StemRegenin 1 Mitigates Radiation-Mediated Hematopoietic Injury by Modulating Radioresponse of Hematopoietic Stem/Progenitor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimentation and Radiation Exposure
2.2. Radiomitigation by SR1 in Lethally Irradiated Mice
2.3. Blood Cell Counts in Mice
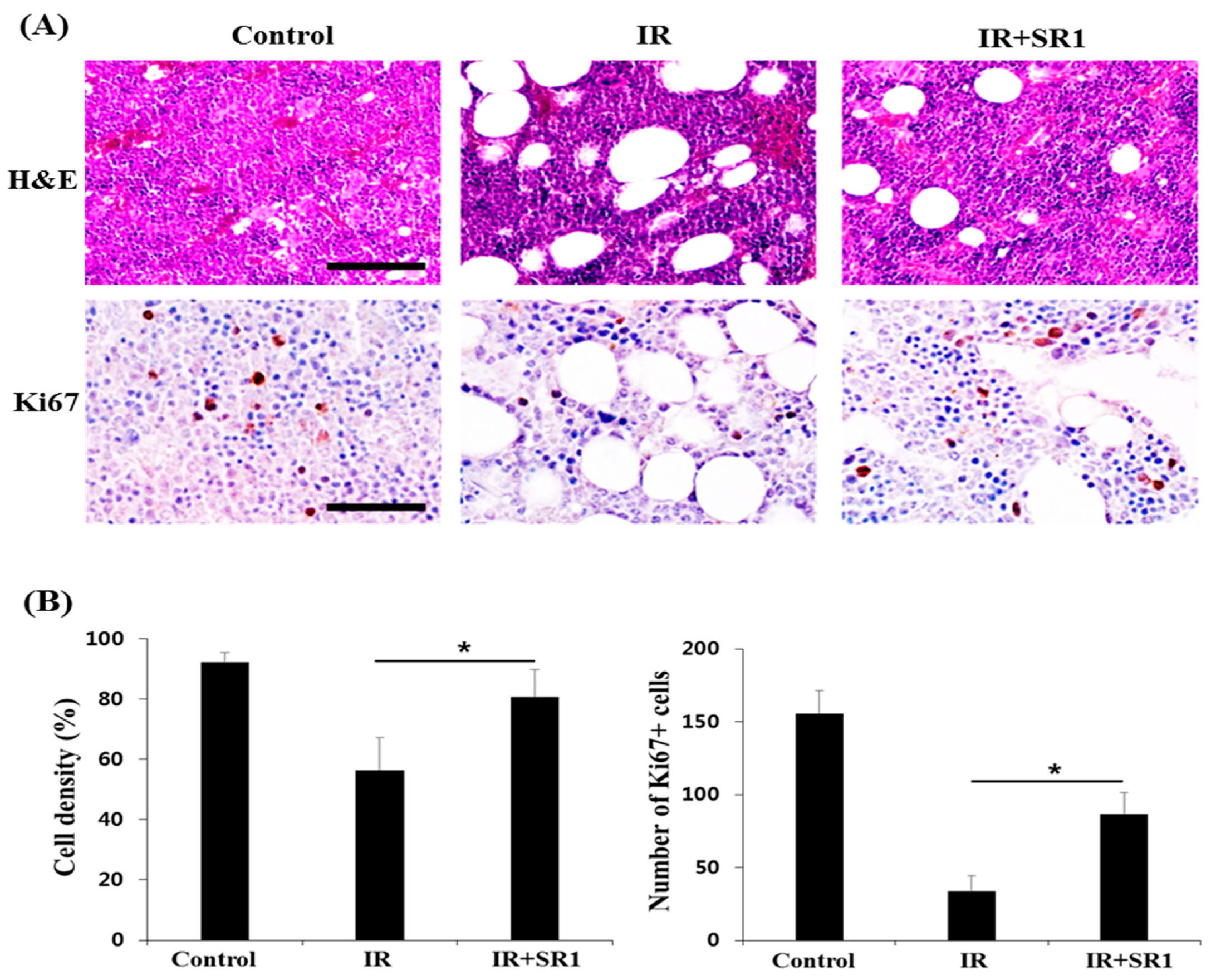
2.4. Histologic Analysis of Bone Marrow (BM)
2.5. Culture and Treatment of HSPCs
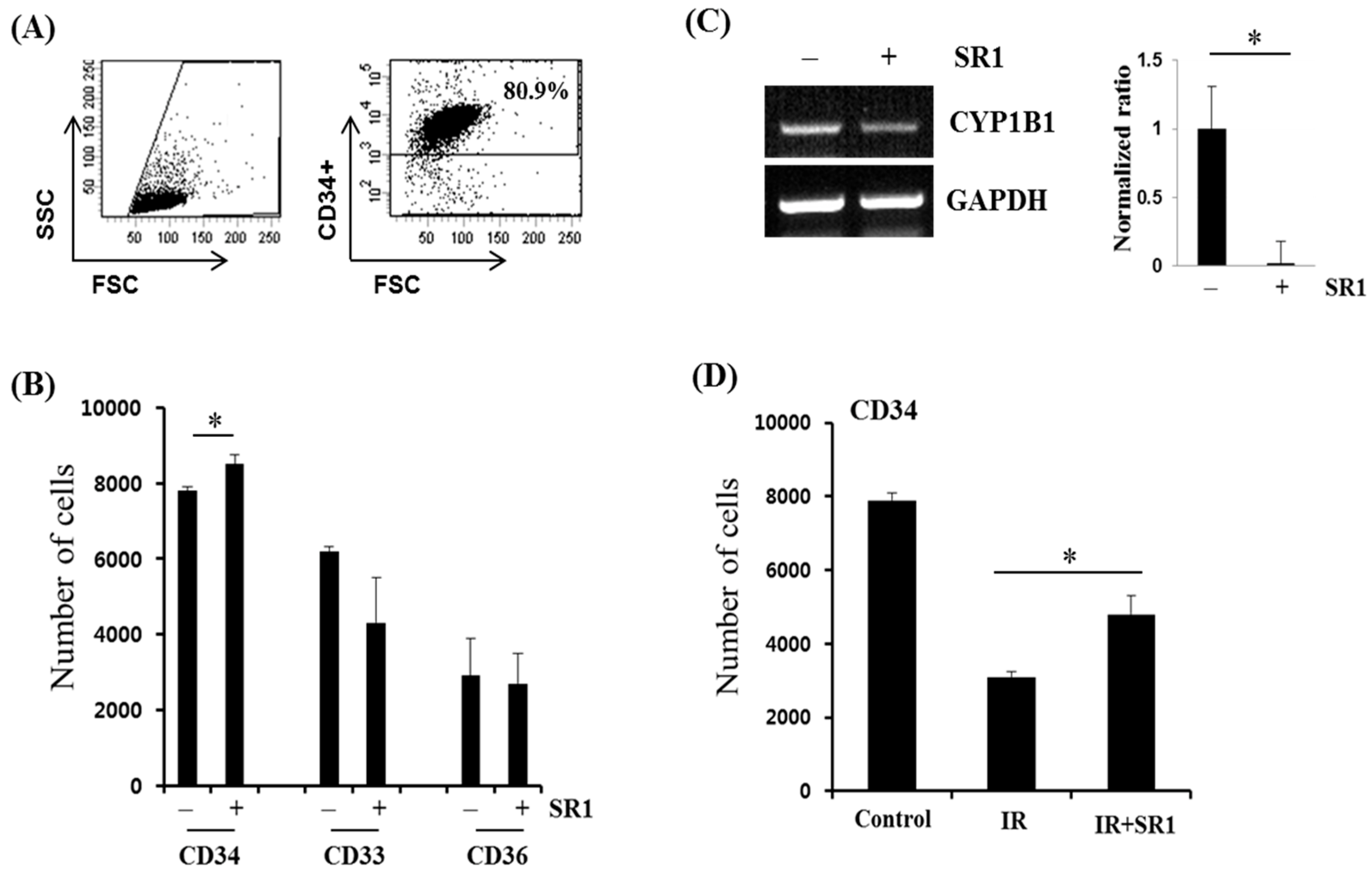
2.6. HSPC Population Analysis by FACS
2.7. Apoptosis Assay
2.8. Immunocytochemistry (ICC)
2.9. Comet Assay
2.10. RT-PCR
2.11. Statistical Analysis
3. Results
3.1. StemRegenin 1 (SR1) Mitigated Total Body Irradiation (TBI)-Induced Lethality and Pancytopenia in Mice
3.2. SR1 Treatment Promoted HSPC Expansion and Attenuated the Radiation-Induced Reduction in the HSC Population
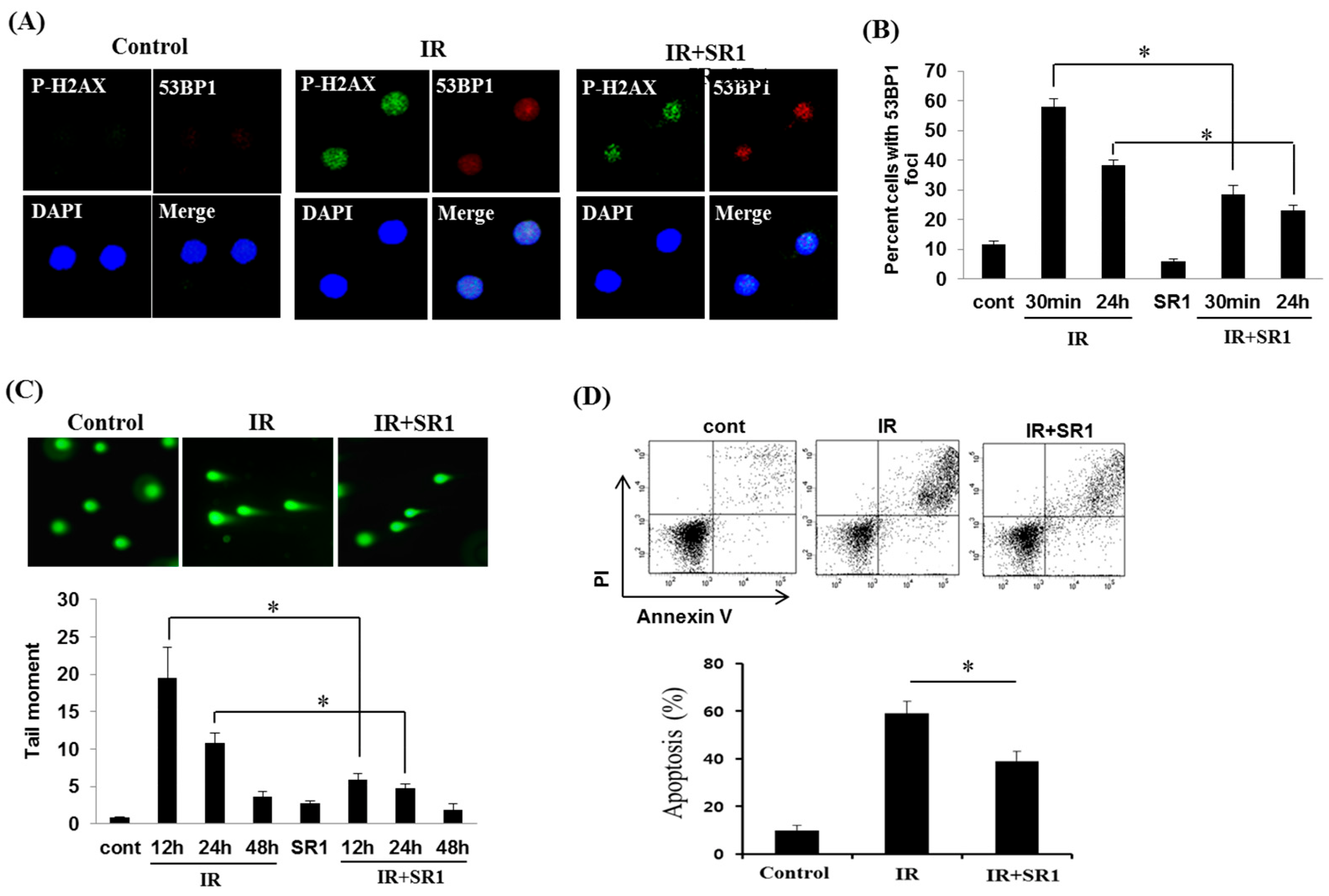
3.3. SR1 Decreased the Genomic Instability and Apoptosis of HSPCs in Response to Radiation
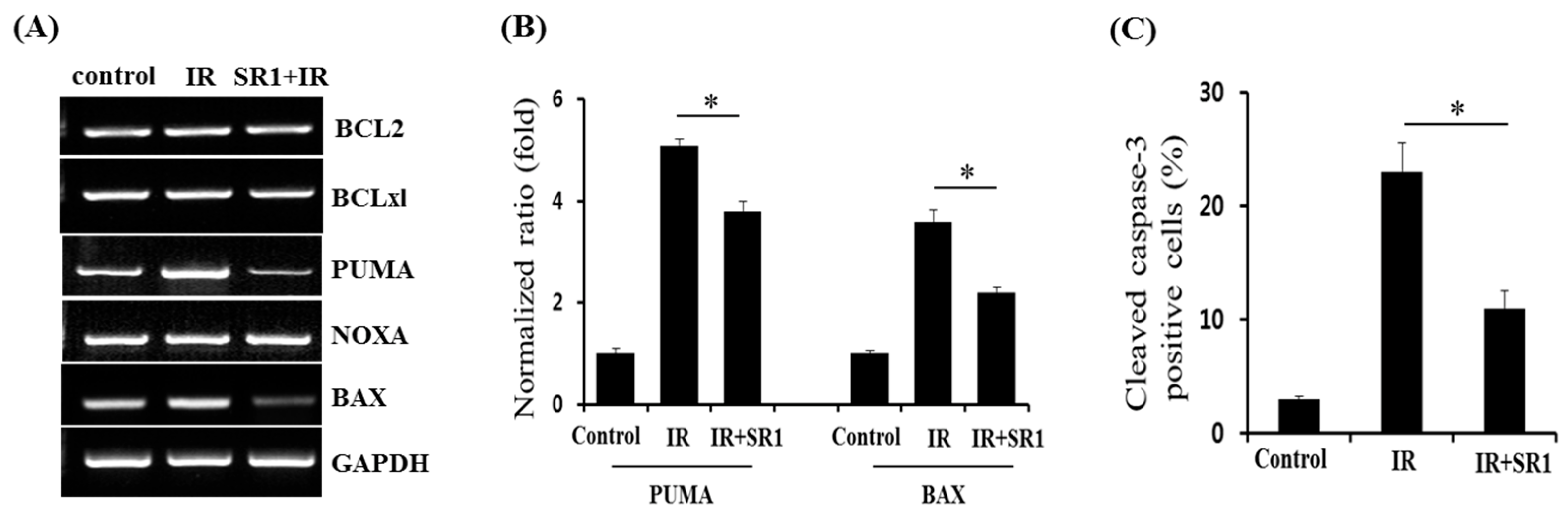
3.4. Members of the BCL-2 Family Were Involved in SR1-Mediated Alleviation of HSPC Apoptosis Following Irradiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waselenko, J.K.; MacVittie, T.J.; Blakely, W.F.; Pesik, N.; Wiley, A.L.; Dickerson, W.E.; Tsu, H.; Confer, D.L.; Coleman, C.N.; Seed, T.; et al. Medical management of the acute radiation syndrome: Recommendations of the Strategic National Stockpile Radiation Working Group. Ann. Intern. Med. 2004, 140, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, N.; Albanese, J. Medical management of acute radiation syndrome. J. Radiol. Prot. 2022, 42, 031002. [Google Scholar] [CrossRef] [PubMed]
