Insulin-like Growth Factor-1 Prevents Hypoxia/Reoxygenation-Induced White Matter Injury in Sickle Cell Mice
Abstract
1. Introduction
2. Materials and Methods
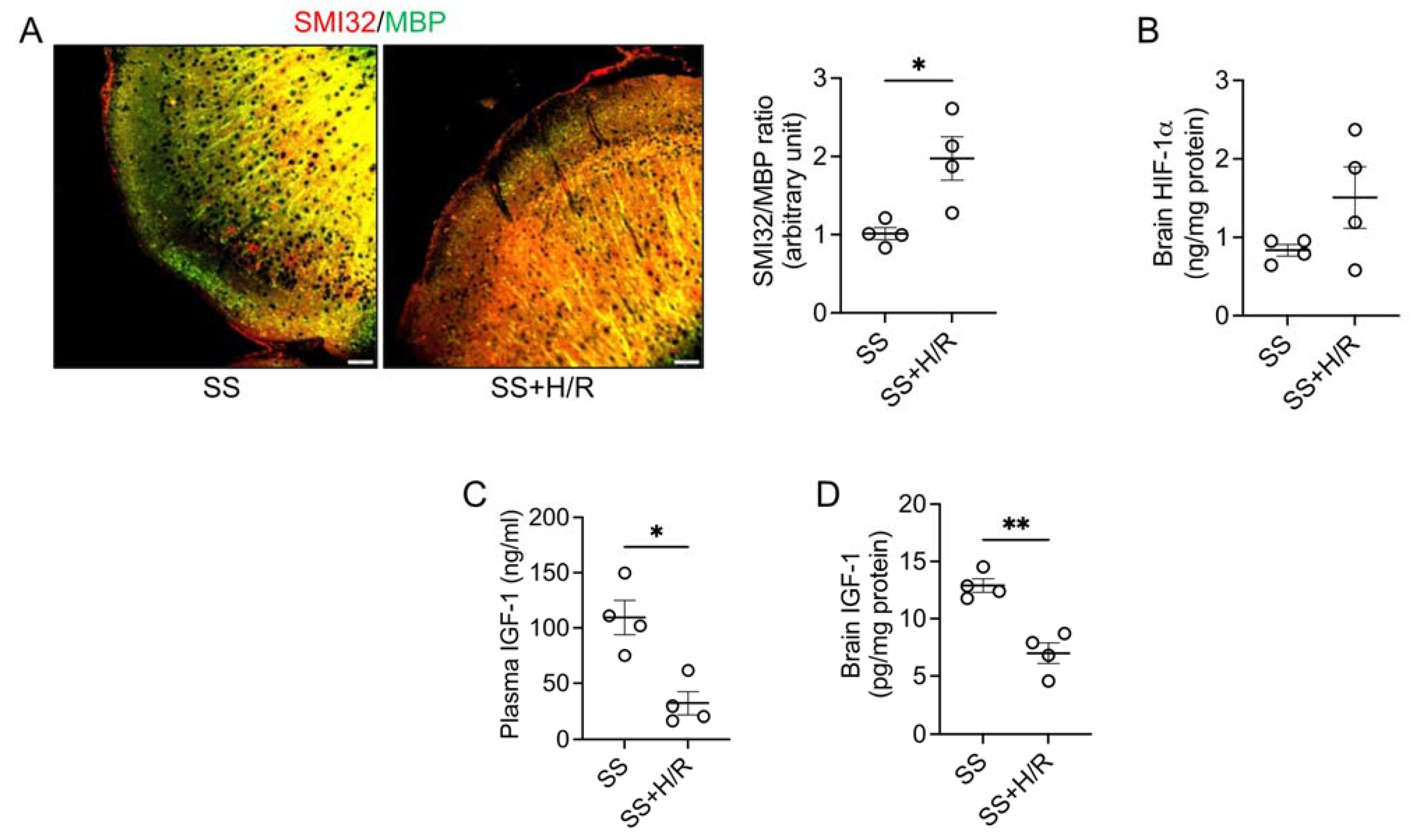
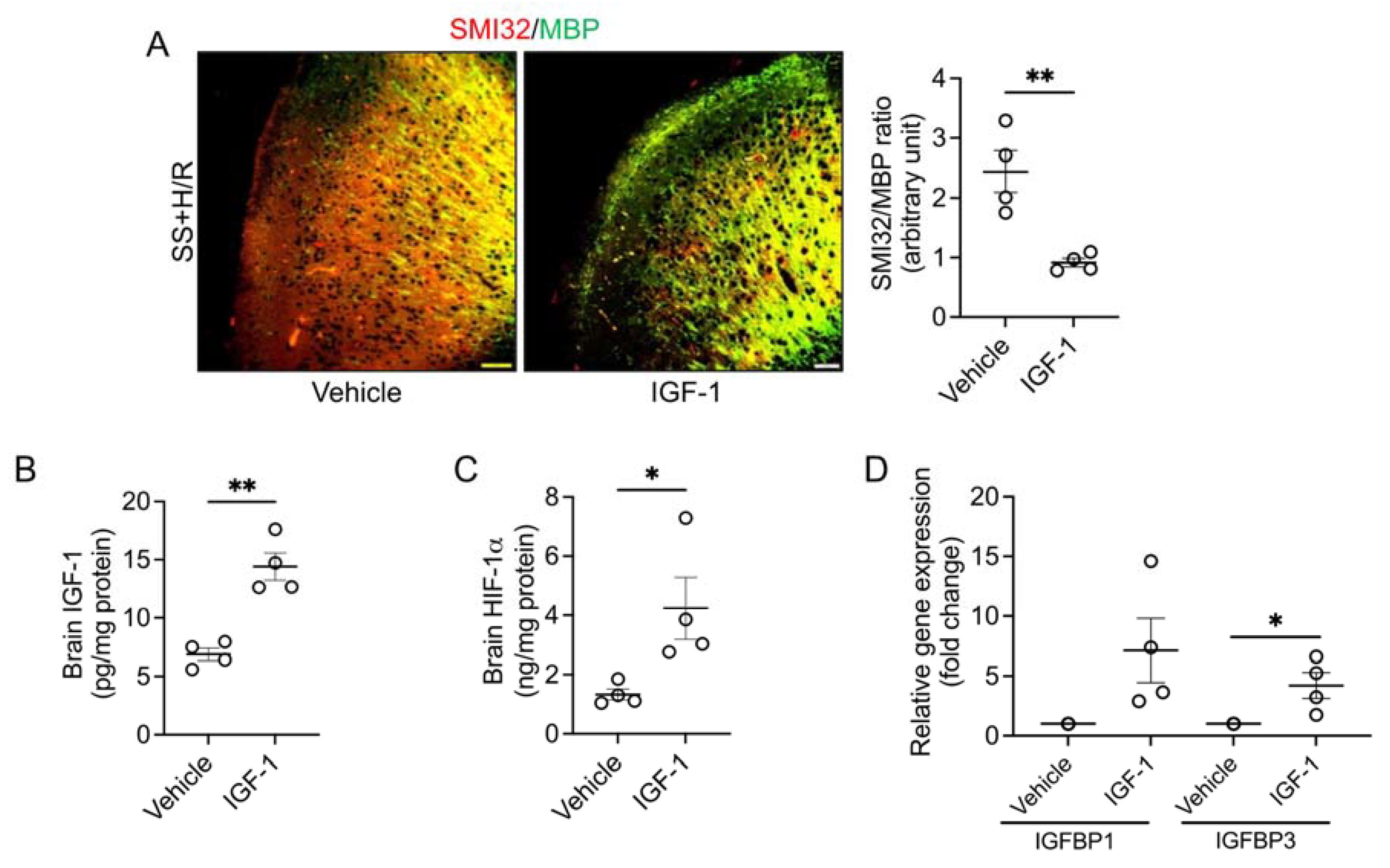
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piel, F.B.; Hay, S.I.; Gupta, S.; Weatherall, D.J.; Williams, T.N. Global burden of sickle cell anaemia in children under five, 2010-2050: Modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013, 10, e1001484. [Google Scholar] [CrossRef] [PubMed]
- Powars, D.R.; Chan, L.S.; Hiti, A.; Ramicone, E.; Johnson, C. Outcome of sickle cell anemia: A 4-decade observational study of 1056 patients. Medicine 2005, 84, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Hebbel, R.P.; Belcher, J.D.; Vercellotti, G.M. The multifaceted role of ischemia/reperfusion in sickle cell anemia. J. Clin. Investig. 2020, 130, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Gavins, F.N.E. Ischemia-Reperfusion Injury in Sickle Cell Disease: From Basics to Therapeutics. Am. J. Pathol. 2019, 189, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Ohene-Frempong, K.; Weiner, S.J.; Sleeper, L.A.; Miller, S.T.; Embury, S.; Moohr, J.W.; Wethers, D.L.; Pegelow, C.H.; Gill, F.M. Cerebrovascular accidents in sickle cell disease: Rates and risk factors. Blood 1998, 91, 288–294. [Google Scholar]
- Stotesbury, H.; Kawadler, J.M.; Hales, P.W.; Saunders, D.E.; Clark, C.A.; Kirkham, F.J. Vascular Instability and Neurological Morbidity in Sickle Cell Disease: An Integrative Framework. Front. Neurol. 2019, 10, 871. [Google Scholar] [CrossRef]
- Brousse, V.; Pondarre, C.; Kossorotoff, M.; Arnaud, C.; Kamdem, A.; de Montalembert, M.; Boutonnat-Faucher, B.; Allali, S.; Bourdeau, H.; Charlot, K.; et al. Brain injury pathophysiology study by a multimodal approach in children with sickle cell anemia with no intra or extra cranial arteriopathy. Haematologica 2022, 107, 958–965. [Google Scholar] [CrossRef]
- Bernaudin, F.; Verlhac, S.; Arnaud, C.; Kamdem, A.; Vasile, M.; Kasbi, F.; Hau, I.; Madhi, F.; Fourmaux, C.; Biscardi, S.; et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood 2015, 125, 1653–1661. [Google Scholar] [CrossRef]
