Unexpected Absence of Skeletal Responses to Dietary Magnesium Depletion: Basis for Future Perspectives?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Treatment
2.2. Serum Mg Concentration
2.3. RNA Extraction, cDNA Preparation, and qPCR
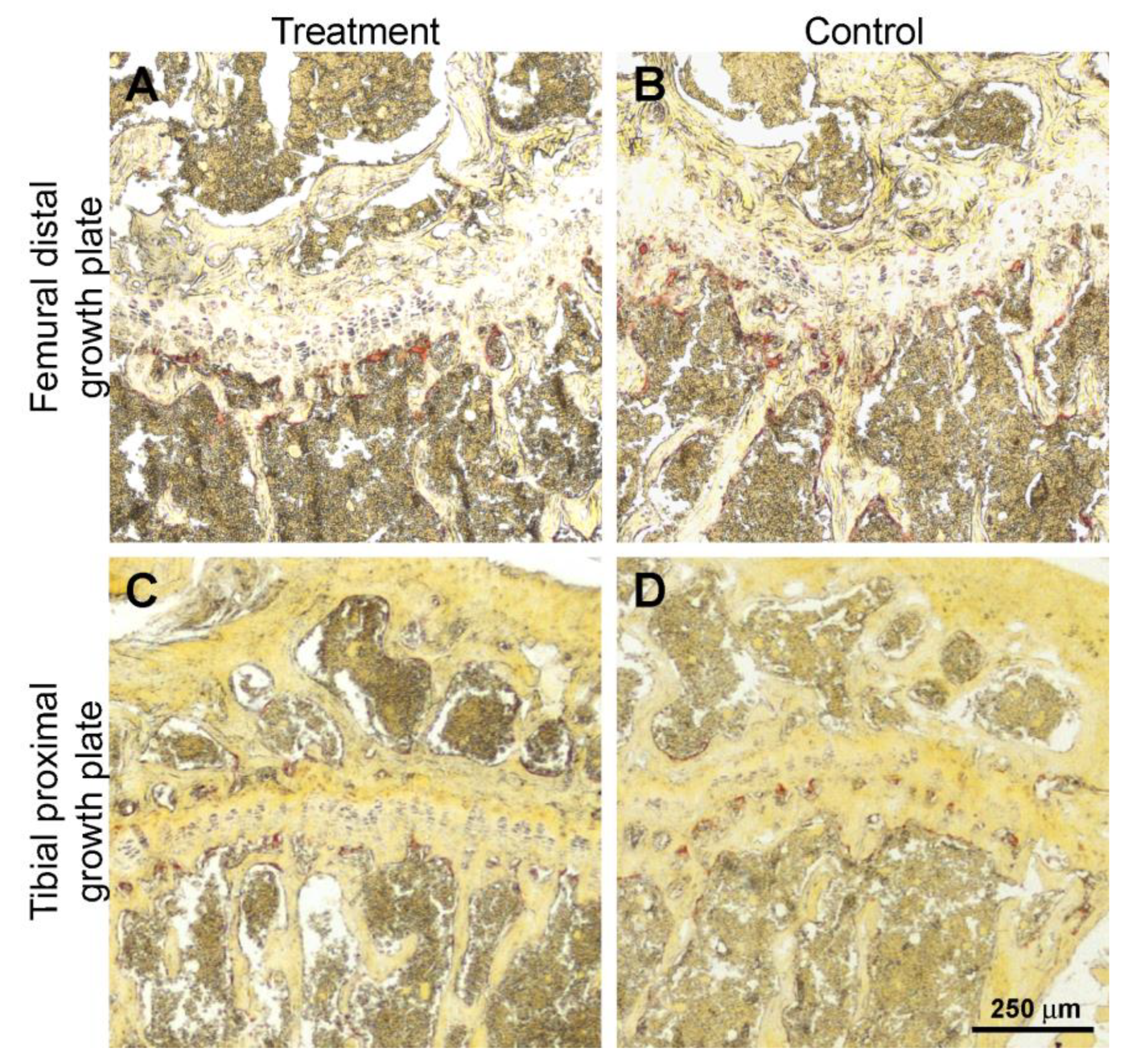
2.4. Histology and Histomorphometry
2.4.1. Resin-Embedded Specimens
- -
- trabecular bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular separation (Tb.Sp) in the L5 body, femoral distal, and tibial proximal metaphyses;
- -
- anterolateral cortical thickness (Ct.Th) measuring it at 10 random points in the vertebral body.
