Inflammation Related to Association of Low Uric Acid and Progression to Severe Disease in Patients Hospitalized for Non-Severe Coronavirus Disease 2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Determination of Uric Acid, CRP, KL-6, and D-Dimer Blood Levels
2.4. Diagnosis of COVID-19
2.5. Classification of COVID-19 Severity Level
2.6. COVID-19 Patient Management during Hospitalization
2.7. Outcome
2.8. Other Clinical Assessments
2.9. Statistical Analysis
3. Results
3.1. Study Population
3.2. Clinical Characteristics of Patients
3.3. Medications Given for COVID-19 after Hospitalization
3.4. Progression from Non-Severe to Severe COVID-19
3.5. Serum Uric Acid Level Associated with Progression from Non-Severe to Severe COVID-19
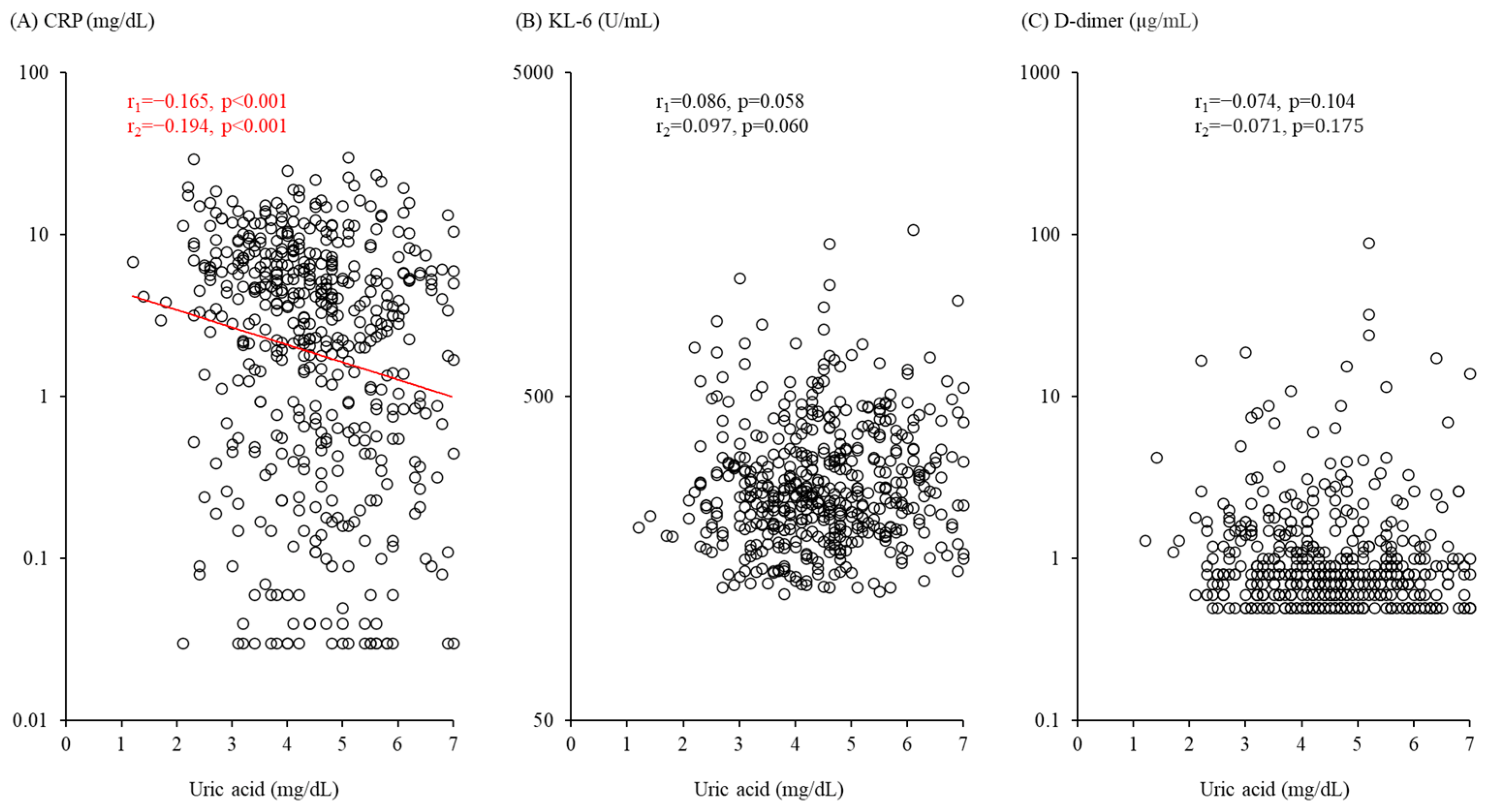
3.6. Serum Uric Acid Level Associated with Inflammation but Not Alveolar Damage or Coagulation Abnormality Markers
3.7. Inflammation Shows Stronger Relationship with Progression from Non-Severe to Severe COVID-19 as Compared to Alveolar Damage and Coagulation Abnormality Markers
3.8. Inflammation Marker Found to Influence Association of Serum Uric Acid Level with Severe COVID-19 Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Morawiec, E.; Bednarska-Czerwinska, A.; Pudelko, A.; Strychalska, A.; Broncel, M.; Sagan, D.; Madej, A.; Jasinska-Balwierz, A.; Staszkiewicz, R.; Sobanski, D.; et al. A Retrospective Population Study of 385 191 Positive Real-Time Reverse Transcription-Polymerase Chain Reaction Tests For SARS-CoV-2 from a Single Laboratory in Katowice, Poland from April 2020 to July 2022. Med. Sci. Monit. 2023, 29, e938872. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Mamun, A.; Dominic, A.; Le, N.T. SARS-CoV-2 Mediated Endothelial Dysfunction: The Potential Role of Chronic Oxidative Stress. Front. Physiol. 2020, 11, 605908. [Google Scholar] [CrossRef]
- Wieczfinska, J.; Kleniewska, P.; Pawliczak, R. Oxidative Stress-Related Mechanisms in SARS-CoV-2 Infections. Oxidative Med. Cell. Longev. 2022, 2022, 5589089. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. Inflammation and thrombosis in COVID-19 pathophysiology: Proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets. Physiol. Rev. 2021, 101, 545–567. [Google Scholar] [CrossRef]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Boardman, N.T.; Falck, A.T.; Lund, T.; Chu, X.; Martin-Armas, M.; Norvik, J.V.; Jenssen, T.G.; Ytrehus, K. Human concentrations of uric acid scavenges adaptive and maladaptive reactive oxygen species in isolated rat hearts subjected to ischemic stress. Can. J. Physiol. Pharmacol. 2020, 98, 139–146. [Google Scholar] [CrossRef]
- Yin, W.; Zhou, Q.L.; OuYang, S.X.; Chen, Y.; Gong, Y.T.; Liang, Y.M. Uric acid regulates NLRP3/IL-1beta signaling pathway and further induces vascular endothelial cells injury in early CKD through ROS activation and K+ efflux. BMC Nephrol. 2019, 20, 319. [Google Scholar] [CrossRef]
