Identification of Bacterial Strains and Development of anmRNA-Based Vaccine to Combat Antibiotic Resistance in Staphylococcus aureus via In Vitro and In Silico Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Acquisition
2.2. Culture of Strains
2.2.1. DNA Isolation and 16s rRNA Characterization
2.2.2. Gel Electrophoresis Procedure
2.2.3. Strain Identification
2.2.4. Amplification of LukD, FmhA, Spa and Delta Hemolysin
2.2.5. Phylogenetic Analysis
2.3. Chimeric Structure Design
2.4. Immune Cells Epitopes Prediction
2.4.1. Homology Analysis of Predicted Proteins
2.4.2. Antigenicity and Allergenicity Analysis of Epitopes
2.4.3. Population Coverage Analysis
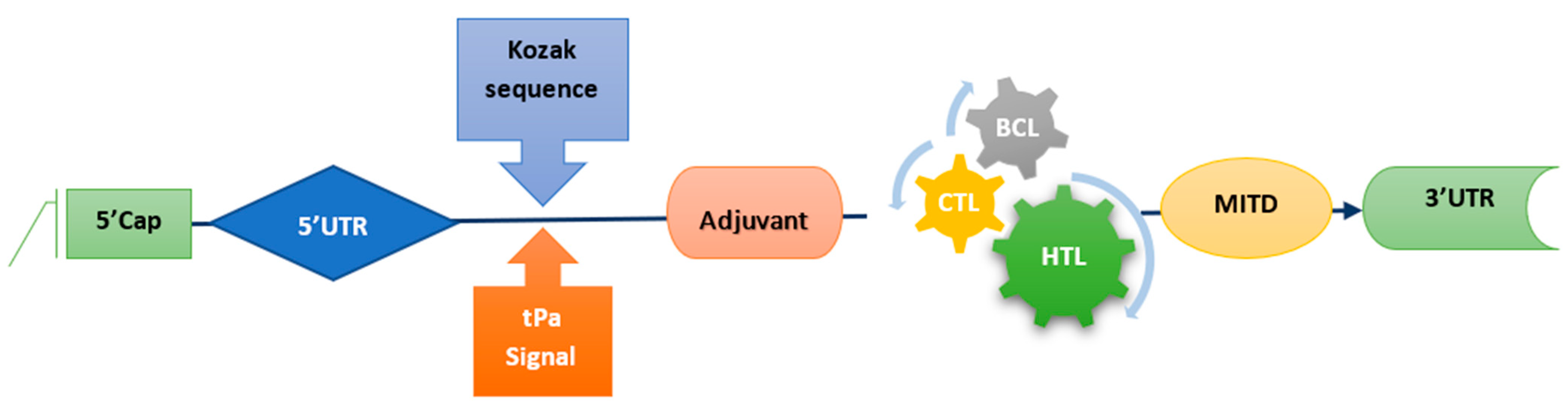
2.5. mRNA Vaccine Construct
2.6. Prediction of Physicochemical Properties of Vaccine Construct
2.6.1. Immune Simulation of Vaccine Construct
2.6.2. Structures Prediction and Validation of Vaccine Construct
2.7. Docking of Vaccine Construct with TLR-3 Receptor
2.8. Molecular Dynamic Simulation
2.9. Computational Expression Studies
3. Results
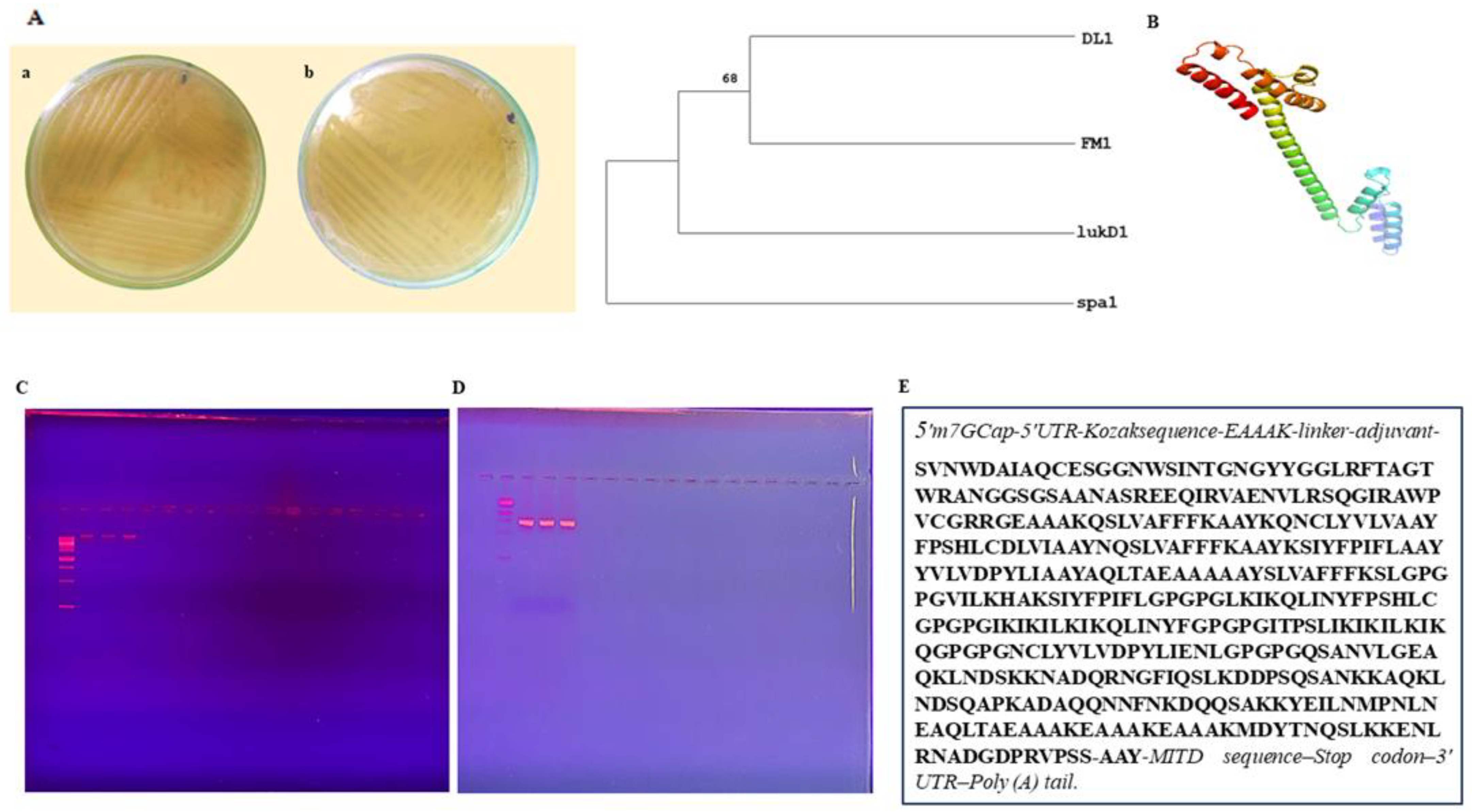
3.1. Sample Collection and Strain Identification
3.2. Phylogenetic Analysis
3.3. Chimeric Design
3.4. Immune Cells Prediction and Estimation
3.5. mRNA Vaccine Construct and Physiochemical Analysis
3.6. Predicted Population Coverage
3.7. Immune Simulation
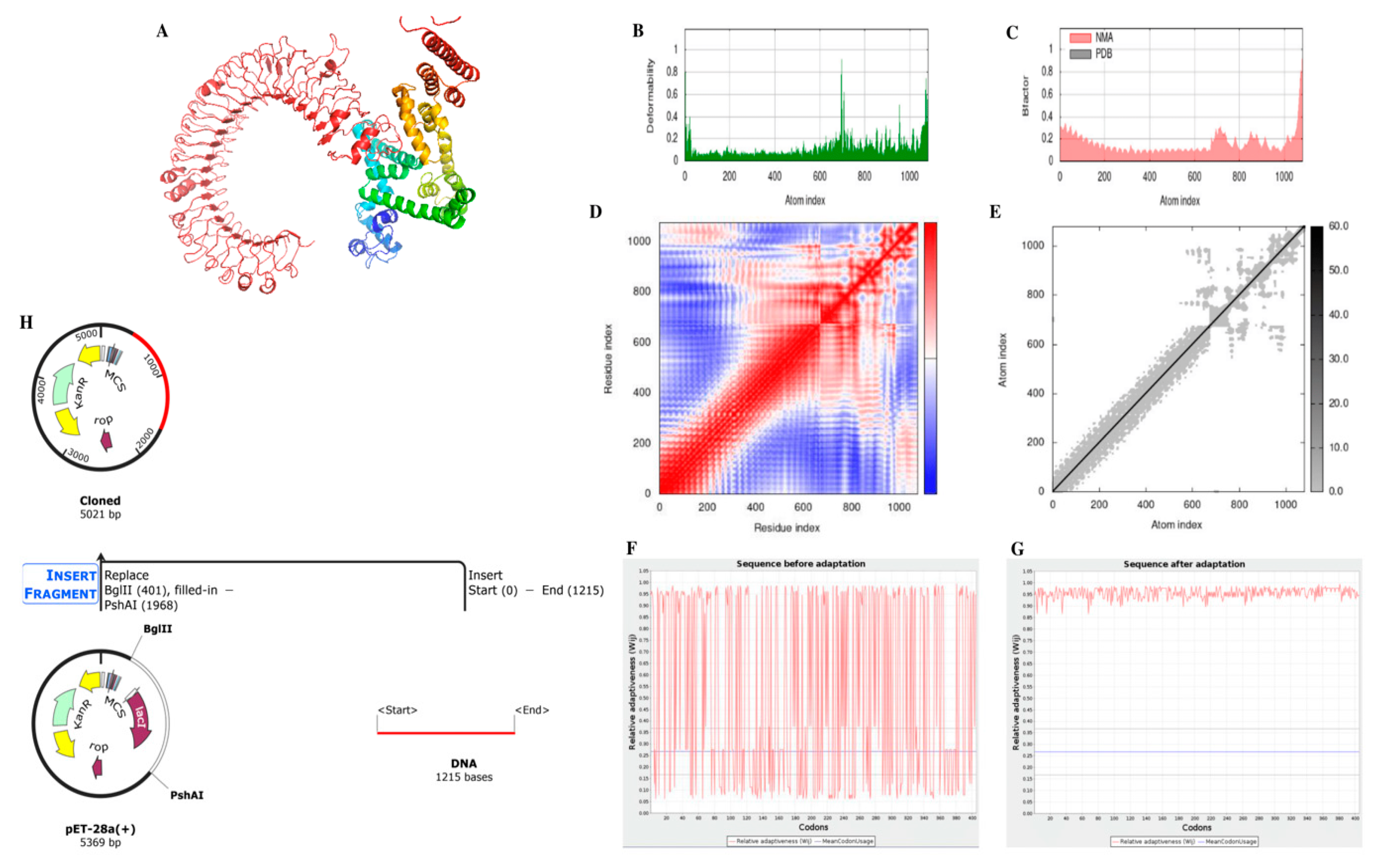
3.8. Structures of mRNA Vaccine Construct
3.9. Molecular Docking of Vaccine Construct with TLR-3
3.10. Molecular Dynamic Simulations
3.11. Computational Expression Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bindu, S.; Dandapat, S.; Manikandan, R.; Dinesh, M.; Subbaiyan, A.; Mani, P.; Dhawan, M.; Tiwari, R.; Bilal, M.; Emran, T.B.; et al. Prophylactic and therapeutic insights into trained immunity: A renewed concept of innate immune memory. Hum. Vaccines Immunother. 2022, 18, 2040238. [Google Scholar] [CrossRef]
- Al-Bdery, A.S.J.; Mohammad, G.J.; Hussen, B. Vancomycin and linezolid resistance among multidrug-resistant Staphylococcus aureus clinical isolates and interaction with neutrophils. Gene Rep. 2020, 21, 100804. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.-S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect. Drug Resist. 2020, 13, 3255. [Google Scholar] [CrossRef]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Duca, K.; Jarrah, A.; Laubenbacher, R.; Hochberg, D.; Thorley-Lawson, D. Simulating epstein-barr virus infection with c-immsim. Bioinformatics 2007, 23, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: An interim analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef]
