietic Stem Cells in Vitro. Cryobiology 2021, 98, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The Impact of Storage on Extracellular Vesicles: A Systematic Study. J. Extracell. Vesicles 2022, 11, 12162. [Google Scholar] [CrossRef]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.M. Trehalose Prevents Aggregation of Exosomes and Cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef] [PubMed]
- Kreke, M.; Smith, R.; Hanscome, P.; Peck, K.; Ibrahim, A. Processes for Producing Stable Exosome Formulations. US Patent US20160158291A, 3 December 2015. [Google Scholar]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of Exosomes at Room Temperature Using Lyophilization. Int. J. Pharm. 2018, 553, 32. [Google Scholar] [CrossRef]
- Richter, M.; Fuhrmann, K.; Fuhrmann, G. Evaluation of the Storage Stability of Extracellular Vesicles. J. Vis. Exp. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- el Baradie, K.B.Y.; Nouh, M.; O’Brien, F.; Liu, Y.; Fulzele, S.; Eroglu, A.; Hamrick, M.W. Freeze-Dried Extracellular Vesicles From Adipose-Derived Stem Cells Prevent Hypoxia-Induced Muscle Cell Injury. Front. Cell Dev. Biol. 2020, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; di Bella, M.A.; et al. Citrus Limon-Derived Nanovesicles Inhibit Cancer Cell Proliferation and Suppress CML Xenograft Growth by Inducing TRAIL-Mediated Cell Death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A Review on Exosomes Application in Clinical Trials: Perspective, Questions, and Challenges. Cell Commun. Signal 2022, 20, 145. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, E.Y.; Chiou, T.W.; Harn, H.J. Exosomes in Clinical Trial and Their Production in Compliance with Good Manufacturing Practice. Tzu. Chi. Med. J. 2020, 32, 113–120. [Google Scholar] [CrossRef]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.; Kumar, A.; Sundaram, K.; et al. Plant-Derived Exosomal MicroRNAs Inhibit Lung Inflammation Induced by Exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Jun, Y.; et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas Gingivalis. Iscience 2019, 21, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Sriwastva, M.K.; Deng, Z.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like Nanoparticles from Mulberry Bark Prevent DSS-induced Colitis via the AhR/COPS8 Pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Han, J.; Wu, T.; Jin, J.; Li, Z.; Cheng, W.; Dai, X.; Yang, K.; Zhang, H.; Zhang, Z.; Zhang, H.; et al. Exosome-like Nanovesicles Derived from Phellinus Linteus Inhibit Mical2 Expression through Cross-Kingdom Regulation and Inhibit Ultraviolet-Induced Skin Aging. J. Nanobiotechnology 2022, 20, 455. [Google Scholar] [CrossRef] [PubMed]
- Kantarcioglu, M.; Yildirim, G.; Akpinar Oktar, P.; Yanbakan, S.; Ozer, Z.B.; Yurtsever Sarica, D.; Tasdelen, S.; Bayrak, E.; Akin Bali, D.F.; Ozturk, S.; et al. Coffee-Derived Exosome-Like Nanoparticles: Are They the Secret Heroes? Turk. J. Gastroenterol. 2022, 34, 161–169. [Google Scholar] [CrossRef]