2.4.2. Paraffin-Embedded Specimens
2.5. Statistical Analysis
3. Results
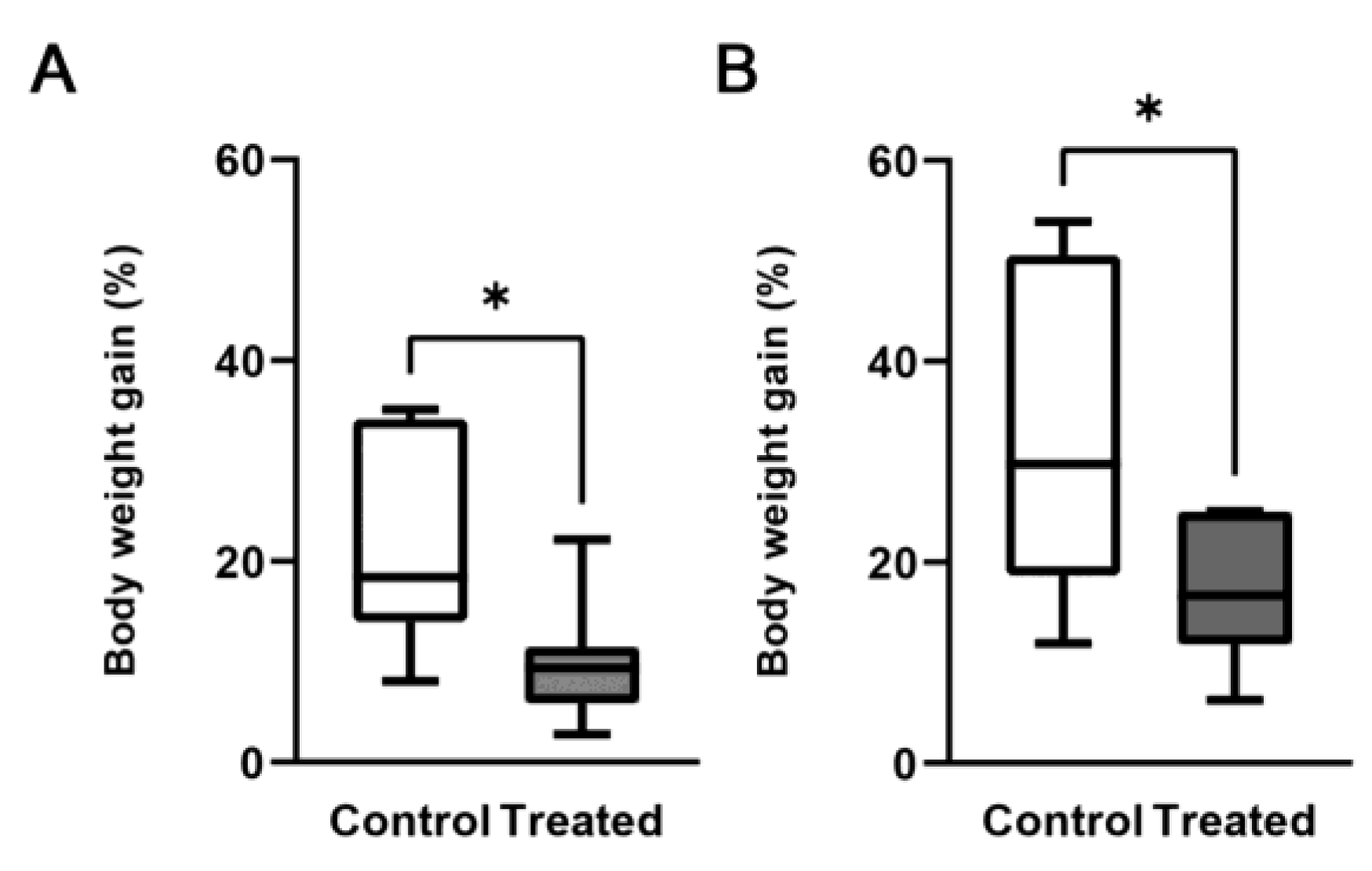
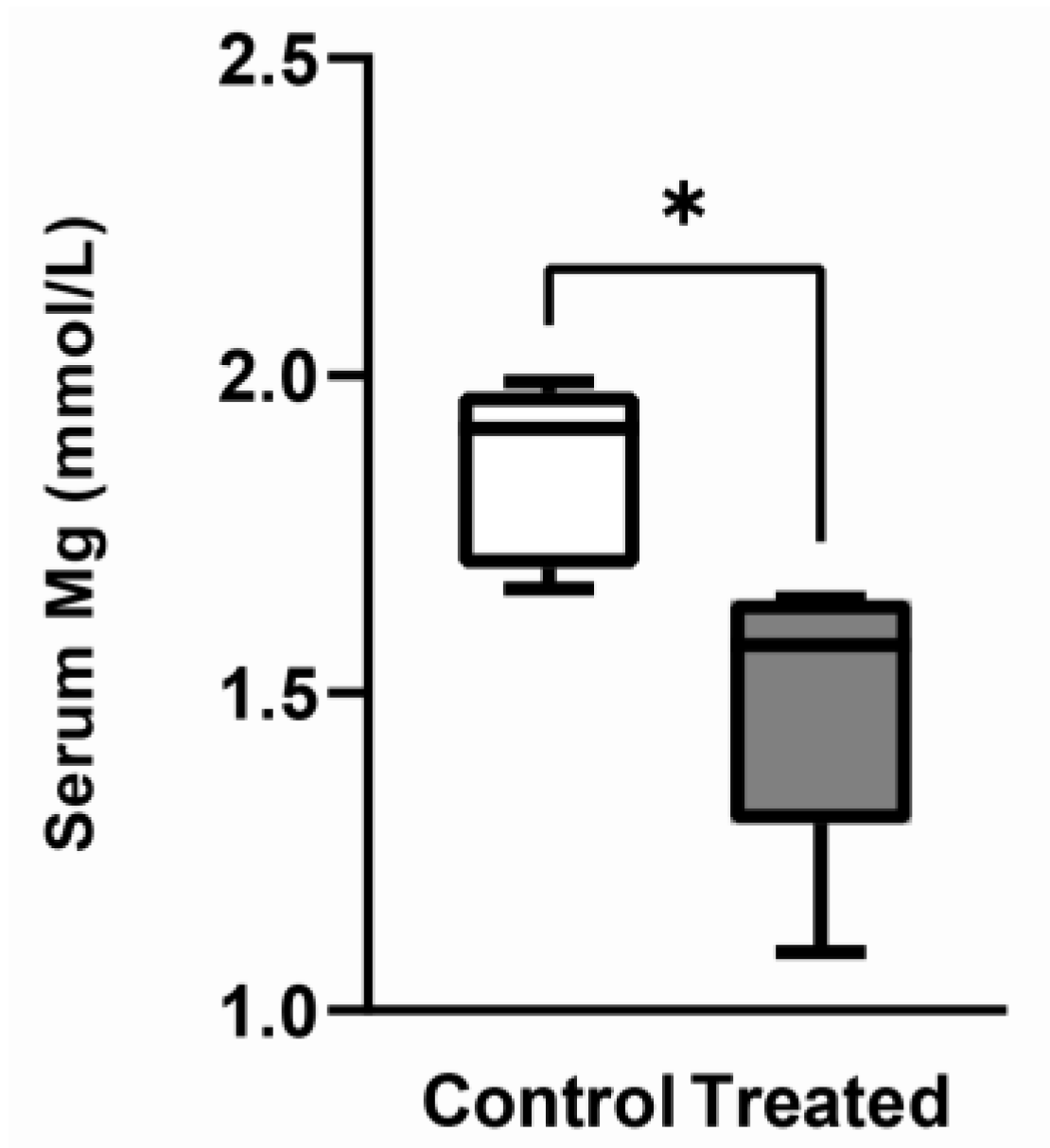
3.1. Effects of the Mg-Deprived Diet on Body Weight and Mg Serum Concentration
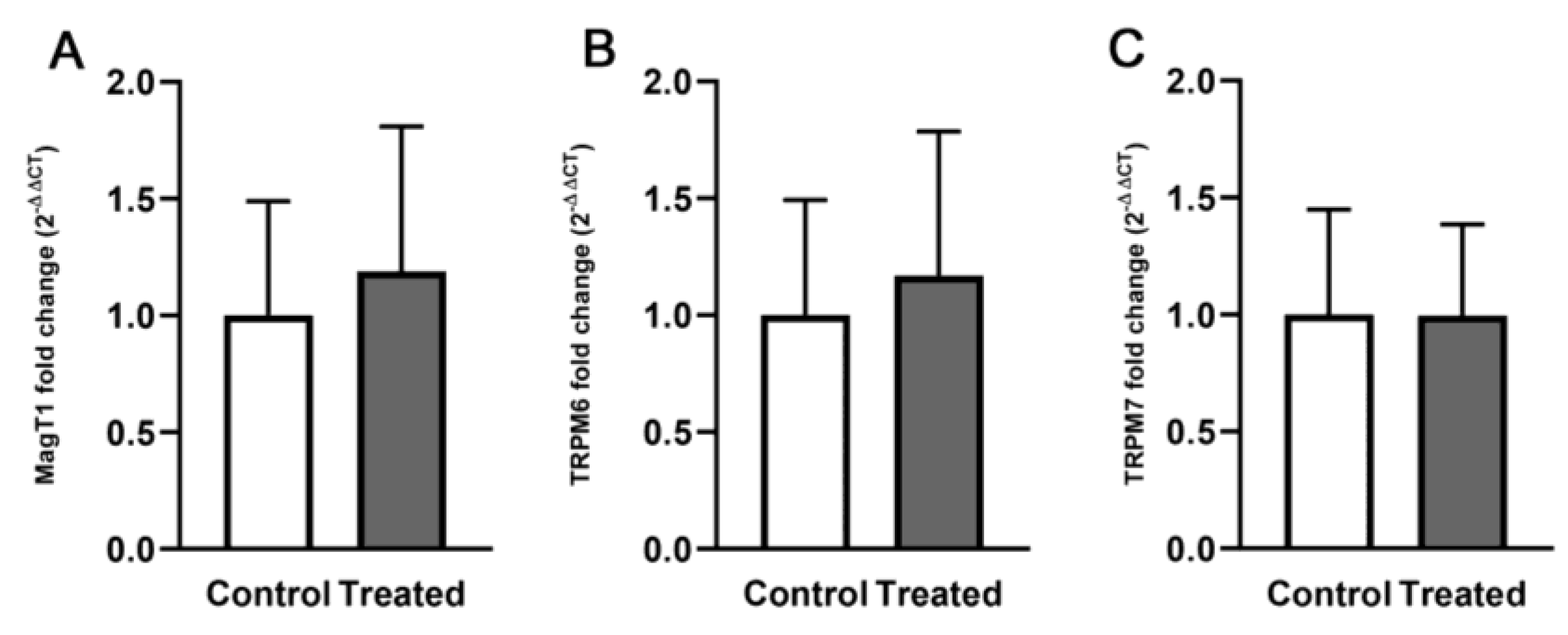
3.2. Expression of TRPM6, TRPM7 and MagT1
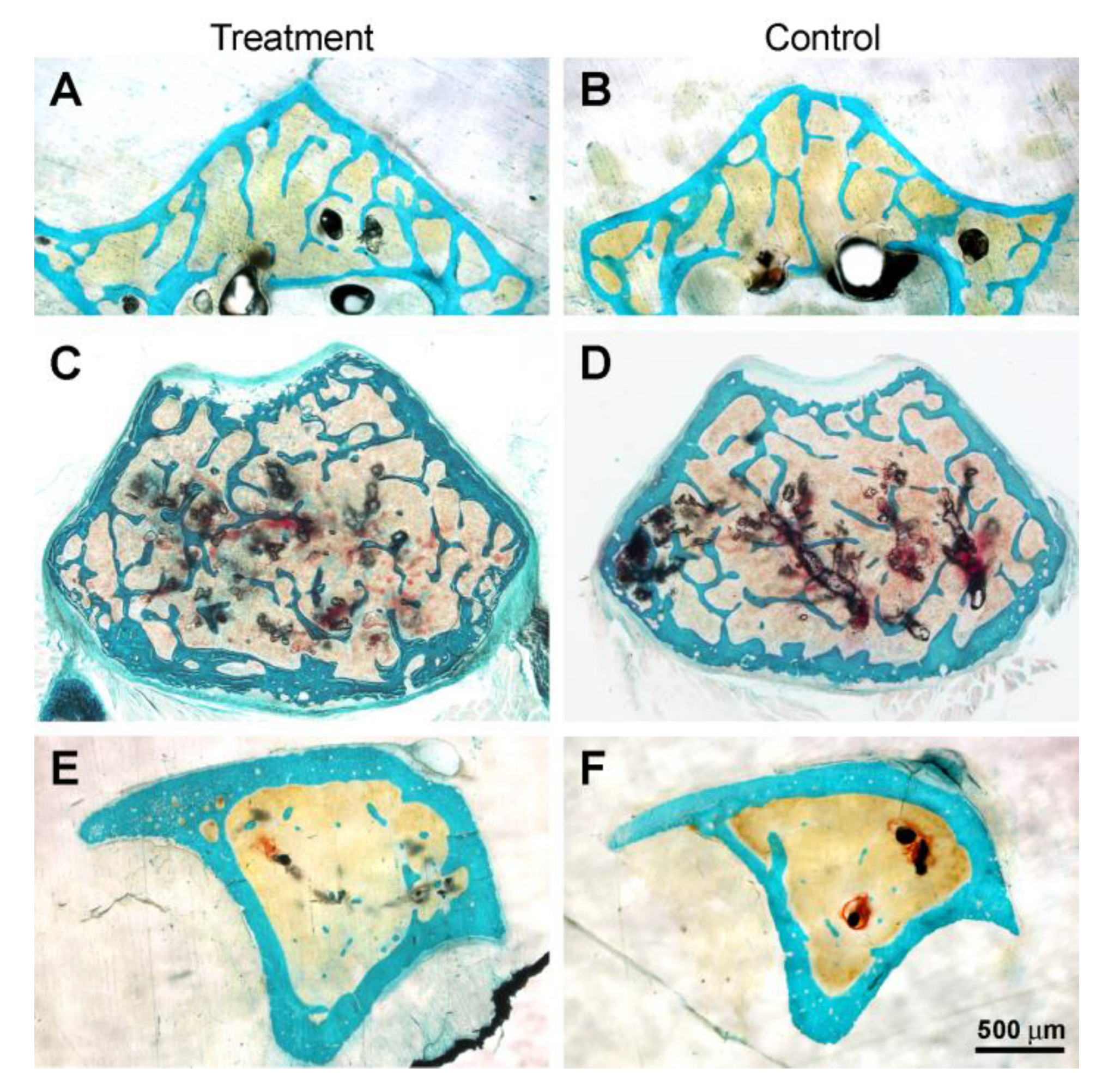
3.3. Histology and Histomorphometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maguire, M.E.; Cowan, J.A. Magnesium Chemistry and Biochemistry. BioMetals 2002, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Cittadini, A. Chemistry and Biochemistry of Magnesium. Mol. Asp. Med. 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Rapisarda, F.; Portale, G.; Ferrario, S.; Sessa, C.; Aliotta, R.; Zanoli, L.; Fatuzzo, P. Magnesium, Calcium and Potassium: “No One Was Born Alone”. Ital. J. Nefrol. 2016, 33, 1–5. [Google Scholar]
- Cowan, J.A. Introduction to the Biological Chemistry of Magnesium. In The Biological Chemistry of Magnesium; Cowan, J.A., Ed.; VCH Publishers: New York, NY, USA, 1995; pp. 1–24. [Google Scholar]
- Black, C.B.; Cowan, J.A. Magnesium Dependent Enzymes in Nucleic Acid Biochemistry (Chapter 6) and Magnesium Dependent Enzymes in General Metabolism (Chapter 7). In The Biological Chemistry of Magnesium; Cowan, J.A., Ed.; VCH Publishers: New York, NY, USA, 1995; pp. 137–158. [Google Scholar]
- Li, F.-Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second Messenger Role for Mg2+ Revealed by Human T-Cell Immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Marier, J.R. Dietary Magnesium and Drinking Water: Effects on Human Health Status. Met. Ions Biol. Syst. 1990, 26, 85–104. [Google Scholar]
- Wallach, S. Effects of Magnesium on Skeletal Metabolism. Magnes. Trace Elem. 1990, 9, 1–14. [Google Scholar]
