Positive Correlation between Relative Concentration of Spermine to Spermidine in Whole Blood and Skeletal Muscle Mass Index: A Possible Indicator of Sarcopenia and Prognosis of Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data and Sample Collection
2.3. Blood and Biochemical Tests
2.4. Determination of Polyamine Concentrations in Whole Blood
2.5. Determination of Polyamine Concentrations in Dialysate
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Comparison of Blood Polyamine Levels
3.3. Polyamine Concentrations in Dialysate
3.4. Analyses of Various Measurements in HD Patients
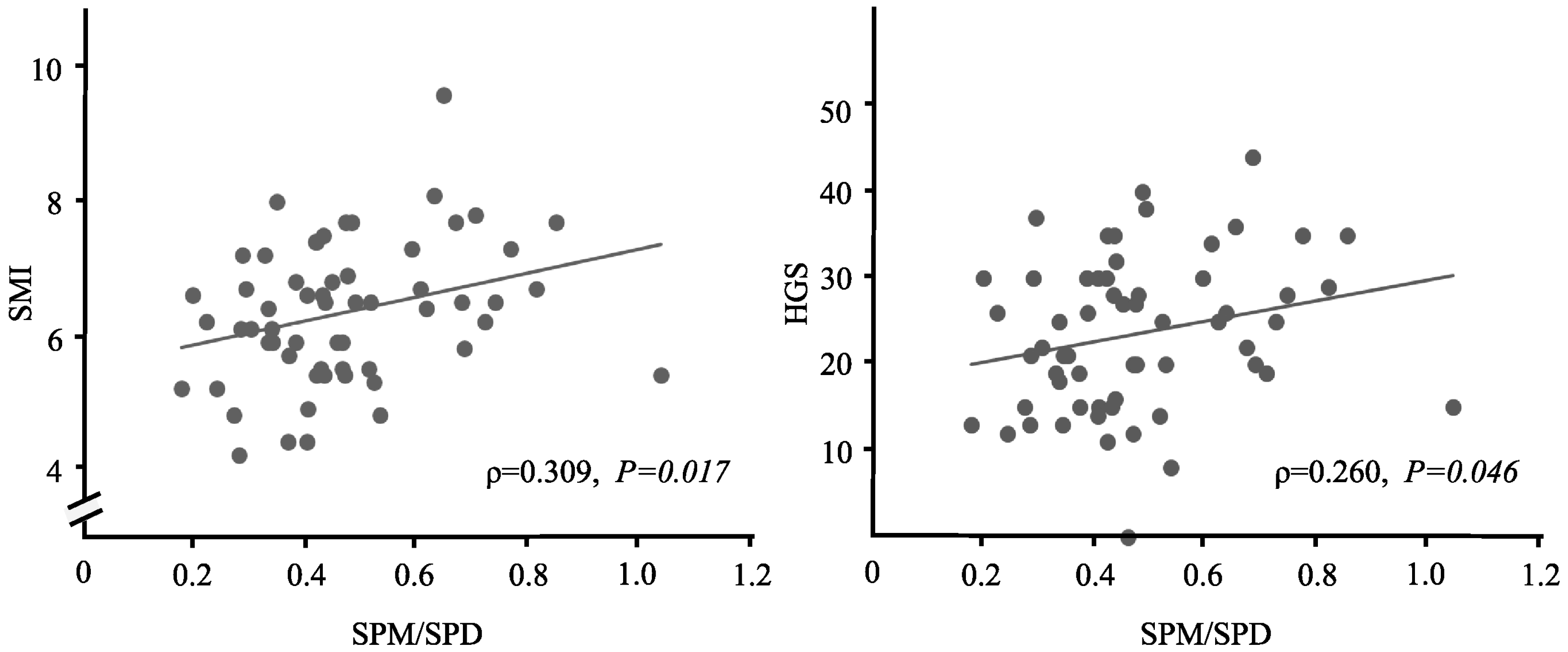
3.5. The Relationship between Polyamines and Various Measurements
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soda, K. Overview of polyamines as nutrients for human healthy long life and effect of increased polyamine intake on DNA methylation. Cells 2022, 11, 164. [Google Scholar] [CrossRef]
- Despotović, D.; Longo, L.M.; Aharon, E.; Kahana, A.; Scherf, T.; Gruic-Sovulj, I.; Tawfik, D.S. Polyamines mediate folding of primordial hyperacidic helical proteins. Biochemistry 2020, 59, 4456–4462. [Google Scholar] [CrossRef]
- Pekar, T.; Wendzel, A.; Flak, W.; Kremer, A.; Pauschenwein-Frantsich, S.; Gschaider, A.; Wantke, F.; Jarisch, R. Spermidine in dementia: Relation to age and memory performance. Wien Klin Wochenschr. 2020, 132, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Uemura, T.; Sanayama, H.; Igarashi, K.; Fukui, T. Polyamine-rich diet elevates blood spermine levels and inhibits pro-inflammatory status: An interventional study. Med. Sci. 2021, 9, 22. [Google Scholar] [CrossRef]
- Soda, K. The mechanisms by which polyamines accelerate tumor spread. J. Exp. Clin. Cancer Res. 2011, 30, 95. [Google Scholar] [CrossRef] [PubMed]
- Swendseid, M.E.; Panaqua, M.; Kopple, J.D. Polyamine concentrations in red cells and urine of patients with chronic renal failure. Life Sci. 1980, 26, 533–539. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, S.G.; Oh, J.E.; Lee, Y.K.; Noh, J.W.; Kim, H.J.; Song, Y.R. Impact of sarcopenia on long-term mortality and cardiovascular events in patients undergoing hemodialysis. Korean J. Intern. Med. 2019, 34, 599–607. [Google Scholar] [CrossRef]