- Cai, H.; Huang, L.Y.; Hong, R.; Song, J.X.; Guo, X.J.; Zhou, W.; Hu, Z.L.; Wang, W.; Wang, Y.L.; Shen, J.G.; et al. Momordica Charantia Exosome-Like Nanoparticles Exert Neuroprotective Effects Against Ischemic Brain Injury via Inhibiting Matrix Metalloproteinase 9 and Activating the AKT/GSK3β Signaling Pathway. Front. Pharm. 2022, 13, 908830. [Google Scholar] [CrossRef]
- Mahdipour, E. Beta Vulgaris Juice Contains Biologically Active Exosome-like Nanoparticles. Tissue Cell 2022, 76, 101800. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Kuranaga, Y.; Heishima, K.; Sugito, N.; Morikawa, K.; Ito, Y.; Soga, T.; Ito, T. Plant Hvu-MIR168-3p Enhances Expression of Glucose Transporter 1 (SLC2A1) in Human Cells by Silencing Genes Related to Mitochondrial Electron Transport Chain Complex I. J. Nutr. Biochem. 2022, 101, 108922. [Google Scholar] [CrossRef]
- Zhao, W.; Bian, Y.; Wang, Q.; Yin, F.; Yin, L.; Zhang, Y.; Liu, J. Blueberry-Derived Exosomes-like Nanoparticles Ameliorate Nonalcoholic Fatty Liver Disease by Attenuating Mitochondrial Oxidative Stress. Acta Pharm. Sin. 2022, 43, 645–658. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Gao, L.; Cong, M.; Sun, J.; Xu, J.; Wang, Y.; Hu, Y.; Asghar, S.; Hu, L.; et al. Engineering Exosome-like Nanovesicles Derived from Asparagus Cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int. J. Nanomed. 2021, 16, 1575–1586. [Google Scholar] [CrossRef]
- de Robertis, M.; Sarra, A.; D’oria, V.; Mura, F.; Bordi, F.; Postorino, P.; Fratantonio, D. Blueberry-Derived Exosome-like Nanoparticles Counters the Response to TNF-α-Induced Change on Gene Expression in Ea.Hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef]
- Liu, B.; Lu, Y.; Chen, X.; Muthuraj, P.G.; Li, X.; Pattabiraman, M.; Zempleni, J.; Kachman, S.D.; Natarajan, S.K.; Yu, J. Protective Role of Shiitake Mushroom-Derived Exosome-like Nanoparticles in d-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Nutrients 2020, 12, 477. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of Exosome-like Nanoparticle-Derived MicroRNAs from 11 Edible Fruits and Vegetables. PeerJ 2018, 2018, 5186. [Google Scholar] [CrossRef]
- Li, X.; Liang, Z.; Du, J.; Wang, Z.; Mei, S.; Li, Z.; Zhao, Y.; Zhao, D.; Ma, Y.; Ye, J.; et al. Herbal Decoctosome Is a Novel Form of Medicine. Sci. China Life Sci. 2019, 62, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.; Guo, P. Arrowtail RNA for Ligand Display on Ginger Exosome-like Nanovesicles to Systemic Deliver SiRNA for Cancer Suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies Communication between Plant and Mouse Gut Host Cells through Edible Plant Derived Exosome-like Nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, X.; Xu, K.; Zhang, Y.; Ren, X.; Qi, B.; Liang, Q.; Yang, X.; Li, L.; Li, S. Digestion of Plant Dietary MiRNAs Starts in the Mouth under the Protection of Coingested Food Components and Plant-Derived Exosome-like Nanoparticles. J. Agric. Food Chem. 2022, 70, 4316–4327. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the MicroRNA Profile of Ginger Exosome-like Nanoparticles and Their Anti-Inflammatory Effects in Intestinal Caco-2 Cells. J. Agric. Food Chem. 2022, 70, 4725–4734. [Google Scholar] [CrossRef] [PubMed]