- Rude, R.K. Magnesium Deficiency: A Cause of Heterogeneous Disease in Humans. J. Bone Miner. Res. 1998, 13, 749–758. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Mackowiak, S.J.; Troiano, N.; Gundberg, C.M. Osteocalcin and Its Message: Relationship to Bone Histology in Magnesium-Deprived Rats. Am. J. Physiol. Endocrinol. Metab. 1992, 263, E107–E114. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A.; Mills, B.G. Magnesium Deficiency: Effect on Bone and Mineral Metabolism in the Mouse. Calcif. Tissue Int. 2003, 72, 32–41. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Mills, B.G. Bone Loss Induced by Dietary Magnesium Reduction to 10% of the Nutrient Requirement in Rats Is Associated with Increased Release of Substance P and Tumor Necrosis Factor-Alpha. J. Nutr. 2004, 134, 79–85. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Reduction of Dietary Magnesium by Only 50% in the Rat Disrupts Bone and Mineral Metabolism. Osteoporos. Int. 2006, 17, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Rude, R.K.; Wei, L.; Frausto, A.; Mills, B.G.; Norton, H.J. Magnesium Deficiency: Effect on Bone Mineral Density in the Mouse Appendicular Skeleton. BMC Musculoskelet Disord 2003, 4, 7. [Google Scholar] [CrossRef]
- Rude, R.K.; Kirchen, M.E.; Gruber, H.E.; Meyer, M.H.; Luck, J.S.; Crawford, D.L. Magnesium Deficiency-Induced Osteoporosis in the Rat: Uncoupling of Bone Formation and Bone Resorption. Magnes. Res. 1999, 12, 257–267. [Google Scholar]
- Liu, C.C.; Yeh, J.K.; Aloia, J.F. Magnesium Directly Stimulates Osteoblast Proliferation. J. Bone Miner. Res. 1988, 3, S104. [Google Scholar]
- Sgambato, A.; Wolf, F.I.; Faraglia, B.; Cittadini, A. Magnesium Depletion Causes Growth Inhibition, Reduced Expression of Cyclin D1, and Increased Expression of P27 KIP1 in Normal But Not in Transformed Mammary Epithelial Cells. J. Cell. Physiol. 1999, 180, 245–254. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Dietary Magnesium Reduction to 25% of Nutrient Requirement Disrupts Bone and Mineral Metabolism in the Rat. Bone 2005, 37, 211–219. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A. Immunolocalization of RANKL Is Increased and OPG Decreased during Dietary Magnesium Deficiency in the Rat. Nutr. Metab. 2005, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Brugnara, C.; Beuzard, Y. Dietary Magnesium Supplementation Ameliorates Anemia in a Mouse Model of β-Thalassemia. Blood 1997, 90, 1283–1290. [Google Scholar] [CrossRef]