- Kittiskulnam, P.; Chertow, G.M.; Carrero, J.J.; Delgado, C.; Kaysen, G.A.; Johansen, K.L. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int. 2017, 92, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Saiki, S.; Sasazawa, Y.; Fujimaki, M.; Kamagata, K.; Kaga, N.; Taka, H.; Li, Y.; Souma, S.; Hatano, T.; Imamichi, Y.; et al. A metabolic profile of polyamines in parkinson disease: A promising biomarker. Ann. Neurol. 2019, 86, 251–263. [Google Scholar] [CrossRef]
- Cooper, K.D.; Shukla, J.B.; Rennert, O.M. Polyamine distribution in cellular compartments of blood and in aging erythrocytes. Clin. Chim. Acta 1976, 73, 71–88. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K.; Hamasaki, H.; Miura, A.; Kakegawa, T.; Hirose, S.; Matsuzaki, S. Formation of a compensatory polyamine by Escherichia coli polyamine-requiring mutants during growth in the absence of polyamines. J. Bacteriol. 1986, 166, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Moulinoux, J.P.; Le Pogamp, P.; Quémener, V.; Le Calvé, M.; Joyeux, V.; Chevet, D. Red cell free polyamine concentrations in patients on maintenance hemodialysis. Life Sci. 1981, 29, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Ueda, S.; Yoshida, K.; Kashiwagi, K. Polyamines in renal failure. Amino Acids 2006, 31, 477–483. [Google Scholar] [CrossRef]
- Yoshida, K.; Yoneda, T.; Kimura, S.; Fujimoto, K.; Okajima, E.; Hirao, Y. Polyamines as an inhibitor on erythropoiesis of hemodialysis patients by in vitro bioassay using the fetal mouse liver assay. Ther. Apher. Dial. 2006, 10, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Elworthy, P.; Hitchcock, E. Polyamine levels in red blood cells from patient groups of different sex and age. Biochim. Biophys. Acta 1989, 993, 212–216. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension 2003, 42, 1050–1065. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Epidemiology of cardiovascular disease in chronic renal disease. J. Am. Soc. Nephrol. 1998, 9 (Suppl. 12), S16–S23. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Mori, K.; Nishide, K.; Okuno, S.; Shoji, T.; Emoto, M.; Tsuda, A.; Nakatani, S.; Imanishi, Y.; Ishimura, E.; Yamakawa, T.; et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 2019, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Sousa-Victor, P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020, 287, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. Biological Effects of Polyamines on the Prevention of Aging-associated Diseases and on Lifespan Extension. Food Sci. Technol. Res. 2015, 21, 145–157. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef]