- Boccia, E.; Alfieri, M.; Belvedere, R.; Santoro, V.; Colella, M.; del Gaudio, P.; Moros, M.; Dal Piaz, F.; Petrella, A.; Leone, A.; et al. Plant Hairy Roots for the Production of Extracellular Vesicles with Antitumor Bioactivity. Commun. Biol. 2022, 5, 848. [Google Scholar] [CrossRef]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.G.; Qiao, H. Technology Insight: Plant-Derived Vesicles—How Far from the Clinical Biotherapeutics and Therapeutic Drug Carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-Derived Exosome-like Nanoparticles and Their Therapeutic Activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Díez-Sainz, E.; Lorente-Cebrián, S.; Aranaz, P.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021, 8, 586564. [Google Scholar] [CrossRef] [PubMed]
- di Gioia, S.; Hossain, M.N.; Conese, M. Biological Properties and Therapeutic Effects of Plant-Derived Nanovesicles. Open. Med. 2020, 15, 1096–1122. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; He, X.; Zheng, J. Exosomes and Regenerative Medicine: State of the Art and Perspectives. In Translational Research; Mosby Inc.: Maryland Heights, MO, USA, 2018; pp. 1–16. [Google Scholar] [CrossRef]
- Moghadasi, S.; Elveny, M.; Rahman, H.S.; Suksatan, W.; Jalil, A.T.; Abdelbasset, W.K.; Yumashev, A.V.; Shariatzadeh, S.; Motavalli, R.; Behzad, F.; et al. A Paradigm Shift in Cell-Free Approach: The Emerging Role of MSCs-Derived Exosomes in Regenerative Medicine. J. Transl. Med. 2021, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Bapat, A.; Jain, R.; Jeyaraman, N.; Jeyaraman, M. Exosomal Therapy—A New Frontier in Regenerative Medicine. Stem Cell Investig. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell–Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharm. 2021, 11, 590470. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting Tissue Regeneration by Modulating the Immune System. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Clevers, H. Reparative Inflammation Takes Charge of Tissue Regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Gawriluk, T.R.; Simkin, J.; Hacker, C.K.; Kimani, J.M.; Kiama, S.G.; Ezenwa, V.O.; Seifert, A.W. Complex Tissue Regeneration in Mammals Is Associated With Reduced Inflammatory Cytokines and an Influx of T Cells. Front. Immunol. 2020, 11, 1695. [Google Scholar] [CrossRef]
- Cavalieri, D.; Rizzetto, L.; Tocci, N.; Rivero, D.; Asquini, E.; Si-Ammour, A.; Bonechi, E.; Ballerini, C.; Viola, R. Plant MicroRNAs as Novel Immunomodulatory Agents. Sci. Rep. 2016, 6, 25761. [Google Scholar] [CrossRef]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and Its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef]
- Sen, C.K.; Ghatak, S. MiRNA Control of Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2629–2640. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.H. Isolation of Aloe Saponaria-Derived Extracellular Vesicles and Investigation of Their Potential for Chronic Wound Healing. Pharmaceutics 2022, 14, 1905. [Google Scholar] [CrossRef]
- Xu, X.H.; Yuan, T.J.; Dad, H.A.; Shi, M.Y.; Huang, Y.Y.; Jiang, Z.H.; Peng, L.H. Plant Exosomes As Novel Nanoplatforms for MicroRNA Transfer Stimulate Neural Differentiation of Stem Cells in Vitro and In Vivo. Nano Lett. 2021, 21, 8151–8159. [Google Scholar] [CrossRef]