- Eidaroos, N.H.; Youssef, A.I.; El-Sebae, A.; Enany, M.E.; Farid, D.S. Genotyping of enterotoxigenic methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA) among commensal rodents in North Sinai, Egypt. J. Appl. Microbiol. 2022, 132, 2331–2341. [Google Scholar] [CrossRef]
- Gajdács, M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics 2019, 8, 52. [Google Scholar] [CrossRef]
- Gasteiger, E. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Grote, A.; Hiller, K.; Scheer, M.; Munch, R.; Nortemann, B.; Hempel, D.C.; Jahn, D. JCat: A novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res. 2005, 33, W526–W531. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P.S. In Silico Approach for Predicting Toxicity of Peptides and Proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kaushik, V. Insilico identification of epitope-based peptide vaccine for Nipah virus. Int. J. Pept. Res. Ther. 2020, 26, 1147–1153. [Google Scholar] [CrossRef]
- Khan, T.; Khan, A.; Ansari, J.K.; Najmi, M.H.; Wei, D.-Q.; Muhammad, K.; Waheed, Y. Potential Immunogenic Activity of Computationally Designed mRNA-and Peptide-Based Prophylactic Vaccines against MERS, SARS-CoV, and SARS-CoV-2: A Reverse Vaccinology Approach. Molecules 2022, 27, 2375. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Ahn, K.; Leung, D.Y. Interactions between atopic dermatitis and Staphylococcus aureus infection: Clinical implications. Allergy Asthma Immunol. Res. 2019, 11, 593–603. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 1–23. [Google Scholar] [CrossRef]
- Leman, J.K.; Weitzner, B.D.; Lewis, S.M.; Adolf-Bryfogle, J.; Alam, N.; Alford, R.F.; Aprahamian, M.; Baker, D.; Barlow, K.A.; Barth, P. Macromolecular modeling and design in Robetta: Recent methods and frameworks. Nat. Methods 2020, 17, 665–680. [Google Scholar] [CrossRef]
- Buchan, D.W.; Jones, D.T. The PSIPRED protein analysis workbench: 20 years on. Nucleic Acids Res. 2019, 47, W402–W407. [Google Scholar] [CrossRef]