- Fengler, V.H.; Macheiner, T.; Goessler, W.; Ratzer, M.; Haybaeck, J.; Sargsyan, K. Hepatic Response of Magnesium-Restricted Wild Type Mice. Metabolites 2021, 11, 762. [Google Scholar] [CrossRef]
- Labots, M.; Zheng, X.; Moattari, G.; Lozeman-van’t Klooster, J.G.; Baars, J.M.; Hesseling, P.; Lavrijsen, M.; Kirchhoff, S.; Ohl, F.; van Lith, H.A. Substrain and Light Regime Effects on Integrated Anxiety-Related Behavioral z-Scores in Male C57BL/6 Mice-Hypomagnesaemia Has Only a Small Effect on Avoidance Behavior. Behav. Brain Res. 2016, 306, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Yamato, F.; Tsuji, S.; Kaneko, K. Low Calcium Diet Increases Radical Production of Polymorphonuclear Leukocytes from Mice. Int. J. Vitam. Nutr. Res. 2013, 79, 375–380. [Google Scholar] [CrossRef]
- de Franceschi, L.; Beuzard, Y.; Jouault, H.; Brugnara, C. Modulation of Erythrocyte Potassium Chloride Cotransport, Potassium Content, and Density by Dietary Magnesium Intake in Transgenic SAD Mouse. Blood 1996, 88, 2738–2744. [Google Scholar] [CrossRef]
- Mekada, K.; Abe, K.; Murakami, A.; Nakamura, S.; Nakata, H.; Moriwaki, K.; Obata, Y.; Yoshiki, A. Genetic Differences among C57BL/6 Substrains. Exp. Anim. 2009, 58, 141–149. [Google Scholar] [CrossRef]
- Simon, M.M.; Greenaway, S.; White, J.K.; Fuchs, H.; Gailus-Durner, V.; Wells, S.; Sorg, T.; Wong, K.; Bedu, E.; Cartwright, E.J.; et al. A Comparative Phenotypic and Genomic Analysis of C57BL/6J and C57BL/6N Mouse Strains. Genome Biol. 2013, 14, R82. [Google Scholar] [CrossRef]
