Prostate Ultrasound Image Segmentation Based on DSU-Net
Abstract
1. Introduction
Related Work
2. Prostate Segmentation Method Based on DSU-Net
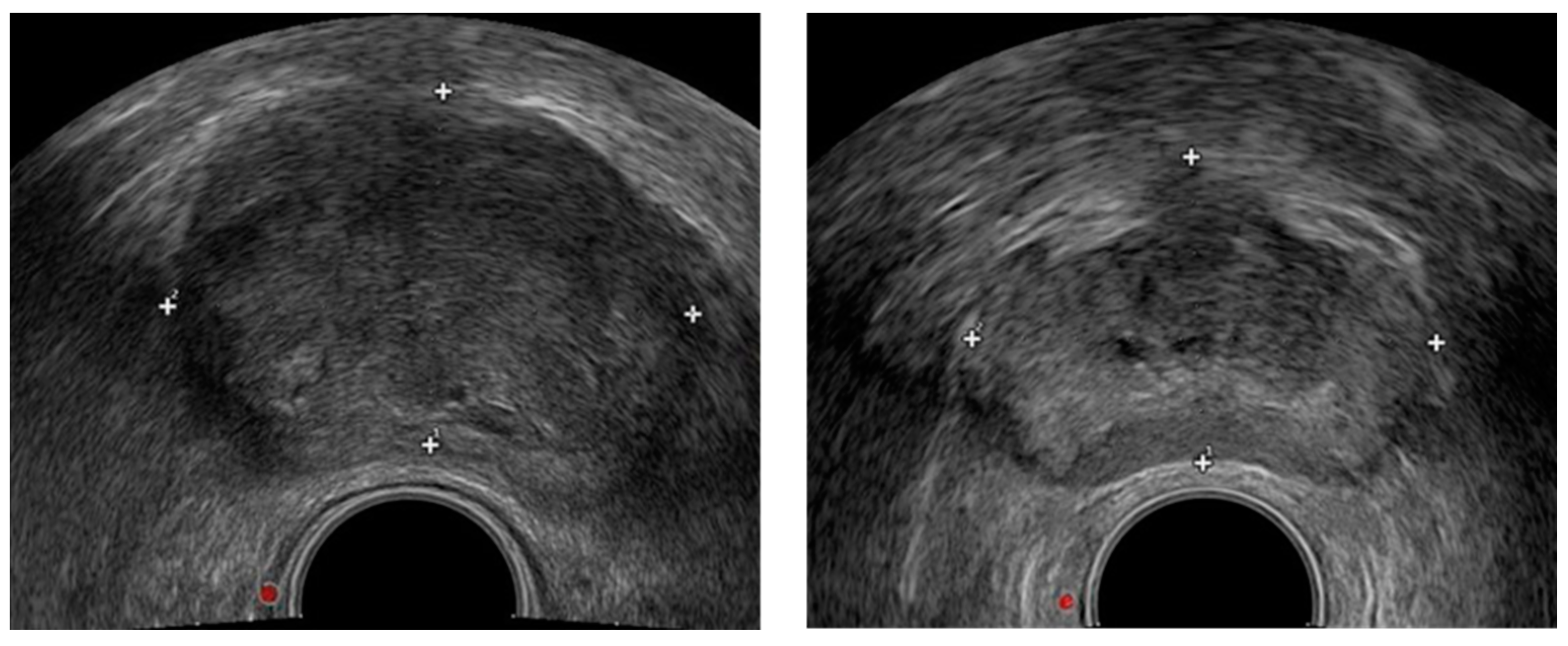
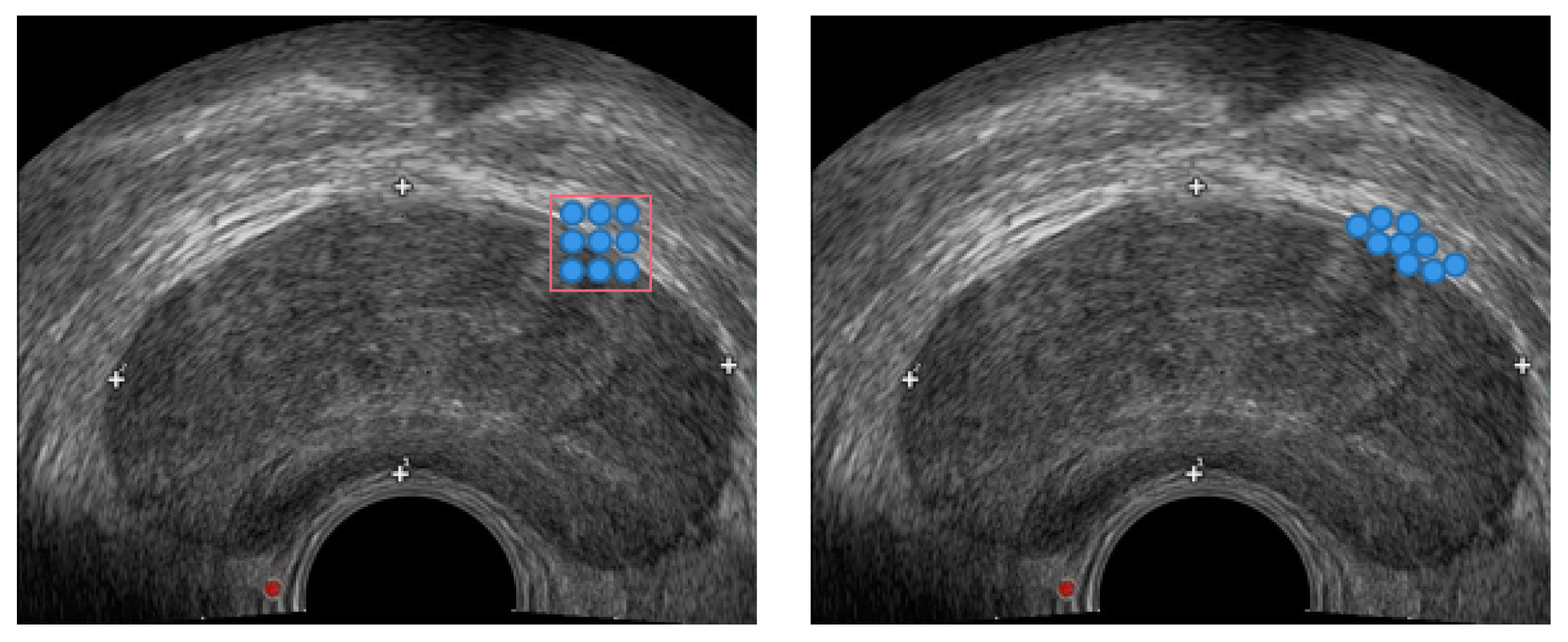
2.1. Data Preprocessing
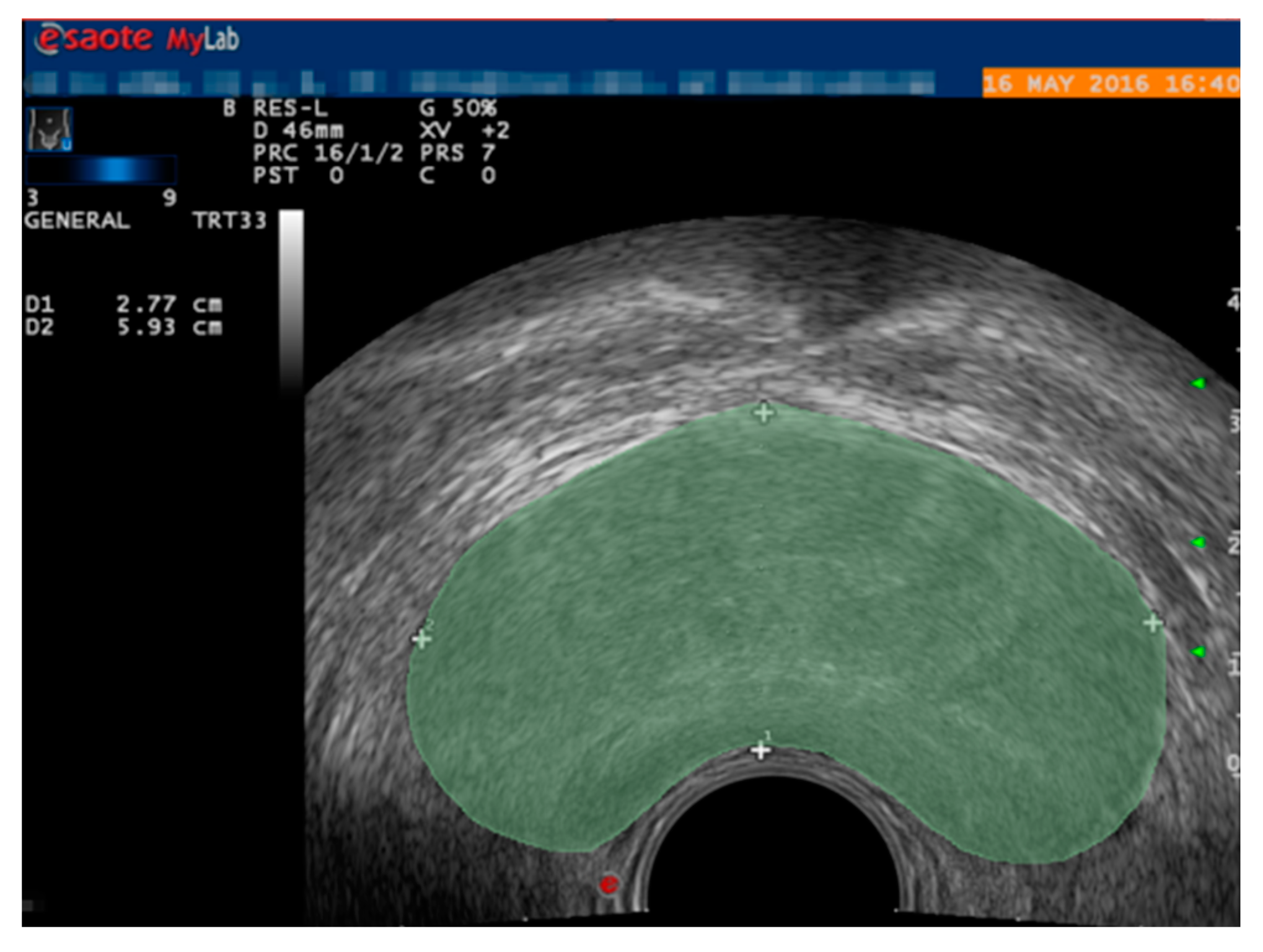
2.2. Prostate Segmentation Method Based on DSU-Net
3. Experiment and Analysis
3.1. Dataset
3.2. Evaluation Index
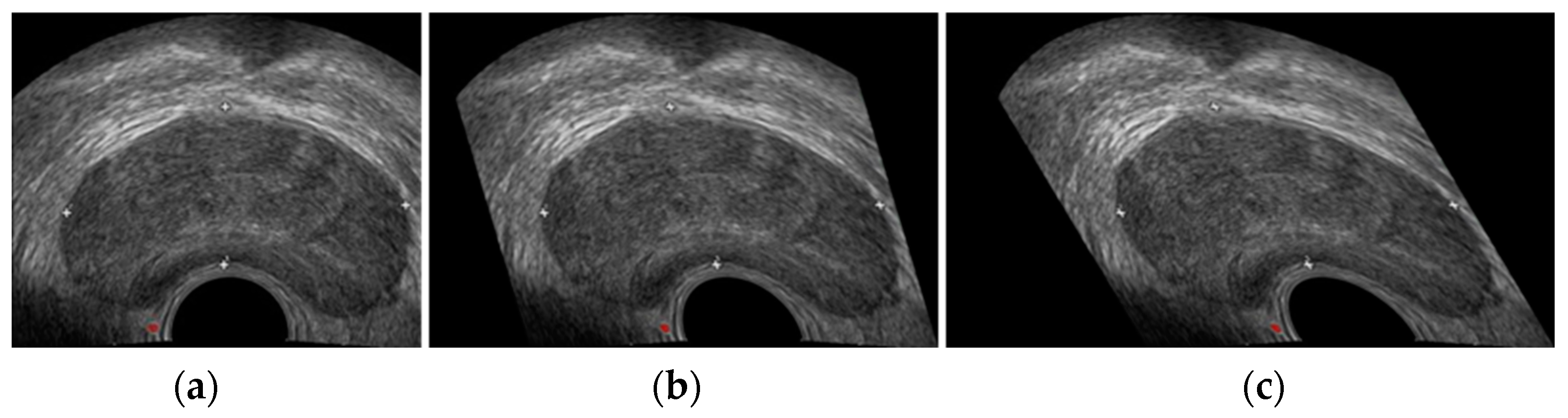
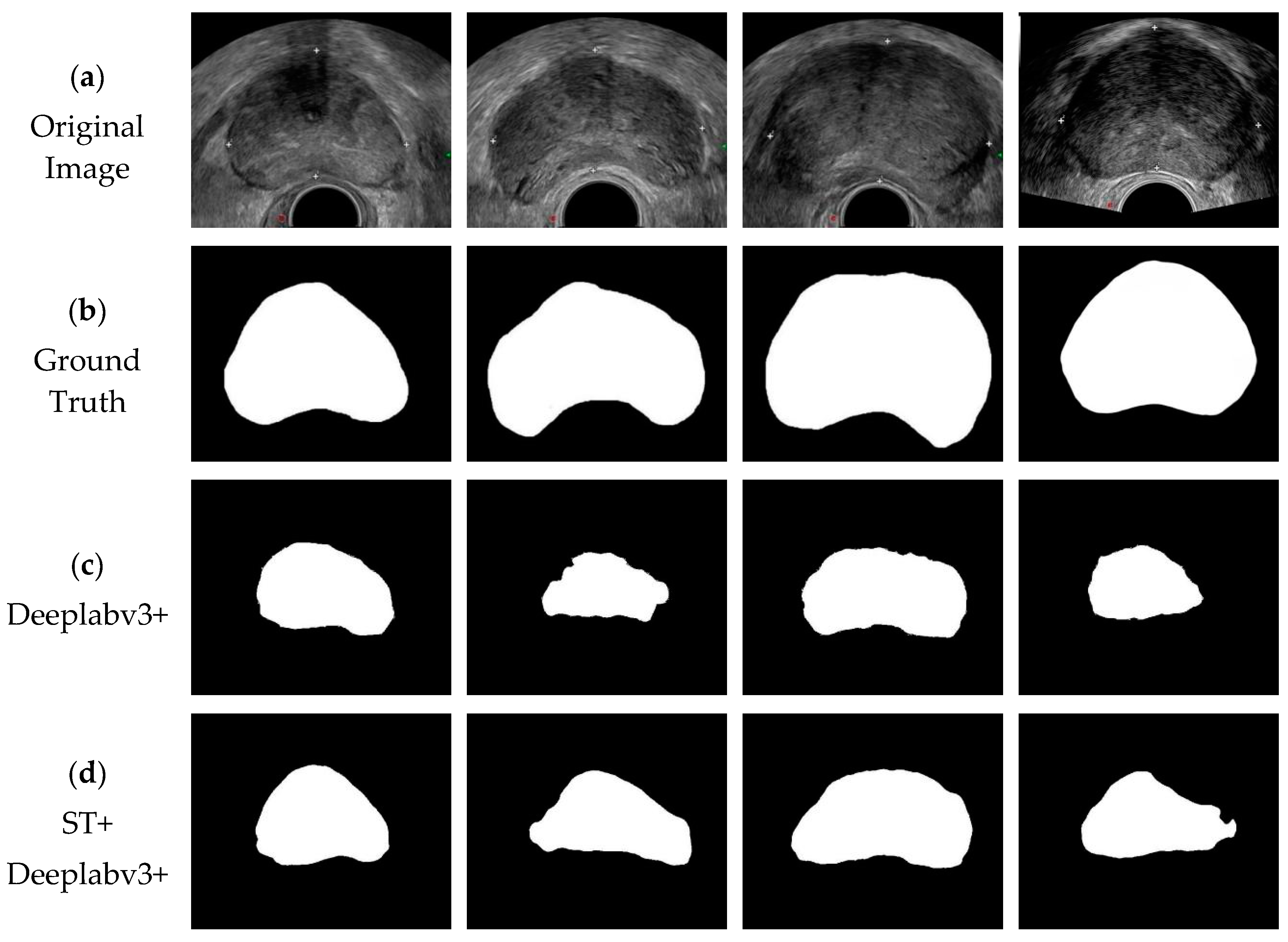
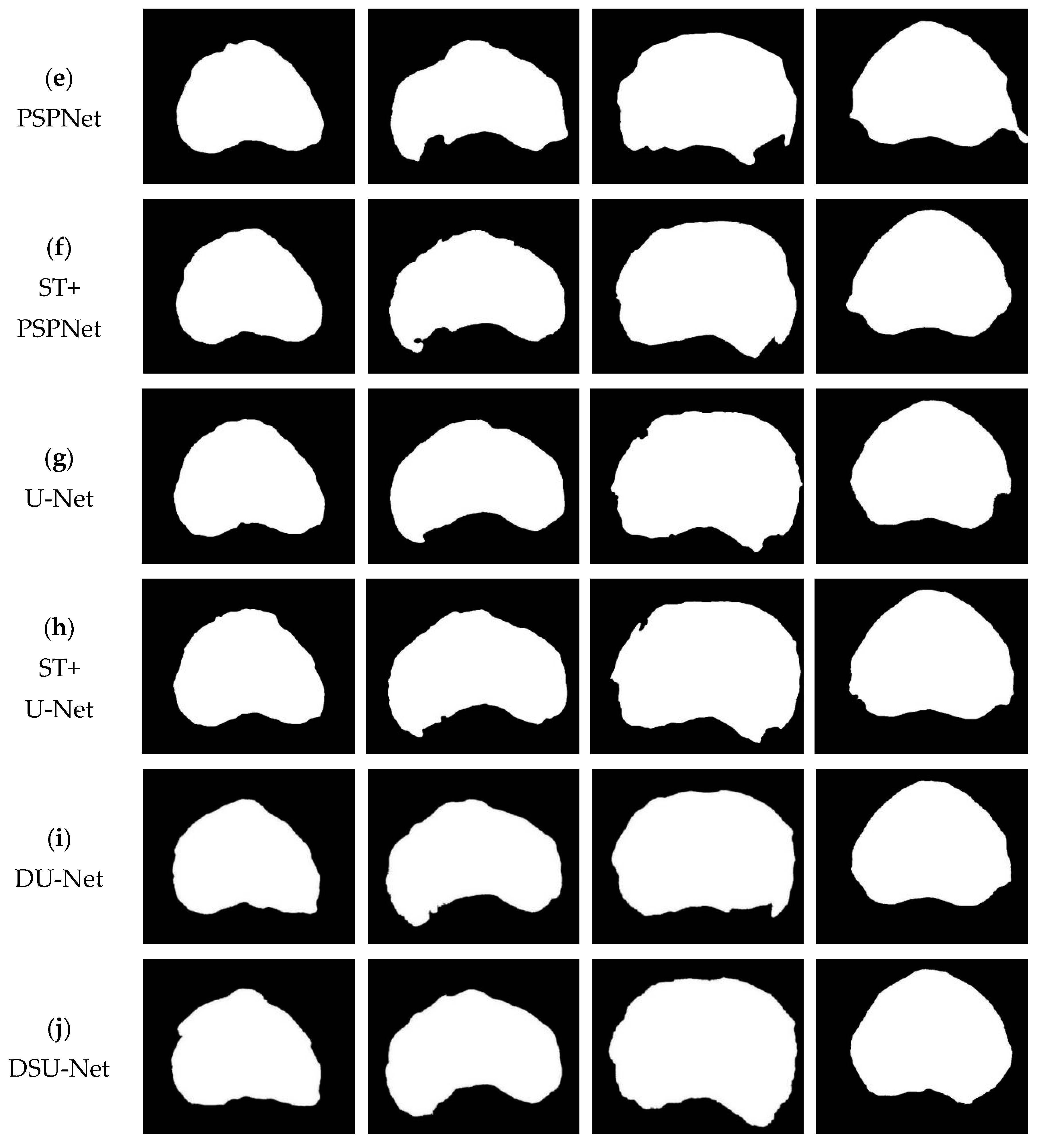
3.3. Experimental Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Z.T.; Guo, X.L.; Zhang, S.W.; Zheng, R.; Zeng, H.; Chen, R.; Wang, S.; Sun, K.; Wei, W.; He, J. Incidence and mortality of prostate cancer in China in 2015. Chin. J. Oncol. 2020, 42, 718–722. [Google Scholar]