- Stenke, L.; Hedman, C.; Lindberg, M.L.; Lindberg, K.; Valentin, J. The acute radiation syndrome—Need for updated medical guidelines. J. Radiol. Prot. 2022, 42, 014004. [Google Scholar] [CrossRef] [PubMed]
- Dainiak, N. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 2002, 30, 513–528. [Google Scholar] [CrossRef]
- Uma Devi, P. Radiosensitivity of the developing haemopoietic system in mammals and its adult consequences: Animal studies. Br. J. Radiol. 2003, 76, 366–372. [Google Scholar] [CrossRef]
- Mason, K.A.; Withers, H.R.; McBride, W.H.; Davis, C.A.; Smathers, J.B. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat. Res. 1989, 117, 480. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; Zon, L. Hematopoiesis. Development 2013, 140, 2463–2467. [Google Scholar] [CrossRef]
- Bakanay, S.M.; Demirer, T. Novel agents and approaches for stem cell mobilization in normal donors and patients. Bone Marrow Transplant. 2011, 47, 1154–1163. [Google Scholar] [CrossRef]
- Zarniko, N.; Skorska, A.; Steinhoff, G.; David, R.; Gaebel, R. Dose-Independent Therapeutic Benefit of Bone Marrow Stem Cell Transplantation after MI in Mice. Biomedicines 2020, 8, 157. [Google Scholar] [CrossRef]
- Mulero-Navarro, S.; Fernandez-Salguero, P.M. New Trends in Aryl Hydrocarbon Receptor Biology. Front. Cell Dev. Biol. 2016, 4, 45. [Google Scholar] [CrossRef]