- Kwiatkowski, J.L.; Zimmerman, R.A.; Pollock, A.N.; Seto, W.; Smith-Whitley, K.; Shults, J.; Blackwood-Chirchir, A.; Ohene-Frempong, K. Silent infarcts in young children with sickle cell disease. Br. J. Haematol. 2009, 146, 300–305. [Google Scholar] [CrossRef]
- Stotesbury, H.; Kawadler, J.M.; Saunders, D.E.; Kirkham, F.J. MRI detection of brain abnormality in sickle cell disease. Expert Rev. Hematol. 2021, 14, 473–491. [Google Scholar] [CrossRef]
- Kawadler, J.M.; Kirkham, F.J.; Clayden, J.D.; Hollocks, M.J.; Seymour, E.L.; Edey, R.; Telfer, P.; Robins, A.; Wilkey, O.; Barker, S.; et al. White Matter Damage Relates to Oxygen Saturation in Children With Sickle Cell Anemia Without Silent Cerebral Infarcts. Stroke 2015, 46, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.J.; Nichols, F.T.; McKie, V.; McKie, K.; Milner, P.; Gammal, T.E. Cerebral infarction in sickle cell anemia: Mechanism based on CT and MRI. Neurology 1988, 38, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Merkel, K.H.; Ginsberg, P.L.; Parker, J.C.; Post, M.J. Cerebrovascular disease in sickle cell anemia: A clinical, pathological and radiological correlation. Stroke 1978, 9, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rothman, S.M.; Fulling, K.H.; Nelson, J.S. Sickle cell anemia and central nervous system infarction: A neuropathological study. Ann. Neurol. 1986, 20, 684–690. [Google Scholar] [CrossRef]
- Sharp, F.R.; Bernaudin, M. HIF1 and oxygen sensing in the brain. Nat. Rev. Neurosci. 2004, 5, 437–448. [Google Scholar] [CrossRef]
- Adams, J.M.; Difazio, L.T.; Rolandelli, R.H.; Lujan, J.J.; Hasko, G.; Csoka, B.; Selmeczy, Z.; Nemeth, Z.H. HIF-1: A key mediator in hypoxia. Acta Physiol. Hung. 2009, 96, 19–28. [Google Scholar] [CrossRef]
- Declercq, W.; Vanden Berghe, T.; Vandenabeele, P. RIP kinases at the crossroads of cell death and survival. Cell 2009, 138, 229–232. [Google Scholar] [CrossRef]
- Bernaudin, M.; Nedelec, A.S.; Divoux, D.; MacKenzie, E.T.; Petit, E.; Schumann-Bard, P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J. Cereb. Blood Flow Metab. 2002, 22, 393–403. [Google Scholar] [CrossRef]
- Amin, N.; Chen, S.; Ren, Q.; Tan, X.; Botchway, B.O.A.; Hu, Z.; Chen, F.; Ye, S.; Du, X.; Chen, Z.; et al. Hypoxia Inducible Factor-1alpha Attenuates Ischemic Brain Damage by Modulating Inflammatory Response and Glial Activity. Cells 2021, 10, 1359. [Google Scholar] [CrossRef]