- López-Blanco, J.R.; Aliaga, J.I.; Quintana-Ortí, E.S.; Chacón, P. iMODS: Internal coordinates normal mode analysis server. Nucleic Acids Res. 2014, 42, W271–W276. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.; Bernhart, S.H.; Höner zu Siederdissen, C.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of protein models with three-dimensional profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Magnan, C.N.; Zeller, M.; Kayala, M.A.; Vigil, A.; Randall, A.; Felgner, P.L.; Baldi, P. High-throughput prediction of protein antigenicity using protein microarray data. Bioinformatics 2010, 26, 2936–2943. [Google Scholar] [CrossRef]
- Mahram, A.; Herbordt, M.C. NCBI BLASTP on high-performance reconfigurable computing systems. ACM Trans. Reconfig. Technol. Syst. (TRETS) 2015, 7, 1–20. [Google Scholar] [CrossRef]
- Malik, A.A.; Ojha, S.C.; Schaduangrat, N.; Nantasenamat, C. ABCpred: A webserver for the discovery of acetyl-and butyryl-cholinesterase inhibitors. Mol. Divers. 2022, 26, 467–487. [Google Scholar] [CrossRef]
- Ogundipe, F.O.; Ojo, O.E.; Feßler, A.T.; Hanke, D.; Awoyomi, O.J.; Ojo, D.A.; Akintokun, A.K.; Schwarz, S.; Maurischat, S. Antimicrobial resistance and virulence of methicillin-resistant Staphylococcus aureus from human, chicken and environmental samples within live bird markets in three Nigerian cities. Antibiotics 2020, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Okwu, M.U.; Olley, M.; Akpoka, A.O.; Izevbuwa, O.E. Methicillin-resistant Staphylococcus aureus (MRSA) and anti-MRSA activities of extracts of some medicinal plants: A brief review. AIMS Microbiol. 2019, 5, 117. [Google Scholar] [CrossRef]
- Palladini, A.; Nicoletti, G.; Pappalardo, F.; Murgo, A.; Grosso, V.; Stivani, V.; Ianzano, M.L.; Antognoli, A.; Croci, S.; Landuzzi, L.; et al. In silico modeling and in vivo efficacy of cancer-preventive vaccinations. Cancer Res. 2010, 70, 7755–7763. [Google Scholar] [CrossRef]
- Park, K.S.; Ki, C.-S.; Kang, C.-I.; Kim, Y.-J.; Chung, D.R.; Peck, K.R.; Song, J.-H.; Lee, N.Y. Evaluation of the GenBank, EzTaxon, and BIBI services for molecular identification of clinical blood culture isolates that were unidentifiable or misidentified by conventional methods. J. Clin. Microbiol. 2012, 50, 1792–1795. [Google Scholar] [CrossRef]