- Boitano, A.E.; Wang, J.; Romeo, R.; Bouchez, L.C.; Parker, A.E.; Sutton, S.E.; Walker, J.R.; Flaveny, C.A.; Perdew, G.H.; Denison, M.S.; et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 2010, 329, 1345–1348. [Google Scholar] [CrossRef]
- Gori, J.L.; Chandrasekaran, D.; Kowalski, J.P.; Adair, J.E.; Beard, B.C.; D’Souza, S.L.; Kiem, H.P. Efficient generation, purification, and expansion of CD34(+) hematopoietic progenitor cells from nonhuman primate-induced pluripotent stem cells. Blood 2012, 120, e35–e44. [Google Scholar] [CrossRef]
- Angelos, M.G.; Ruh, P.N.; Webber, B.R.; Blum, R.H.; Ryan, C.D.; Bendzick, L.; Shim, S.; Yingst, A.M.; Tufa, D.M.; Verneris, M.R.; et al. Aryl hydrocarbon receptor inhibition promotes hematolymphoid development from human pluripotent stem cells. Blood 2017, 129, 3428–3439. [Google Scholar] [CrossRef]
- Walasek, M.A.; van Os, R.; de Haan, G. Hematopoietic stem cell expansion: Challenges and opportunities. Ann. N. Y. Acad. Sci. 2012, 1266, 138–150. [Google Scholar] [CrossRef]
- Wagner, J.E., Jr.; Brunstein, C.G.; Boitano, A.E.; DeFor, T.E.; McKenna, D.; Sumstad, D.; Blazar, B.R.; Tolar, J.; Le, C.; Jones, J.; et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell 2016, 18, 144–155. [Google Scholar] [CrossRef]
- Baverstock, K.; Belyakov, O.V. Classical radiation biology, the bystander effect and paradigms: A reply. Hum. Exp. Toxicol. 2005, 24, 537–542. [Google Scholar] [CrossRef]