- Holbert, C.E.; Dunworth, M.; Foley, J.R.; Dunston, T.T.; Stewart, T.M.; Casero, R.A., Jr. Autophagy induction by exogenous polyamines is an artifact of bovine serum amine oxidase activity in culture serum. J. Biol. Chem. 2020, 295, 9061–9068. [Google Scholar] [CrossRef]
- Holst, C.M.; Nevsten, P.; Johansson, F.; Carlemalm, E.; Oredsson, S.M. Subcellular distribution of spermidine/spermine N1-acetyltransferase. Cell Biol. Int. 2008, 32, 39–47. [Google Scholar] [CrossRef]
- Vujcic, S.; Liang, P.; Diegelman, P.; Kramer, D.L.; Porter, C.W. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem. J. 2003, 370 Pt 1, 19–28. [Google Scholar] [CrossRef]
- Wang, Y.; Devereux, W.; Woster, P.M.; Stewart, T.M.; Hacker, A.; Casero, R.A., Jr. Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001, 61, 5370–5373. [Google Scholar]
- Pegg, A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar] [CrossRef]
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Irie, Y.; Murotani, N.; Igarashi, K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 2003, 305, 143–149. [Google Scholar] [CrossRef]
- Gomes-Trolin, C.; Nygren, I.; Aquilonius, S.M.; Askmark, H. Increased red blood cell polyamines in ALS and Parkinson’s disease. Exp. Neurol. 2002, 177, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Els, T.; Bruckmann, J.; Röhn, G.; Daffertshofer, M.; Mönting, J.S.; Ernestus, R.I.; Hennerici, M. Spermidine: A predictor for neurological outcome and infarct size in focal cerebral ischemia? Stroke 2001, 32, 43–46. [Google Scholar] [CrossRef]
- Uchitomi, R.; Hatazawa, Y.; Senoo, N.; Yoshioka, K.; Fujita, M.; Shimizu, T.; Miura, S.; Ono, Y.; Kamei, Y. Metabolomic analysis of skeletal muscle in aged mice. Sci. Rep. 2019, 9, 10425. [Google Scholar] [CrossRef] [PubMed]
- Turchanowa, L.; Rogozkin, V.A.; Milovic, V.; Feldkoren, B.I.; Caspary, W.F.; Stein, J. Influence of physical exercise on polyamine synthesis in the rat skeletal muscle. Eur. J. Clin. Investig. 2000, 30, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Abukhalaf, I.K.; von Deutsch, D.A.; Wineski, L.E.; Silvestrov, N.A.; Abera, S.A.; Sahlu, S.W.; Potter, D.E. Effect of hindlimb suspension and clenbuterol treatment on polyamine levels in skeletal muscle. Pharmacology 2002, 65, 145–154. [Google Scholar] [CrossRef]
- Cervelli, M.; Leonetti, A.; Duranti, G.; Sabatini, S.; Ceci, R.; Mariottini, P. Skeletal muscle pathophysiology: The emerging role of spermine oxidase and spermidine. Med. Sci. 2018, 6, 14. [Google Scholar] [CrossRef]