- Parvizpour, S.; Pourseif, M.M.; Razmara, J.; Rafi, M.A.; Omidi, Y. Epitope-based vaccine design: A comprehensive overview of bioinformatics approaches. Drug Discov. Today 2020, 25, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Perilla, J.R.; Goh, B.C.; Cassidy, C.K.; Liu, B.; Bernardi, R.C.; Rudack, T.; Yu, H.; Wu, Z.; Schulten, K. Molecular dynamics simulations of large macromolecular complexes. Curr. Opin. Struct. Biol. 2015, 31, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Mrochen, D.M.; Al’Sholui, F.; Heuser, E.; Ryll, R.; Pritchett-Corning, K.R.; Jacob, J.; Walther, B.; Matuschka, F.-R.; Richter, D. Molecular epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in wild, captive and laboratory rats: Effect of habitat on the nasal S. aureus population. Toxins 2020, 12, 80. [Google Scholar] [CrossRef]
- Rasheed, N.A.; Hussein, N.R. Staphylococcus aureus: An Overview of Discovery, Characteristics, Epidemiology, Virulence Factors and Antimicrobial Sensitivity. Eur. J. Mol. Clin. Med. 2021, 8, 1160–1183. [Google Scholar]
- Samad, A.; Ahammad, F.; Nain, Z.; Alam, R.; Imon, R.R.; Hasan, M.; Rahman, M.S. Designing a multi-epitope vaccine against SARS-CoV-2: An immunoinformatics approach. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Starostina, E.V.; Sharabrin, S.V.; Antropov, D.N.; Stepanov, G.A.; Shevelev, G.Y.; Lemza, A.E.; Rudometov, A.P.; Borgoyakova, M.B.; Rudometova, N.B.; Marchenko, V.Y. Construction and immunogenicity of modified mRNA-vaccine variants encoding influenza virus antigens. Vaccines 2021, 9, 452. [Google Scholar] [CrossRef]
- Stothard, P. The Sequence Manipulation Suite: JavaScript Programs for Analyzing and Formatting Protein and DNA Sequences. BioTechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Peters, B.; Bulik, S.; Schoor, O.; Lemmel, C.; Schatz, M.M.; Kloetzel, P.-M.; Rammensee, H.-G.; Schild, H.; Holzhütter, H.-G. Modeling the MHC class I pathway by combining predictions of proteasomal cleavage, TAP transport and MHC class I binding. Cell. Mol. Life Sci. CMLS 2005, 62, 1025–1037. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 1, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Ura, T.; Takeuchi, M.; Kawagoe, T.; Mizuki, N.; Okuda, K.; Shimada, M. Current Vaccine Platforms in Enhancing T-Cell Response. Vaccines 2022, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bogdan, P.; Nazarian, S. An in silico deep learning approach to multi-epitope vaccine design: A SARS-CoV-2 case study. Sci. Rep. 2021, 11, 3238. [Google Scholar] [CrossRef]