- Kurajoh, M.; Fukumoto, S.; Yoshida, S.; Akari, S.; Murase, T.; Nakamura, T.; Ishii, H.; Yoshida, H.; Nagata, Y.; Morioka, T.; et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci. Rep. 2021, 11, 7378. [Google Scholar] [CrossRef]
- Fukushima, T.; Chubachi, S.; Namkoong, H.; Otake, S.; Nakagawara, K.; Tanaka, H.; Lee, H.; Morita, A.; Watase, M.; Kusumoto, T.; et al. U-shaped association between abnormal serum uric acid levels and COVID-19 severity: Reports from the Japan COVID-19 Task Force. Int. J. Infect. Dis. 2022, 122, 747–754. [Google Scholar] [CrossRef]
- Chen, B.; Lu, C.; Gu, H.Q.; Li, Y.; Zhang, G.; Lio, J.; Luo, X.; Zhang, L.; Hu, Y.; Lan, X.; et al. Serum Uric Acid Concentrations and Risk of Adverse Outcomes in Patients With COVID-19. Front. Endocrinol. 2021, 12, 633767. [Google Scholar] [CrossRef]
- Li, G.; Wu, X.; Zhou, C.L.; Wang, Y.M.; Song, B.; Cheng, X.B.; Dong, Q.F.; Wang, L.L.; You, S.S.; Ba, Y.M. Uric acid as a prognostic factor and critical marker of COVID-19. Sci. Rep. 2021, 11, 17791. [Google Scholar] [CrossRef]
- Wada, A.; Higashiyama, M.; Kurihara, C.; Ito, S.; Tanemoto, R.; Mizoguchi, A.; Nishii, S.; Inaba, K.; Sugihara, N.; Hanawa, Y.; et al. Protective Effect of Luminal Uric Acid Against Indomethacin-Induced Enteropathy: Role of Antioxidant Effect and Gut Microbiota. Dig. Dis. Sci. 2022, 67, 121–133. [Google Scholar] [CrossRef]
- Naderi, N.; Rahimzadeh, M. Krebs von den Lungen-6 (KL-6) as a clinical marker for severe COVID-19: A systematic review and meta-analyses. Virology 2022, 566, 106–113. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Varshney, S.; Dutt, N.; Munikumar, M.; Asfahan, S.; Kulkarni, P.P.; Gupta, P. D-dimer, disease severity, and deaths (3D-study) in patients with COVID-19: A systematic review and meta-analysis of 100 studies. Sci. Rep. 2021, 11, 21888. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Hisatome, I.; Li, P.; Miake, J.; Taufiq, F.; Mahati, E.; Maharani, N.; Utami, S.B.; Kuwabara, M.; Bahrudin, U.; Ninomiya, H. Uric Acid as a Risk Factor for Chronic Kidney Disease and Cardiovascular Disease—Japanese Guideline on the Management of Asymptomatic Hyperuricemia. Circ. J. 2021, 85, 130–138. [Google Scholar] [CrossRef]
- Oda, Y.; Shiraishi, S.; Shimada, M.; Kurai, O. Clinical profiles and outcome of patients with COVID-19 in a specialized hospital in Japan. J. Anesth. 2021, 35, 405–411. [Google Scholar] [CrossRef]
- Numaguchi, R.; Kurajoh, M.; Hiura, Y.; Imai, T.; Morioka, T.; Saito, M.; Shiraishi, S.; Emoto, M.; Nishiguchi, Y. Glycated hemoglobin level on admission associated with progression to severe disease in hospitalized patients with non-severe coronavirus disease 2019. J. Diabetes Investig. 2022, 13, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Japanese Ministry of Health, Labour and Welfare. Clinical Management of Patients with COVID-19: A Guide for Front-Line Healthcare Workers, Version 3; Japanese Ministry of Health, Labour and Welfare: Tokyo, Japan, 2020.