- Tu, S.J.; Wang, S.P.; Cheng, F.C.; Weng, C.E.; Huang, W.T.; Chang, W.J.; Chen, Y.J. Attenuating Trabecular Morphology Associated with Low Magnesium Diet Evaluated Using Micro Computed Tomography. PLoS ONE 2017, 12, e0174806. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, C.; Ramazzina, I.; Russo, I.; Filippini, A.; Bettuzzi, S.; Rizzi, F. The Down-Regulation of Clusterin Expression Enhances the αSynuclein Aggregation Process. Int. J. Mol. Sci. 2020, 21, 7181. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Bayle, D.; Coudy-Gandilhon, C.; Gueugneau, M.; Castiglioni, S.; Zocchi, M.; Maj-Zurawska, M.; Palinska-Saadi, A.; Mazur, A.; Béchet, D.; Maier, J.A. Magnesium Deficiency Alters Expression of Genes Critical for Muscle Magnesium Homeostasis and Physiology in Mice. Nutrients 2021, 13, 2169. [Google Scholar] [CrossRef]
- Quamme, G.A.; Rouffignac, D.C. Epithelial Magnesium Transport and Regulation by the Kidney. Front. Biosci. 2000, 5, 694–711. [Google Scholar] [CrossRef]
- Rondón, L.J.; Tiel Groenestege, W.M.; Rayssiguier, Y.; Mazur, A. Relationship between Low Magnesium Status and TRPM6 Expression in the Kidney and Large Intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, 2001–2007. [Google Scholar] [CrossRef]
- Groenestege, W.M.T.; Hoenderop, J.G.; van den Heuvel, L.; Knoers, N.; Bindels, R.J. The Epithelial Mg2+ Channel Transient Receptor Potential Melastatin 6 Is Regulated by Dietary Mg2+ Content and Estrogens. J. Am. Soc. Nephrol. 2006, 17, 1035–1043. [Google Scholar] [CrossRef]
- Schäffers, O.J.M.; Hoenderop, J.G.J.; Bindels, R.J.M.; de Baaij, J.H.F. The Rise and Fall of Novel Renal Magnesium Transporters. Am. J. Physiol. Renal. Physiol. 2018, 314, F1027–F1033. [Google Scholar] [CrossRef]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Nejsum, L.N.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with Secondary Hypocalcemia Is Caused by Mutations in TRPM6, a New Member of the TRPM Gene Family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Walder, R.Y.; Landau, D.; Meyer, P.; Shalev, H.; Tsolia, M.; Borochowitz, Z.; Boettger, M.B.; Beck, G.E.; Englehardt, R.K.; Carmi, R.; et al. Mutation of TRPM6 Causes Familial Hypomagnesemia with Secondary Hypocalcemia. Nat. Genet. 