- Naveed, M.; Makhdoom, S.I.; Ali, U.; Jabeen, K.; Aziz, T.; Khan, A.A.; Jamil, S.; Shahzad, M.; Alharbi, M.; Alshammari, A. Immunoinformatics Approach to Design Multi-Epitope-Based Vaccine against Machupo Virus Taking Viral Nucleocapsid as a Potential Candidate. Vaccines 2022, 10, 1732. [Google Scholar] [CrossRef]
- Naveed, M.; Sheraz, M.; Amin, A.; Waseem, M.; Aziz, T.; Khan, A.A.; Ghani, M.; Shahzad, M.; Alruways, M.W.; Dablool, A.S.; et al. Designing a Novel Peptide-Based Multi-Epitope Vaccine to Evoke a Robust Immune Response against Pathogenic Multidrug-Resistant Providencia heimbachae. Vaccines 2022, 10, 1300. [Google Scholar] [CrossRef] [PubMed]

| Ingredient | Amount (μL) |
|---|---|
| Primer1 (Forward) | 1 |
| Primer2 (Reverse) | 1 |
| ddH2O | 8.5 |
| Master mix | 12.5 |
| Sample of DNA | 2 |
| Total Amount | 25 |
| Profile for 16S rRNA |
|---|
| Adjust the initial temperature to 95 °C for 5 min. |
| Adjust the time for denaturation to 30 s at 95 °C. |
| Set the annealing temperature for 1 min and 30 s at 54 °C. |
| Extension temperature set for 60 s at 72 °C. |
| Set final extension for 5 min at 72 °C. |
| Adjust 35 cycles for reaction at the end. |
| Profile for LukD amplification |
| Set 95 °C initial temperature for 320 s. |
| Set the time for denaturation to 60 s at 95 °C. |
| Adjust the annealing temperature to 58 °C for 90 s. |
| Allow 60 s for the extension at 72 °C. |
| Adjust to 600 s for the final extension at 72 °C temperature. |
| Then adjust 35 cycles for the PCR reaction. |
| Adjust the storage temperature to 4 °C at the end. |
| Profile for spa amplification |
| Set 95 °C initial temperature for 300 s. |
| Set the time for denaturation to 60 s at 95 °C. |
| Adjust the annealing temperature to 56 °C for 90 s. |
| Allow 60 s for the extension at 72 °C. |
| Adjust to 600 s for the final extension at 72 °C temperature. |
| Then adjust 35 cycles for the PCR reaction. |
| Adjust the storage temperature to 4 °C at the end. |
| Profile for FmhA amplification |
| Set 95 °C initial temperature for 320 s. |
| Set the time for denaturation to 60 s at 95 °C. |
| Adjust the annealing temperature to 54 °C for 90 s. |
| Allow 90 s for the extension at 72 °C. |
| Adjust to 300 s for the final extension at 72 °C temperature. |