- Xia, Q.; Ding, T.; Zhang, G.; Li, Z.; Zeng, L.; Zhu, Y.; Guo, J.; Hou, J.; Zhu, T.; Zheng, J.; et al. Circular RNA expression profiling identifies prostate cancer-specific circRNAs in prostate cancer. Cell. Physiol. Biochem. 2018, 50, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Ai, Q.; Li, H.; Ma, X.; Zhang, X. Extraperitoneal Laparoscopic Radical Prostatectomy. In Laparoscopic and Robotic Surgery in Urology; Springer: Singapore, 2020; pp. 233–247. [Google Scholar]
- Wei, Y.; Wu, J.; Gu, W.; Qin, X.; Dai, B.; Lin, G.; Gan, H.; Freedland, S.J.; Zhu, Y.; Ye, D. Germline DNA Repair Gene Mutation Landscape in Chinese Prostate Cancer Patients. Eur. Urol. 2019, 76, 280–283. [Google Scholar] [CrossRef] [PubMed]
- van der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: A large prospective multicenter clinical study. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [PubMed]
- Narula, J.; Chandrashekhar, Y.; Braunwald, E. Time to add a fifth pillar to bedside physical examination: Inspection, palpation, percussion, auscultation, and insonation. JAMA Cardiol. 2018, 3, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yuan, X.; Yang, Y. Research progress in the physiology-psycho-social model of prostate cancer patients nursing. Shanghai Nurs. 2022, 22, 46–49. [Google Scholar]
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic review of complications of prostate biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Kutluhan, M.A.; Toprak, T.; Topaktaş, R. Evaluation