- Japanese Ministry of Health, Labour and Welfare. Clinical Management of Patients with COVID-19: A Guide for Front-Line Healthcare Workers, Version 4.2; Japanese Ministry of Health, Labour and Welfare: Tokyo, Japan, 2021.
- Ichii, M.; Kurajoh, M.; Okute, Y.; Ihara, Y.; Imai, T.; Morioka, T.; Mori, K.; Shoji, T.; Tsujimoto, Y.; Ubai, T.; et al. Reduced Risk of Progression from Non-Severe to Severe COVID-19 in Hospitalized Dialysis Patients by Full COVID-19 Vaccination. J. Clin. Med. 2022, 11, 6348. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Kinoshita, M.; Yokote, K.; Arai, H.; Iida, M.; Ishigaki, Y.; Ishibashi, S.; Umemoto, S.; Egusa, G.; Ohmura, H.; Okamura, T.; et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J. Atheroscler. Thromb. 2018, 25, 846–984. [Google Scholar] [CrossRef]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Imai, K.; Keele, L.; Tingley, D. A general approach to causal mediation analysis. Psychol. Methods 2010, 15, 309–334. [Google Scholar] [CrossRef]
- Abou-Mourad, N.N.; Chamberlain, B.E.; Ackerman, N.B. Poor prognosis of patients with intra-abdominal sepsis and hypouricemia. Surg. Gynecol. Obstet. 1979, 148, 358–360. [Google Scholar]
- Zhou, Y.; Li, W.; Huang, Y. A low serum uric acid concentration predicts a poor prognosis in adult patients with candidemia. Open Med. 2022, 17, 1077–1083. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Mesta, F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Tsermpini, E.E.; Glamoclija, U.; Ulucan-Karnak, F.; Redensek Trampuz, S.; Dolzan, V. Molecular Mechanisms Related to Responses to Oxidative Stress and Antioxidative Therapies in COVID-19: A Systematic Review. Antioxidants 2022, 11, 1609. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef]
- Ivanciuc, T.; Sbrana, E.; Casola, A.; Garofalo, R.P. Protective Role of Nuclear Factor Erythroid 2-Related Factor 2 Against Respiratory Syncytial Virus and Human Metapneumovirus Infections. Front. Immunol. 2018, 9, 854. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C. Front. Pharmacol. 2022, 13, 899198. [Google Scholar] [CrossRef]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef]
- Elshazli, R.M.; Toraih, E.A.; Elgaml, A.; El-Mowafy, M.; El-Mesery, M.; Amin, M.N.; Hussein, M.H.; Killackey, M.T.; Fawzy, M.S.; Kandil, E. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS ONE 2020, 15, e0238160. [Google Scholar] [CrossRef]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Omer, A.K.; Khorshidi, S.; Mortazavi, N.; Rahman, H.S. A Review on the Antiviral Activity of Functional Foods Against COVID-19 and Viral Respiratory Tract Infections. Int. J. Gen. Med. 2022, 15, 4817–4835. [Google Scholar] [CrossRef] [PubMed]