- Verma, Y.K.; Gangenahalli, G.U.; Singh, V.K.; Gupta, P.; Chandra, R.; Sharma, R.K.; Raj, H.G. Cell death regulation by B-cell lymphoma protein. Apoptosis 2006, 11, 459–471. [Google Scholar] [CrossRef]
- Cao, X.; Wen, P.; Fu, Y.; Gao, Y.; Qi, X.; Chen, B.; Tao, Y.; Wu, L.; Xu, A.; Lu, H.; et al. Radiation induces apoptosis primarily through the intrinsic pathway in mammalian cells. Cell. Signal. 2019, 62, 109337. [Google Scholar] [CrossRef]
- Kale, J.; Liu, Q.; Leber, B.; Andrews, D.W. Shedding light on apoptosis at subcellular membranes. Cell 2012, 151, 1179–1184. [Google Scholar] [CrossRef]
- Kollek, M.; Müller, A.; Egle, A.; Erlacher, M. Bcl-2 proteins in development, health, and disease of the hematopoietic system. FEBS J. 2016, 283, 2779–2810. [Google Scholar] [CrossRef]
- Shim, S.; Lee, S.B.; Lee, J.G.; Jang, W.S.; Lee, S.J.; Park, S.; Lee, S.S. Mitigating effects of hUCB-MSCs on the hematopoietic syndrome resulting from total body irradiation. Exp. Hematol. 2013, 41, 346–353.e2. [Google Scholar] [CrossRef] [PubMed]
- Tratwal, J.; Bekri, D.; Boussema, C.; Sarkis, R.; Kunz, N.; Koliqi, T.; Rojas-Sutterlin, S.; Schyrr, F.; Tavakol, D.N.; Campos, V.; et al. MarrowQuant Across Aging and Aplasia: A Digital Pathology Workflow for Quantification of Bone Marrow Compartments in Histological Sections. Front. Endocrinol. 2020, 24, 480. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.S.; Fackler, M.J.; Civin, C.I.; May, W.S. CD34: Structure, biology, and clinical utility. Blood 1996, 87, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Feuillard, J.; Jacob, M.