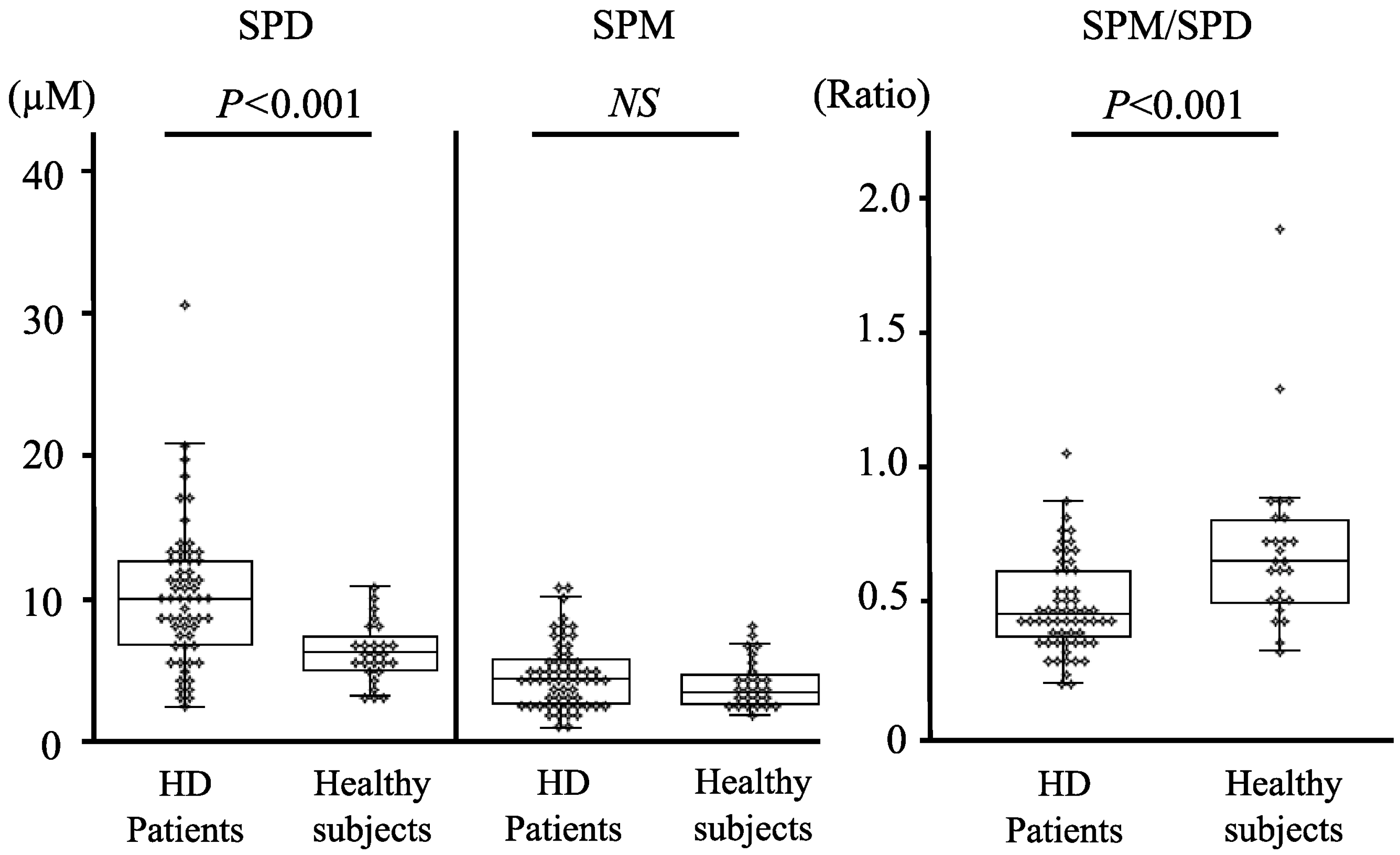

| Characteristics | HD Patients | Controls |
|---|---|---|
| Number of patients n, (men/women) | 59 (35/24) | 26 (11/15) |
| Age (years) | 70.0 (62.0–75.0) | 44.5 (37.0–52.0) |
| HD duration (months) | 62.0 (29.0–142.0) | |
| Dialysis time (min) | 246 (246–247) | |
| Dry Weight (kg), mean ± SD | 57.2 ± 11.4 | |
| Interdialytic weight gain (kg), mean ± SD | 2.6 ± 1.0 | |
| Interdialytic weight gain/dry weight (%), mean ± SD | 4.6 ± 1.7 | |
| BMI (kg/m2), mean ± SD | 22.4 ± 3.7 | 22.8 ± 3.1 |
| SMI (kg/m2), mean ± SD | 6.3 ± 1.1 | 8.0 ± 1.2 |
| HGS (kg), mean ± SD | 23.5 ± 9.0 | 35.3 ± 9.6 |
| Primary Disease, n (%) | ||
| Chronic glomerulonephritis | 20 (34) | |
| Diabetes mellitus | 18 (31) | |
| Nephrosclerosis | 6 (10) | |
| Others | 15 (25) | |
| Past Medical History, n (%) | ||
| Diabetes mellitus | 22 (37) | 0 (0) |
| Dyslipidemia | 27 (44) | 0 (0) |
| Hypertension | 55 (93) | 0 (0) |
| Cardiovascular disease | 8 (14) | 0 (0) |
| Cerebrovascular disease | 12 (20) | 0 (0) |
| Age | HD Duration | BMI | SMI | HGS | Hb | Alb | BUN | Cre | CRP | Ca | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1.000 | |||||||||||
| HD duration | −0.099 | 1.000 | ||||||||||
| BMI | −0.247 | −0.420 * | 1.000 | |||||||||
| SMI | −0.493 ** | −0.249 * | 0.377 †* | 1.000 | ||||||||
| HGS | −0.422 * | −0.293 * | 0.203 † | 0.629 †** | 1.000 | |||||||
| Hb | −0.083 | 0.158 | −0.252 † | 0.022 † | 0.086 † | 1.000 | ||||||
| Alb | −0.276 * | −0.049 | 0.213 | 0.281 * | 0.372 * | 0.143 | 1.000 | |||||
| BUN | −0.210 | −0.068 | 0.092 | 0.153 | 0.237 | 0.192 | 0.136 | 1.000 | ||||
| Cre | −0.427 * | 0.325 * | 0.051 | 0.350 * | 0.405 * | 0.164 | 0.210 | 0.354 * | 1.000 | |||
| CRP | 0.170 | 0.132 | −0.083 | −0.156 | −0.184 | −0.284 * | −0.480 ** | −0.038 | −0.163 | 1.000 | ||
| Ca | −0.067 | 0.184 | −0.052 † | −0.036 † | −0.024 † | 0.229 † | 0.463 ** | 0.135 | 0.051 | −0.077 | 1.000 | |
| P | −0.132 | −0.018 | 0.183 | 0.119 | 0.107 | −0.032 | −0.026 | 0.390 * | 0.186 | 0.066 | 0.039 | 1.000 |
| vs. SPD Values in Simple Linear Regression | vs. SPM Values in Simple Linear Regression | vs. SPM/SPD Values in Simple Linear Regression | ||||
|---|---|---|---|---|---|---|
| ρ | p value | ρ | p value | ρ | p value | |
| Age | −0.055 | 0.680 | −0.137 | 0.301 | −0.145 | 0.275 |