- Haas de Mello, A.; Liu, T.; Garofalo, R.P.; Casola, A. Hydrogen Sulfide Donor GYY4137 Rescues NRF2 Activation in Respiratory Syncytial Virus Infection. Antioxidants 2022, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Checconi, P.; De Angelis, M.; Marcocci, M.E.; Fraternale, A.; Magnani, M.; Palamara, A.T.; Nencioni, L. Redox-Modulating Agents in the Treatment of Viral Infections. Int. J. Mol. Sci. 2020, 21, 4084. [Google Scholar] [CrossRef] [PubMed]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: Implications for uncoupling endothelial nitric oxide synthase. Biochem. Pharmacol. 2005, 70, 343–354. [Google Scholar] [CrossRef]
- Werion, A.; Belkhir, L.; Perrot, M.; Schmit, G.; Aydin, S.; Chen, Z.; Penaloza, A.; De Greef, J.; Yildiz, H.; Pothen, L.; et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020, 98, 1296–1307. [Google Scholar] [CrossRef]
- Wu, V.C.; Huang, J.W.; Hsueh, P.R.; Yang, Y.F.; Tsai, H.B.; Kan, W.C.; Chang, H.W.; Wu, K.D.; SARS Research Group of National Taiwan University College of Medicine. Renal hypouricemia is an ominous sign in patients with severe acute respiratory syndrome. Am. J. Kidney Dis. 2005, 45, 88–95. [Google Scholar] [CrossRef]
- Balawender, K.; Pliszka, A.; Krowiak, A.; Sito, M.; Grabarek, B.O.; Boron, D. Does SARS-CoV-2 Affect Male Urogenital System? Curr. Pharm. Biotechnol. 2022, 23, 1792–1799. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- El-Kassas, M.; Alboraie, M.; Elbadry, M.; El Sheemy, R.; Abdellah, M.; Afify, S.; Madkour, A.; Zaghloul, M.; Awad, A.; Wifi, M.N.; et al. Non-pulmonary involvement in COVID-19: A systemic disease rather than a pure respiratory infection. World J. Clin. Cases 2023, 11, 493–505. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Age (all subjects), years | 76.0 (57.0–82.0) |
| Male, n | 254 (52.0%) |
| Age (males), years | 71.5 (55.0–80.0) |
| Females, n | 234 (48.0%) |
| Age (females), years | 79.0 (61.5–85.0) |
| BMI, kg/m2 | 23.1 (21.0–25.7) |
| Smoker, n | 193 (39.5%) |
| eGFR, mL/min/1.73 m2 | 67.0 (54.0–84.0) |
| Coexisting diseases | |
| Diabetes mellitus, n | 167 (34.2%) |
| Hypertension, n | 270 (55.3%) |
| Dyslipidemia>, n | 264 (54.1%) |
| Cerebrovascular/cardiovascular disease, n | 91 (18.6%) |
| Chronic respiratory disease, n | 64 (13.1%) |
| Days from onset of illness to hospital admission | 5.0 (3.0–8.0%) |
| COVID-19 severity | |
| Mild, n | 211 (43.2%) |
| Moderate I, n | 167 (34.2%) |
| Moderate II, n | 110 (22.5%) |
| Uric acid, mg/dL | 4.4 (3.6–5.4) |
| Use of uric acid-lowering agents, n | 54 (11.1%) |
| CRP, mg/dL | 3.33 (0.58–6.77) |
| >KL-6, U/mL | 252.0 (198.0–337.0) |
| D-dimer, µ>g/mL | 0.8 (0.6–1.2) |
| Variables | HR (95% CI) | p Value |
|---|---|---|
| Age (per 10-year increase) | 1.342 (1.096–1.642) | 0.004 |
| Male (ref. female) | 2.103 (1.232–3.589) | 0.006 |
| BMI (per 1 kg/m2 increase) | 1.053 (0.995–1.115) | 0.074 |
| Smoker (ref. non-smoker) | 1.324 (0.823–2.131) | 0.247 |