- Shafi, S.; Ansari, H.R.; Bahitham, W.; Aouabdi, S. The Impact of Natural Antioxidants on the Regenerative Potential of Vascular Cells. Front. Cardiovasc. Med. 2019, 6, 28. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Kahroba, H.; Davatgaran-Taghipour, Y. Exosomal Nrf2: From Anti-Oxidant and Anti-Inflammation Response to Wound Healing and Tissue Regeneration in Aged-Related Diseases. Biochimie 2020, 171–172, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe Vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, T.; Gomes, A. MicroRNAs in the Regulation of Cellular Redox Status and Its Implications in Myocardial Ischemia-Reperfusion Injury. Redox. Biol. 2020, 36, 101607. [Google Scholar] [CrossRef]
- Lee, Y.; Im, E. Regulation of Mirnas by Natural Antioxidants in Cardiovascular Diseases: Focus on Sirt1 and Enos. Antioxidants 2021, 10, 377. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, S.; Li, M.; Gui, X.; Li, P. Isolation of Exosome-Like Nanoparticles and Analysis of MicroRNAs Derived from Coconut Water Based on Small RNA High-Throughput Sequencing. J. Agric. Food Chem. 2018, 66, 2749–2757. [Google Scholar] [CrossRef]
- Collino, F.; Pomatto, M.; Bruno, S.; Lindoso, R.S.; Tapparo, M.; Sicheng, W.; Quesenberry, P.; Camussi, G. Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells. Stem. Cell Rev. Rep. 2017, 13, 226–243. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells in Vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, D.; Li, H. Bioglass Enhances the Production of Exosomes and Improves Their Capability of Promoting Vascularization. Bioact. Mater. 2021, 6, 823–835. [Google Scholar] [CrossRef]
- Kang, M.; Lee, C.S.; Lee, M. Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering 2021, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Brunet, M.Y.; Federici, A.S.; Hoey, D.A.; Cox, S.C. An ECM-Mimetic Hydrogel to Promote the Therapeutic Efficacy of Osteoblast-Derived Extracellular Vesicles for Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 829969. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Chen, A.; Tian, H.; Yang, N.; Zhang, Z.; Yang, G.-Y.; Cui, W.; Tang, Y. Towards Extracellular Vesicle Delivery Systems for Tissue Regeneration: Material Design at the Molecular Level. Extracell. Vesicles Circ. Nucl. Acids. 2022, 3, 306–339. [Google Scholar] [CrossRef]
- Youseflee, P.; Ranjbar, F.E.; Bahraminasab, M.; Ghanbari, A.; Faradonbeh, D.R.; Arab, S.; Alizadeh, A.; Nooshabadi, V.T. Exosome Loaded Hydroxyapatite (HA) Scaffold Promotes Bone Regeneration in Calvarial Defect: An in Vivo Study. Cell Tissue Bank. 2022, 1–12. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Wang, X.; Shah, F.A.; Vazirisani, F.; Johansson, A.; Palmquist, A.; Omar, O.; Ekström, K.; Thomsen, P. Exosomes Influence the Behavior of Human Mesenchymal Stem Cells on Titanium Surfaces. Biomaterials 2020, 230, 119571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, M.; Yin, X.; Yao, P.; Sun, H. Mechanism of Exosomes Involved in Osteoimmunity Promoting Osseointegration Around Titanium Implants With Small-Scale Topography. Front. Bioeng. Biotechnol. 2021, 9, 682384. [Google Scholar] [CrossRef]