-C.; Valensi, F.; Maynadié, M.; Gressin, R.; Chaperot, L.; Arnoulet, C.; Brignole-Baudouin, F.; Drénou, B.; Duchayne, E.; et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood 2002, 99, 1556–1563. [Google Scholar] [CrossRef]
- Edelman, P.; Vinci, G.; Villeval, J.L.; Vainchenker, W.; Henri, A.; Miglierina, R.; Rouger, P.; Reviron, J.; Breton-Gorius, J.; Sureau, C. A monoclonal antibody against an erythrocyte ontogenic antigen identifies fetal and adult erythroid progenitors. Blood 1986, 67, 56–63. [Google Scholar] [CrossRef]
- Czechowicz, A.; Kraft, D.; Weissman, I.L.; Bhattacharya, D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 2007, 318, 1296–1299. [Google Scholar] [CrossRef]
- Kawajiri, K.; Fujii-Kuriyama, Y. Cytochrome P450 gene regulation and physiological functions mediated by the aryl hydrocarbon receptor. Arch. Biochem. Biophys. 2007, 464, 207–212. [Google Scholar] [CrossRef]
- Little, J.B. Radiation-induced genomic instability. Int. J. Radiat. Biol. 1998, 74, 663–671. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Tartier, L.; Gilchrist, S.; Burdak-Rothkamm, S.; Folkard, M.; Prise, K.M. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007, 67, 5872–5879. [Google Scholar] [CrossRef]
- Shao, L.; Sun, Y.; Zhang, Z.; Feng, W.; Gao, Y.; Cai, Z.; Wang, Z.Z.; Look, A.T.; Wu, W.-S. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood 2010, 115, 4707–4714. [Google Scholar] [CrossRef]