2002, 31, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Cavani, F.; Smargiassi, A.; Roli, L.; Palumbo, C. Mineral and Skeletal Homeostasis Influence the Manner of Bone Loss in Metabolic Osteoporosis Due to Calcium-Deprived Diet in Different Sites of Rat Vertebra and Femur. Biomed. Res. Int. 2015, 2015, 304178. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Cavani, F.; Roli, L.; Checchi, M.; Magarò, M.S.; Bertacchini, J.; Palumbo, C. Interaction among Calcium Diet Content, PTH (1-34) Treatment and Balance of Bone Homeostasis in Rat Model: The Trabecular Bone as Keystone. Int. J. Mol. Sci. 2019, 20, 753. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ingram, J.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Mills, B.G.; Rude, R.K. Alterations in Growth Plate and Articular Cartilage Morphology Are Associated with Reduced SOX9 Localization in the Magnesium-Deficient Rat. Biotech. Histochem. 2004, 79, 45–52. [Google Scholar] [CrossRef]
- Abdelmoez, A.M.; Puig, L.S.; Smith, J.A.B.; Gabriel, B.M.; Savikj, M.; Dollet, L.; Chibalin, A.V.; Krook, A.; Zierath, J.R.; Pillon, N.J. Comparative Profiling of Skeletal Muscle Models Reveals Heterogeneity of Transcriptome and Metabolism. Am. J. Physiol. Cell Physiol. 2020, 318, 615–626. [Google Scholar] [CrossRef]
- Carlson, B.M. Muscle Regeneration in Animal Models. In Skeletal Muscle Repair and Regeneration; Carlson, B.M., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 163–180. [Google Scholar]
- Fox, J.G.; Barthold, S.W.; Davisson, M.T.; Newcomer, C.E.; Quimby, F.W.; Smith, A.L. Mouse Strain and Genetic Nomenclature: An Abbreviated Guide. In The Mouse in Biomedical Research. History, Wild Mice, and Genetics, 2nd ed.; Elsevier: London, UK, 2007; Volume 1, pp. 79–98. [Google Scholar]
- Freeman, H.C.; Hugill, A.; Dear, N.T.; Ashcroft, F.M.; Cox, R.D. Deletion of Nicotinamide Nucleotide Transhydrogenase A New Quantitive Trait Locus Accounting for Glucose Intolerance in C57BL/6J Mice. Diabetes 2006, 55, 2153–2156. [Google Scholar] [CrossRef]