- Lan, Y.; Jin, Q.; Xie, H.; Yan, C.; Ye, Y.; Zhao, X.; Chen, Z.; Xie, Z. Exosomes Enhance Adhesion and Osteogenic Differentiation of Initial Bone Marrow Stem Cells on Titanium Surfaces. Front. Cell Dev. Biol. 2020, 8, 583234. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, B.; Zhai, D.; Wu, C. Three-Dimensional Printing of Bioceramic-Induced Macrophage Exosomes: Immunomodulation and Osteogenesis/Angiogenesis. NPG Asia Mater. 2021, 13, 72. [Google Scholar] [CrossRef]
- Al-Sowayan, B.; Alammari, F.; Alshareeda, A. Preparing the Bone Tissue Regeneration Ground by Exosomes: From Diagnosis to Therapy. Molecules 2020, 25, 4205. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Yang, Y.; Liu, J.; Li, H.; Li, R.; Cao, C.; Shi, L.; Wu, W.; He, K. A Timely Review of Cross-Kingdom Regulation of Plant-Derived MicroRNAs. Front. Genet. 2021, 12, 613197. [Google Scholar] [CrossRef]
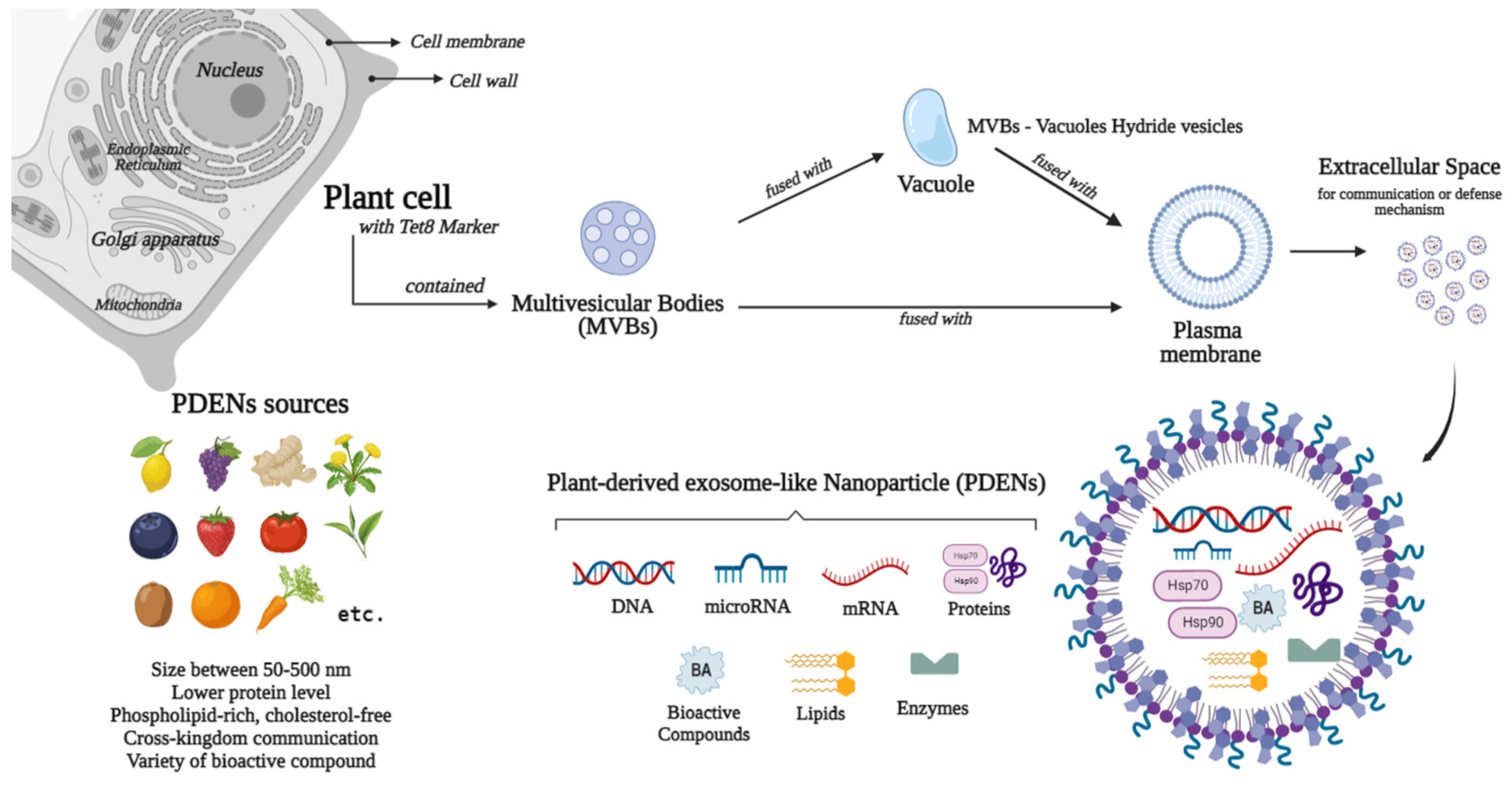
| Sources of PDENs | Compounds | Remarks |
|---|---|---|
| Ginger | 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol | [88,108] |
| Grapefruit | Naringenin | [65] |
| Lemon | Citrate, vitamin C, and galacturonic acid-enriched pectin-type polysaccharide | [66,110] |
| Strawberry | Vitamin C | [88] |
| Tea flower | Epigallo-catechin gallate, epicatechin gallate, epicatechin, vitexin, myricetin-3-O-rhamnoside, kaempferol-3-O-galactoside, and myricetin | [111] |
| Oat | Fiber β-glucan | [112] |
| Broccoli | Sulforaphane | [112] |
| Type of Cells | References |
|---|---|
| Human keratinocyte line (Ha-CaT), human skin fibroblasts (HSFs), human embryonic kidney cells (HEK293T) | [188,198] |
| Fibrotic cell line (LX2), hepatocellular cell line (HEP40) | [189] |
| Cancer cells (MCF-7, HeLa, and N2A), human umbilical vein endothelial cells (HUVEC), primary fibroblasts cells | [191,197] |
| Human colorectal cancer cell line (DLD-1), human neuroblastoma cell line (NB19), human leukemia cell line (K562), ASF-4-1 cells, RD cells | [192] |
| Human alveolar basal epithelial cells (A549) | [182,185] |
| Monocytic U937 cell lines | [185] |
| Human hepatoblastoma cells (HepG2) | [193,194] |
| SMMC-7721 cells, Hep3B cells, normal hepatocyte cell line (LO-2) | [194] |
| Adipose-derived mesenchymal stem cells (ADMSCs) | [88] |
| Human stabilized endothelial cell line (EA.hy926) | [195] |
| Human telomerase immortalized keratinocytes (TIGKs) | [186] |
| Human epithelial colorectal adenocarcinoma cells (Caco-2) | [101,202] |
| Human chronic myeloid leukemia cell line (LAMA84), human colorectal adenocarcinoma cell line (SW480), human bone marrow-derived stromal cell line (HS5) | [182] |
| Human fetal lung fibroblast (MRC-5), human lung adenocarcinoma (A549), human acute monocytic leukemia (THP-1) | [198] |
| KB cells | [199] |
| Mouse hippocampal neuronal cells (HT22) | [190] |
| Mouse C57BL/6 lung carcinoma cells (LLC1), monkey kidney Vero E6 cell | [185] |
| Mouse bone marrow-derived macrophages (BMDMs) | [196] |
| C57BL/6 murine colon adenocarcinoma cells (MC-38) | [101] |