| Then adjust 38 cycles for the PCR reaction. |
| Adjust the storage temperature to 4 °C at the end. |
| Profile for delta hemolysin gene amplification |
| Set 95 °C initial temperature for 300 s. |
| Set the time for denaturation to 60 s at 95 °C. |
| Adjust the annealing temperature to 60 °C for 90 s. |
| Allow 60 s for the extension at 72 °C. |
| Adjust to 300 s for the final extension at 72 °C temperature. |
| Then adjust 38 cycles for the PCR reaction. |
| Adjust the storage temperature to 4 °C at the end. |
| Serial. No. | Epitope | Antigenicity |
|---|---|---|
| 1 | NADQRNGFIQSLKDDPSQSAN | 0.591 |
| 2 | AQKLNDSQAPKADAQQNNFN | 0.987 |
| 3 | KDQQSA | 1.462 |
| 4 | YEILNMPNLNEAQLTAEAAA | 0.576 |
| 5 | KEAAAKEAAAKMDYTNQSL | 0.846 |
| 6 | ENLRNADGDPRVPSS | 0.964 |
| Alleles | Epitopes | Antigenicity |
|---|---|---|
| HLA-A*11:01 | QSLVAFFFK | 0.624 |
| HLA-A*68:01 | ||
| HLA-A*31:01 | ||
| HLA-A*02:06 | KQNCLYVLV | 0.636 |
| HLA-A*02:03 | ||
| HLA-A*02:06 | AQLTAEAAA | 0.854 |
| HLA-B*53:01 | FPSHLCDLVI | 1.017 |
| HLA-A*11:01 | NQSLVAFFFK | 0.636 |
| HLA-A*68:01 | ||
| HLA-A*02:01 | SLVAFFFKSL | 0.501 |
| HLA-A*02:06 | ||
| HLA-A*30:01 | KSIYFPIFL | 2.125 |
| HLA-B*58:01 | ||
| HLA-A*02:01 | YVLVDPYLI | 2.058 |
| Alleles | Epitopes | Antigenicity |
|---|---|---|
| HLA-DRB1*07:01 | VILKHAKSIYFPIFL | 0.981 |
| HLA-DRB1*15:01 | ||
| HLA-DRB1*01:01 | ||
| HLA-DRB1*13:02 | ||
| HLA-DRB1*12:01 | ||
| HLA-DPA1*01:03/DPB1*02:01, HLA-DPA1*01:03/DPB1*04:01, HLA-DRB1*09:01 | ||
| HLA-DRB1*08:02 | ||
| HLA-DRB5*01:01 | ||
| HLA-DRB1*15:01 | LKIKQLINYFPSHLC | 0.632 |
| HLA-DRB1*08:02 | ||
| HLA-DRB1*13:02 | ||
| HLA-DRB1*12:01 | ||
| HLA-DRB1*07:01 | ||
| HLA-DRB1*01:01 | ||
| HLA-DRB1*04:05 | ||
| HLA-DRB1*09:01 | ||
| HLA-DRB1*04:01 | ||
| HLA-DRB1*12:01 | IKIKILKIKQLINYF | 0.591 |
| HLA-DRB4*01:01 | ||
| HLA-DRB1*15:01 | ||
| HLA-DRB1*11:01 | ||
| HLA-DRB1*01:01 | ||
| HLA-DPA1*03:01/DPB1*04:02 HLA-DRB1*08:02 | ||
| HLA-DPA1*03:01/DPB1*04:02, HLA-DRB4*01:01 | ITPSLIKIKILKIKQ | 0.834 |
| HLA-DRB1*11:01 | ||
| HLA-DRB1*12:01 | ||
| HLA-DRB1*15:01 | ||
| HLA-DRB1*01:01 | ||
| HLA-DQA1*01:01/DQB1*05:01 | ||
| NCLYVLVDPYLIENL HLA-DQA1*01:01/DQB1*05:01, HLA-DRB3*01:01 | ||
| HLA-DRB1*01:01 | ||
| HLA-DRB1*07:01 | ||
| HLA-DRB1*04:05 | ||
| HLA-DPA1*01:03/DPB1*02:01, HLA-DPA1*03:01/DPB1*04:02, HLA-DRB1*15:01 | ||