- Khisti, R.T.; Wolstenholme, J.; Shelton, K.L.; Miles, M.F. Characterization of the Ethanol-Deprivation Effect in Substrains of C57BL/6 Mice. Alcohol 2006, 40, 119–126. [Google Scholar] [CrossRef]
- Diwan, B.A.; Blackman, K.E. Differential Susceptibility of 3 Sublines of C57BL6 Mice to the Induction of Colorectal Tumors by 1,2-Dimethylhydrazine. Cancer Lett. 1980, 9, 111–115. [Google Scholar] [CrossRef] [PubMed]
- van Angelen, A.A.; San-Cristobal, P.; Pulskens, W.P.; Hoenderop, J.G.; Bindels, R.J. The Impact of Dietary Magnesium Restriction on Magnesiotropic and Calciotropic Genes. Nephrol. Dial. Transplant. 2013, 28, 2983–2993. [Google Scholar] [CrossRef] [PubMed]
- Liron, T.; Raphael, B.; Hiram-Bab, S.; Bab, I.A.; Gabet, Y. Bone Loss in C57BL/6J-OlaHsd Mice, a Substrain of C57BL/6J Carrying Mutated Alpha-Synuclein and Multimerin-1 Genes. J. Cell. Physiol. 2018, 233, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, J.S.; Varshney, M.; Judex, S. Differences in Bone Structure and Unloading-Induced Bone Loss between C57BL/6N and C57BL/6J Mice. Mamm. Genome 2017, 28, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Rendina-Ruedy, E.; Hembree, K.D.; Sasaki, A.; Davis, M.R.; Lightfoot, S.A.; Clarke, S.L.; Lucas, E.A.; Smith, B.J. A Comparative Study of the Metabolic and Skeletal Response of C57BL/6J and C57BL/6N Mice in a Diet-Induced Model of Type 2 Diabetes. J. Nutr. Metab. 2015, 2015, 758080. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| MagT1 | 5′-CCAAAGGGGCTGATACATA-3 | 5′-ATAGAAGAACGATGTGTG-3′ |
| TRPM6 | 5′-AAAGCCATGCGAGTTATCAGC-3′ | 5′-CTTCACAATGAAAACCTGCCC-3′ |
| TRPM7 | 5′-GGTTCCTCCTCCTGTGGTGCCTT-3′ | 5′-CCCCATGTCGTCTCTGTCGT-3′ |
| GAPDH | 5′-TCAAGCTCATTTCCTGGTAT-3′ | 5′- GTCCAGGGTTTCTTACTCCT-3′ |
| L5 Vertebral Body | Treatment | Control |
| BV/TV | 13.7 ± 3.3 | 14.5 ± 4.2 |
| Tb. Th | 34.2 ± 5.1 | 38.8 ± 6.1 |
| Tb. N | 4.1 ± 1 | 3.7 ± 0.6 |
| Tb. S | 225 ± 67 | 237 ± 52 |
| Ct Th | 68 ± 14.2 | 60 ± 6.1 |