| HD duration | −0.032 | 0.807 | −0.106 | 0.424 | −0.170 | 0.198 |
| BMI | 0.113 | 0.395 | 0.244 | 0.063 | 0.172 | 0.194 |
| SMI | −0.068 | 0.610 | 0.084 | 0.528 | 0.309 | 0.017 * |
| HGS | −0.159 | 0.230 | −0.063 | 0.636 | 0.260 | 0.046 * |
| Laboratory findings | ||||||
| Hb | −0.168 | 0.203 | −0.075 | 0.570 | 0.187 | 0.157 |
| Alb | −0.238 | 0.070 | −0.215 | 0.102 | 0.068 | 0.610 |
| BUN | 0.019 | 0.885 | 0.044 | 0.743 | 0.046 | 0.727 |
| Cre | 0.024 | 0.857 | 0.126 | 0.340 | 0.115 | 0.385 |
| CRP | 0.219 | 0.095 | 0.124 | 0.351 | −0.058 | 0.663 |
| Ca | −0.072 | 0.588 | −0.068 | 0.607 | 0.034 | 0.799 |
| P | 0.046 | 0.730 | 0.136 | 0.303 | 0.095 | 0.473 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanayama, H.; Ito, K.; Ookawara, S.; Uemura, T.; Imai, S.; Kiryu, S.; Iguchi, M.; Sakiyama, Y.; Sugawara, H.; Morishita, Y.; et al. Positive Correlation between Relative Concentration of Spermine to Spermidine in Whole Blood and Skeletal Muscle Mass Index: A Possible Indicator of Sarcopenia and Prognosis of Hemodialysis Patients. Biomedicines 2023, 11, 746. https://doi.org/10.3390/biomedicines11030746
Sanayama H, Ito K, Ookawara S, Uemura T, Imai S, Kiryu S, Iguchi M, Sakiyama Y, Sugawara H, Morishita Y, et al. Positive Correlation between Relative Concentration of Spermine to Spermidine in Whole Blood and Skeletal Muscle Mass Index: A Possible Indicator of Sarcopenia and Prognosis of Hemodialysis Patients. Biomedicines. 2023; 11(3):746. https://doi.org/10.3390/biomedicines11030746
Chicago/Turabian StyleSanayama, Hidenori, Kiyonori Ito, Susumu Ookawara, Takeshi Uemura, Sojiro Imai, Satoshi Kiryu, Miho Iguchi, Yoshio Sakiyama, Hitoshi Sugawara, Yoshiyuki Morishita, and et al. 2023. "Positive Correlation between Relative Concentration of Spermine to Spermidine in Whole Blood and Skeletal Muscle Mass Index: A Possible Indicator of Sarcopenia and Prognosis of Hemodialysis Patients" Biomedicines 11, no. 3: 746. https://doi.org/10.3390/biomedicines11030746
APA StyleSanayama, H., Ito, K., Ookawara, S., Uemura, T., Imai, S., Kiryu, S., Iguchi, M., Sakiyama, Y., Sugawara, H., Morishita, Y., Tabei, K., Igarashi, K., & Soda, K. (2023). Positive Correlation between Relative Concentration of Spermine to Spermidine in Whole Blood and Skeletal Muscle Mass Index: A Possible Indicator of Sarcopenia and Prognosis of Hemodialysis Patients. Biomedicines, 11(3), 746. https://doi.org/10.3390/biomedicines11030746