- Yu, H.; Shen, H.; Yuan, Y.; XuFeng, R.; Hu, X.; Garrison, S.P.; Zhang, L.; Yu, J.; Zambetti, G.P.; Cheng, T. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood 2010, 115, 3472–3480. [Google Scholar] [CrossRef]
- Jones, J.; Alloush, J.; Sellamuthu, R.; Chua, H.L.; MacVittie, T.J.; Orschell, C.M.; Kane, M.A. Effect of sex on biomarker response in a mouse model of the hematopoietic acute radiation syndrome. Health Phys. 2019, 116, 484–502. [Google Scholar] [CrossRef]
- Mills, K.D.; Ferguson, D.; Alt, F. The role of DNA breaks in genomic instability and tumorigenesis. Immunol. Rev. 2003, 194, 77–95. [Google Scholar] [CrossRef]
- Chopra, M.; Schrenk, D. Dioxin toxicity, aryl hydrocarbon receptor signaling, and apoptosis-persistent pollutants affect programmed cell death. Crit. Rev. Toxicol. 2011, 41, 292–320. [Google Scholar] [CrossRef]
- Li, J.; Phadnis-Moghe, A.S.; Crawford, R.B.; Kaminski, N.E. Aryl hydrocarbon receptor activation by 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs human B lymphopoiesis. Toxicology 2017, 378, 17–24. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Erlacher, M.; Michalak, E.M.; Kelly, P.N.; Labi, V.; Niederegger, H.; Coultas, L.; Adams, J.M.; Strasser, A.; Villunger, A. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 2005, 106, 4131–4138. [Google Scholar] [CrossRef]