| Diabetes mellitus (ref. absence) | 1.014 (0.644–1.595) | 0.953 |
| Hypertension (ref. absence) | 1.144 (0.682–1.920) | 0.611 |
| Dyslipidemia (ref. absence) | 0.936 (0.604–1.450) | 0.768 |
| Cerebrovascular/cardiovascular disease (ref. absence) | 1.080 (0.660–1.768) | 0.759 |
| Chronic respiratory disease (ref. absence) | 0.704 (0.363–1.365) | 0.299 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.934 (0.819–1.066) | 0.311 |
| Days from onset of illness to hospital admission (per 1 day increase) | 0.994 (0.925–1.068) | 0.876 |
| Moderate I COVID-19 on admission (ref. mild) | 2.055 (1.119–3.773) | 0.020 |
| Moderate II COVID-19 on admission (ref. mild) | 5.264 (2.922–9.481) | <0.001 |
| Use of uric acid-lowering agent (ref. absence) | 1.067 (0.579–1.966) | 0.835 |
| Uric acid (per 1 mg/dL decrease) | 1.279 (1.021–1.602) | 0.032 |
| Variable of Interest | Direction | Standardized HR | 95% CI | p Value |
|---|---|---|---|---|
| Uric acid | 1 SD decrease | 1.337 | 1.025–1.743 | 0.032 |
| CRP | 1 SD increase in log scale | 2.079 | 1.389–3.113 | <0.001 |
| KL-6 | 1 SD increase in log scale | 1.292 | 1.040–1.606 | 0.021 |
| D-dimer | 1 SD increase in log scale | 1.183 | 0.966–1.448 | 0.104 |
| Variable Adjusted | Standardized Uric Acid HR | 95% CI | p Value | p * Value |
|---|---|---|---|---|
| CRP | 1.233 | 0.941–1.616 | 0.128 | 0.041 |
| KL-6 | 1.337 | 1.029–1.736 | 0.029 | 0.991 |
| D-dimer | 1.320 | 1.013–1.719 | 0.039 | 0.393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurajoh, M.; Hiura, Y.; Numaguchi, R.; Ihara, Y.; Imai, T.; Morioka, T.; Emoto, M.; Nishiguchi, Y. Inflammation Related to Association of Low Uric Acid and Progression to Severe Disease in Patients Hospitalized for Non-Severe Coronavirus Disease 2019. Biomedicines 2023, 11, 854. https://doi.org/10.3390/biomedicines11030854
Kurajoh M, Hiura Y, Numaguchi R, Ihara Y, Imai T, Morioka T, Emoto M, Nishiguchi Y. Inflammation Related to Association of Low Uric Acid and Progression to Severe Disease in Patients Hospitalized for Non-Severe Coronavirus Disease 2019. Biomedicines. 2023; 11(3):854. https://doi.org/10.3390/biomedicines11030854
Chicago/Turabian StyleKurajoh, Masafumi, Yoshikazu Hiura, Ryutaro Numaguchi, Yasutaka Ihara, Takumi Imai, Tomoaki Morioka, Masanori Emoto, and Yukio Nishiguchi. 2023. "Inflammation Related to Association of Low Uric Acid and Progression to Severe Disease in Patients Hospitalized for Non-Severe Coronavirus Disease 2019" Biomedicines 11, no. 3: 854. https://doi.org/10.3390/biomedicines11030854
APA StyleKurajoh, M., Hiura, Y., Numaguchi, R., Ihara, Y., Imai, T., Morioka, T., Emoto, M., & Nishiguchi, Y. (2023). Inflammation Related to Association of Low Uric Acid and Progression to Severe Disease in Patients Hospitalized for Non-Severe Coronavirus Disease 2019. Biomedicines, 11(3), 854. https://doi.org/10.3390/biomedicines11030854