- Benarroch, E.E. Insulin-like growth factors in the brain and their potential clinical implications. Neurology 2012, 79, 2148–2153. [Google Scholar] [CrossRef]
- Trejo, J.L.; Piriz, J.; Llorens-Martin, M.V.; Fernandez, A.M.; Bolos, M.; LeRoith, D.; Nunez, A.; Torres-Aleman, I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol. Psychiatry 2007, 12, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, S.; Arafa, D.; Tropea, D. Insulin-like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front. Cell Neurosci. 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Saber, H.; Himali, J.J.; Beiser, A.S.; Shoamanesh, A.; Pikula, A.; Roubenoff, R.; Romero, J.R.; Kase, C.S.; Vasan, R.S.; Seshadri, S. Serum Insulin-like Growth Factor 1 and the Risk of Ischemic Stroke: The Framingham Study. Stroke 2017, 48, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- De Geyter, D.; Stoop, W.; Sarre, S.; De Keyser, J.; Kooijman, R. Neuroprotective efficacy of subcutaneous insulin-like growth factor-I administration in normotensive and hypertensive rats with an ischemic stroke. Neuroscience 2013, 250, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Hayes, C.A.; Valcarcel-Ares, M.N.; Ashpole, N.M. Preclinical and clinical evidence of IGF-1 as a prognostic marker and acute intervention with ischemic stroke. J. Cereb. Blood Flow Metab. 2021, 41, 2475–2491. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, H.; Ikuta, T.; Tachibana, T.; Yoneda, Y.; Kawajiri, K. A nuclear localization signal of human aryl hydrocarbon receptor nuclear translocator/hypoxia-inducible factor 1beta is a novel bipartite type recognized by the two components of nuclear pore-targeting complex. J. Biol. Chem. 1997, 272, 17640–17647. [Google Scholar] [CrossRef]
- Kallio, P.J.; Wilson, W.J.; O’Brien, S.; Makino, Y.; Poellinger, L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J. Biol. Chem. 1999, 274, 6519–6525. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, H.; Lin, P.; Zhang, Z.; Chen, M.; Zhang, Y.; Mo, J.; Zhu, Y.; Liu, N.; Chen, X. Insulin-like growth factor binding protein-1 regulates HIF-1alpha degradation to inhibit apoptosis in hypoxic cardiomyocytes. Cell Death Discov. 