- Labi, V.; Erlacher, M.; Krumschnabel, G.; Manzl, C.; Tzankov, A.; Pinon, J.; Egle, A.; Villunger, A. Apoptosis of leukocytes triggered by acute DNA damage promotes lymphoma formation. Genes Dev. 2010, 24, 1602–1607. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.J.; Shin, D.-Y.; Kim, M.-J.; Jang, H.; Kim, S.; Yang, H.; Jang, W.I.; Park, S.; Shim, S.; Lee, S.B. StemRegenin 1 Mitigates Radiation-Mediated Hematopoietic Injury by Modulating Radioresponse of Hematopoietic Stem/Progenitor Cells. Biomedicines 2023, 11, 824. https://doi.org/10.3390/biomedicines11030824
Hwang YJ, Shin D-Y, Kim M-J, Jang H, Kim S, Yang H, Jang WI, Park S, Shim S, Lee SB. StemRegenin 1 Mitigates Radiation-Mediated Hematopoietic Injury by Modulating Radioresponse of Hematopoietic Stem/Progenitor Cells. Biomedicines. 2023; 11(3):824. https://doi.org/10.3390/biomedicines11030824
Chicago/Turabian StyleHwang, You Jung, Dong-Yeop Shin, Min-Jung Kim, Hyosun Jang, Soyeon Kim, Hyunwon Yang, Won Il Jang, Sunhoo Park, Sehwan Shim, and Seung Bum Lee. 2023. "StemRegenin 1 Mitigates Radiation-Mediated Hematopoietic Injury by Modulating Radioresponse of Hematopoietic Stem/Progenitor Cells" Biomedicines 11, no. 3: 824. https://doi.org/10.3390/biomedicines11030824
APA StyleHwang, Y. J., Shin, D.-Y., Kim, M.-J., Jang, H., Kim, S., Yang, H., Jang, W. I., Park, S., Shim, S., & Lee, S. B. (2023). StemRegenin 1 Mitigates Radiation-Mediated Hematopoietic Injury by Modulating Radioresponse of Hematopoietic Stem/Progenitor Cells. Biomedicines, 11(3), 824. https://doi.org/10.3390/biomedicines11030824