| Murine RAW 264.7 cells | [200] |
| Potential Target Tissue or Disease | Prospects | Challenges and Issues | Refs. |
|---|---|---|---|
| Periodontitis | PDENs could steadily carry drugs for oral mucosal delivery to modulate oral immunity to periodontopathogen | PDENs could only load small amounts of drugs, with an unclear mechanism of cellular uptake as it may vary per extraction batch | [69] |
| Colitis, tumor, liver diseases, skin diseases, periodontitis | PDENs could act as a drug-delivery system to deliver RNAs and lipids for the inhibition of inflammation genes, as well as bacterial and tumor growth | No clarity on quality control and evaluation systems, stability, biomarker confirmation, and biochemical characterization | [204] |
| Unspecified | Easier large-scale mass production for their large biodiversity with minimal cytotoxicity for drug-delivery system | Elusive internalization mechanisms and unclarity of specific receptors and ligands for PDENs. Biosafety and toxicity of genetic transfer are unclear | [205] |
| Intestine | PDEN-derived miRNAs could modulate gut microbiome, intestinal permeability, and mucosal immunity | High variability of results in studies, due to the lack of consensus regarding PDEN-derived miRNAs | [206] |
| Inflammatory bowel disease, liver disease, cancer | PDENs could mediate interspecies communications to exert their antioxidant, anti-inflammatory, and regenerative activities | Low amount and different kinds of PDEN-derived proteins compared to MSC-derived exosomes | [207] |
| Colitis | PDENs could transfer exogenous drugs or endogenous cargo to epithelial and bacterial cells for their stability in intestinal fluid | Standardization on mass producing and purification techniques | [72] |
| Unspecified | PDENs stability in the digestive system make it a promising functional food to alleviate inflammation | Instability during isolation and processing with unclear proteomic profiling | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarasati, A.; Syahruddin, M.H.; Nuryanti, A.; Ana, I.D.; Barlian, A.; Wijaya, C.H.; Ratnadewi, D.; Wungu, T.D.K.; Takemori, H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines 2023, 11, 1053. https://doi.org/10.3390/biomedicines11041053
Sarasati A, Syahruddin MH, Nuryanti A, Ana ID, Barlian A, Wijaya CH, Ratnadewi D, Wungu TDK, Takemori H. Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines. 2023; 11(4):1053. https://doi.org/10.3390/biomedicines11041053
Chicago/Turabian StyleSarasati, Andari, Muhammad Hidayat Syahruddin, Archadian Nuryanti, Ika Dewi Ana, Anggraini Barlian, Christofora Hanny Wijaya, Diah Ratnadewi, Triati Dewi Kencana Wungu, and Hiroshi Takemori. 2023. "Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy" Biomedicines 11, no. 4: 1053. https://doi.org/10.3390/biomedicines11041053
APA StyleSarasati, A., Syahruddin, M. H., Nuryanti, A., Ana, I. D., Barlian, A., Wijaya, C. H., Ratnadewi, D., Wungu, T. D. K., & Takemori, H. (2023). Plant-Derived Exosome-like Nanoparticles for Biomedical Applications and Regenerative Therapy. Biomedicines, 11(4), 1053. https://doi.org/10.3390/biomedicines11041053