2021, 7, 242. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Martin, J.L.; Baxter, R.C. Signalling pathways of insulin-like growth factors (IGFs) and IGF binding protein-3. Growth Factors 2011, 29, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, M.; Worthmann, H.; Dengler, R.; Schumacher, H.; Lichtinghagen, R.; Eschenfelder, C.C.; Endres, M.; Ebinger, M. Circulating Insulin-like Growth Factor-1 and Insulin-like Growth Factor Binding Protein-3 predict Three-months Outcome after Ischemic Stroke. Exp. Clin. Endocrinol. Diabetes 2017, 125, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Luporini, S.M.; Bendit, I.; Manhani, R.; Bracco, O.L.; Manzella, L.; Giannella-Neto, D. Growth hormone and insulin-like growth factor I axis and growth of children with different sickle cell anemia haplotypes. J. Pediatr. Hematol. Oncol. 2001, 23, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhou, Y.; Bokoliya, S.; Lin, Q.; Hurley, M. Bone loss is ameliorated by fecal microbiota transplantation through SCFA/GPR41/ IGF1 pathway in sickle cell disease mice. Sci. Rep. 2022, 12, 20638. [Google Scholar] [CrossRef]
- Xiao, L.; Andemariam, B.; Taxel, P.; Adams, D.J.; Zempsky, W.T.; Dorcelus, V.; Hurley, M.M. Loss of Bone in Sickle Cell Trait and Sickle Cell Disease Female Mice Is Associated With Reduced IGF-1 in Bone and Serum. Endocrinology 2016, 157, 3036–3046. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Lee, J.; Huang, H.; Wagner, M.B.; Joiner, C.H.; Archer, D.R.; Kuan, C.Y. Sickle Mice Are Sensitive to Hypoxia/Ischemia-Induced Stroke but Respond to Tissue-Type Plasminogen Activator Treatment. Stroke 2017, 48, 3347–3355. [Google Scholar] [CrossRef]
- Wu, L.C.; Sun, C.W.; Ryan, T.M.; Pawlik, K.M.; Ren, J.; Townes, T.M. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 2006, 108, 1183–1188. [Google Scholar] [CrossRef]
- Xia, Y.; Pu, H.; Leak, R.K.; Shi, Y.; Mu, H.; Hu, X.; Lu, Z.; Foley, L.M.; Hitchens, T.K.; Dixon, C.E.; et al. Tissue plasminogen activator promotes white matter integrity and functional recovery in a murine model of traumatic brain injury. Proc. Natl. Acad. Sci. USA 2018, 115, E9230–E9238. [Google Scholar] [CrossRef]