| HLA-DRB1*01:01 | NCLYVLVDPYLIENL | 1.003 |
| Property | Measurement | Indication |
|---|---|---|
| Total number of amino acids | 405 | Appropriate |
| Molecular weight | 43,773.14 kDa | Appropriate |
| Formula | C1988H3093N533O566S8 | - |
| Theoretical pI | 9.44 | Basic |
| Total number of positively charged residues (Arg + Lys) | 28 | - |
| Total number of negatively charged residues (Asp + Glu) | 45 | - |
| Total number of atoms | 6188 | - |
| Instability index (II) | 32.82 | Stable |
| Aliphatic index | 85.70 | Thermostable |
| Grand Average of Hydropathicity (GRAVY) | −0.195 | Hydrophilic |
| Antigenicity VaxiJen | 0.96 | Antigenic |
| Antigenicity AntigenPro | 0.731 | Antigenic |
| Allergenicity | Non-allergenic | Non-allergenic |
| Toxicity | Nontoxic | Nontoxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naveed, M.; Waseem, M.; Aziz, T.; Hassan, J.u.; Makhdoom, S.I.; Ali, U.; Alharbi, M.; Alsahammari, A. Identification of Bacterial Strains and Development of anmRNA-Based Vaccine to Combat Antibiotic Resistance in Staphylococcus aureus via In Vitro and In Silico Approaches. Biomedicines 2023, 11, 1039. https://doi.org/10.3390/biomedicines11041039
Naveed M, Waseem M, Aziz T, Hassan Ju, Makhdoom SI, Ali U, Alharbi M, Alsahammari A. Identification of Bacterial Strains and Development of anmRNA-Based Vaccine to Combat Antibiotic Resistance in Staphylococcus aureus via In Vitro and In Silico Approaches. Biomedicines. 2023; 11(4):1039. https://doi.org/10.3390/biomedicines11041039
Chicago/Turabian StyleNaveed, Muhammad, Muhammad Waseem, Tariq Aziz, Jawad ul Hassan, Syeda Izma Makhdoom, Urooj Ali, Metab Alharbi, and Abdulrahman Alsahammari. 2023. "Identification of Bacterial Strains and Development of anmRNA-Based Vaccine to Combat Antibiotic Resistance in Staphylococcus aureus via In Vitro and In Silico Approaches" Biomedicines 11, no. 4: 1039. https://doi.org/10.3390/biomedicines11041039
APA StyleNaveed, M., Waseem, M., Aziz, T., Hassan, J. u., Makhdoom, S. I., Ali, U., Alharbi, M., & Alsahammari, A. (2023). Identification of Bacterial Strains and Development of anmRNA-Based Vaccine to Combat Antibiotic Resistance in Staphylococcus aureus via In Vitro and In Silico Approaches. Biomedicines, 11(4), 1039. https://doi.org/10.3390/biomedicines11041039