| Femoral distal metaphysis | ||
| BV/TV | 13.7 ± 3.8 | 13.7 ± 3.5 |
| Tb. Th | 31.7 ± 6.9 | 31.5 ± 5.6 |
| Tb. N | 4.3 ± 0.8 | 4.4 ± 0.7 |
| Tb. S | 203 ± 45 | 199 ± 34 |
| Tibial proximal metaphysis | ||
| BV/TV | 7.3 ± 4.2 | 7.5 ± 3.3 |
| Tb. Th | 26.4 ± 4.8 | 28.5 ± 4.8 |
| Tb. N | 2.68 ± 1.2 | 2.68 ± 0.7 |
| Tb. S | 406 ± 180 | 378 ± 144 |
| Growth Plate Thickness | Treatment | Control |
|---|---|---|
| Femoral distal metaphysis | 98.5 ± 22.5 | 102.3 ± 15.7 |
| Tibial proximal metaphysis | 106.1 ± 11.6 | 110.1 ± 23.4 |
| NOc/BS | Treatment | Control |
|---|---|---|
| Femoral distal metaphysis | 9.3 ± 1.9 | 9 ± 1.9 |
| Tibial proximal metaphysis | 8.4 ± 3.7 | 8.8 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferretti, M.; Cavani, F.; Lo Vasco, V.R.; Checchi, M.; Truocchio, S.; Davalli, P.; Frassineti, C.; Rizzi, F.; Palumbo, C. Unexpected Absence of Skeletal Responses to Dietary Magnesium Depletion: Basis for Future Perspectives? Biomedicines 2023, 11, 655. https://doi.org/10.3390/biomedicines11030655
Ferretti M, Cavani F, Lo Vasco VR, Checchi M, Truocchio S, Davalli P, Frassineti C, Rizzi F, Palumbo C. Unexpected Absence of Skeletal Responses to Dietary Magnesium Depletion: Basis for Future Perspectives? Biomedicines. 2023; 11(3):655. https://doi.org/10.3390/biomedicines11030655
Chicago/Turabian StyleFerretti, Marzia, Francesco Cavani, Vincenza Rita Lo Vasco, Marta Checchi, Serena Truocchio, Pierpaola Davalli, Chiara Frassineti, Federica Rizzi, and Carla Palumbo. 2023. "Unexpected Absence of Skeletal Responses to Dietary Magnesium Depletion: Basis for Future Perspectives?" Biomedicines 11, no. 3: 655. https://doi.org/10.3390/biomedicines11030655
APA StyleFerretti, M., Cavani, F., Lo Vasco, V. R., Checchi, M., Truocchio, S., Davalli, P., Frassineti, C., Rizzi, F., & Palumbo, C. (2023). Unexpected Absence of Skeletal Responses to Dietary Magnesium Depletion: Basis for Future Perspectives? Biomedicines, 11(3), 655. https://doi.org/10.3390/biomedicines11030655