- Ford, A.L.; Ragan, D.K.; Fellah, S.; Binkley, M.M.; Fields, M.E.; Guilliams, K.P.; An, H.; Jordan, L.C.; McKinstry, R.C.; Lee, J.M.; et al. Silent infarcts in sickle cell disease occur in the border zone region and are associated with low cerebral blood flow. Blood 2018, 132, 1714–1723. [Google Scholar] [CrossRef]
- Wang, W.; Enos, L.; Gallagher, D.; Thompson, R.; Guarini, L.; Vichinsky, E.; Wright, E.; Zimmerman, R.; Armstrong, F.D.; Cooperative Study of Sickle Cell Disease. Neuropsychologic performance in school-aged children with sickle cell disease: A report from the Cooperative Study of Sickle Cell Disease. J. Pediatr. 2001, 139, 391–397. [Google Scholar] [CrossRef]
- Van der Land, V.; Hijmans, C.T.; de Ruiter, M.; Mutsaerts, H.J.; Cnossen, M.H.; Engelen, M.; Majoie, C.B.; Nederveen, A.J.; Grootenhuis, M.A.; Fijnvandraat, K. Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br. J. Haematol. 2015, 168, 553–556. [Google Scholar] [CrossRef] [PubMed]
- DeBaun, M.R.; Gordon, M.; McKinstry, R.C.; Noetzel, M.J.; White, D.A.; Sarnaik, S.A.; Meier, E.R.; Howard, T.H.; Majumdar, S.; Inusa, B.P.; et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N. Engl. J. Med. 2014, 371, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Kamura, T.; Sato, S.; Iwai, K.; Czyzyk-Krzeska, M.; Conaway, R.C.; Conaway, J.W. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc. Natl. Acad. Sci. USA 2000, 97, 10430–10435. [Google Scholar] [CrossRef] [PubMed]
- Ohh, M.; Park, C.W.; Ivan, M.; Hoffman, M.A.; Kim, T.Y.; Huang, L.E.; Pavletich, N.; Chau, V.; Kaelin, W.G. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat. Cell Biol. 2000, 2, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Takei, A.; Ekstrom, M.; Mammadzada, P.; Aronsson, M.; Yu, M.; Kvanta, A.; Andre, H. Gene Transfer of Prolyl Hydroxylase Domain 2 Inhibits Hypoxia-inducible Angiogenesis in a Model of Choroidal Neovascularization. Sci. Rep. 2017, 7, 42546. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Uemura, K.; Asada, M.; Maesako, M.; Akiyama, H.; Shimohama, S.; Takahashi, R.; Kinoshita, A. The participation of insulin-like growth factor-binding protein 3 released by astrocytes in the pathology of Alzheimer’s disease. Mol. Brain 2015, 8, 82. [Google Scholar] [CrossRef]
- Guan, J.; Williams, C.E.; Skinner, S.J.; Mallard, E.C.; Gluckman, P.D. The effects of insulin-like growth factor (IGF)-1, IGF-2, and des-IGF-1 on neuronal loss after hypoxic-ischemic brain injury in adult rats: Evidence for a role for IGF binding proteins. Endocrinology 1996, 137, 893–898. [Google Scholar] [CrossRef]
- Baxter, R.C. Insulin-like growth factor (IGF)-binding proteins: Interactions with IGFs and intrinsic bioactivities. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E967–E976. [Google Scholar] [CrossRef]
- Lee, W.H.; Wang, G.M.; Yang, X.L.; Seaman, L.B.; Vannucci, S.I. Perinatal hypoxia-ischemia decreased neuronal but increased cerebral vascular endothelial IGFBP3 expression. Endocrine 1999, 11, 181–188. [Google Scholar] [CrossRef]
- Martin, J.L.; Weenink, S.; Baxter, R. Insulin-like growth factor-binding protein-3 potentiates epidermal growth factor action in MCF-10A mammary epithelial cells. Involvement of p44/42 and p38 mitogen-activated protein kinases. J. Biol. Chem. 2003, 278, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazra, R.; Hubert, H.; Little-Ihrig, L.; Ghosh, S.; Ofori-Acquah, S.; Hu, X.; Novelli, E.M. Insulin-like Growth Factor-1 Prevents Hypoxia/Reoxygenation-Induced White Matter Injury in Sickle Cell Mice. Biomedicines 2023, 11, 692. https://doi.org/10.3390/biomedicines11030692
Hazra R, Hubert H, Little-Ihrig L, Ghosh S, Ofori-Acquah S, Hu X, Novelli EM. Insulin-like Growth Factor-1 Prevents Hypoxia/Reoxygenation-Induced White Matter Injury in Sickle Cell Mice. Biomedicines. 2023; 11(3):692. https://doi.org/10.3390/biomedicines11030692
Chicago/Turabian StyleHazra, Rimi, Holland Hubert, Lynda Little-Ihrig, Samit Ghosh, Solomon Ofori-Acquah, Xiaoming Hu, and Enrico M Novelli. 2023. "Insulin-like Growth Factor-1 Prevents Hypoxia/Reoxygenation-Induced White Matter Injury in Sickle Cell Mice" Biomedicines 11, no. 3: 692. https://doi.org/10.3390/biomedicines11030692
APA StyleHazra, R., Hubert, H., Little-Ihrig, L., Ghosh, S., Ofori-Acquah, S., Hu, X., & Novelli, E. M. (2023). Insulin-like Growth Factor-1 Prevents Hypoxia/Reoxygenation-Induced White Matter Injury in Sickle Cell Mice. Biomedicines, 11(3), 692. https://doi.org/10.3390/biomedicines11030692