of the Patients with Urinary Tract Infection after Transrectal Ultrasound-guided Prostate Biopsy. Haydarpaşa Numune Med. J. 2020, 60, 422–425. [Google Scholar] [CrossRef]
- Yang, X.; Yu, L.; Wu, L.; Wang, Y.; Ni, D.; Qin, J.; Heng, P.A. Fine-grained recurrent neural networks for automatic prostate segmentation in ultrasound images. Proc. AAAI Conf. Artif. Intell. 2017, 31, 1633–1639. [Google Scholar] [CrossRef]
- Mishra, D.; Chaudhury, S.; Sarkar, M.; Soin, A.S. Ultrasound image segmentation: A deeply supervised network with attention to boundaries. IEEE Trans. Biomed. Eng. 2018, 66, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Shelhamer, E.; Darrell, T. Fully convolutional networks for semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 3431–3440. [Google Scholar]
- Sun, Y.; Ji, Y. AAWS-Net: Anatomy-aware weakly-supervised learning network for breast mass segmentation. PLoS ONE 2021, 16, e0256830. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional networks for biomedical image segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Munich, Germany, 5–9 October 2015; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Taylor, L.; Nitschke, G. Improving deep learning with generic data augmentation. In Proceedings of the 2018 IEEE Symposium Series on Computational Intelligence (SSCI), Bengaluru, India, 18–21 November 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1542–1547. [Google Scholar]
- Shorten, C.; Khoshgoftaar, T.M. A survey on image data augmentation for deep learning. J. Big Data 2019, 6, 1–48. [Google Scholar] [CrossRef]
- Li, L.; Wei, M.; Liu, B.; Atchaneeyasakul, K.; Zhou, F.; Pan, Z.; Kumar, S.A.; Zhang, J.Y.; Pu, Y.; Liebeskind, D.S.; et al. Deep learning for hemorrhagic lesion detection and segmentation on brain ct images. IEEE J. Biomed. Health Inform. 2020, 25, 1646–1659. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wang, T.; Zhang, Y.; Liu, J.; Xu, Y. Cloud/shadow segmentation based on global attention feature fusion residual network for remote sensing imagery. Int. J. Remote Sens. 2021, 42, 2022–2045. [Google Scholar] [CrossRef]
- Dai, J.; Qi, H.; Xiong, Y.; Li, Y.; Zhang, G.; Hu, H.; Wei, Y. Deformable convolutional networks. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 764–773. [Google Scholar]
- Miao, Q.; Shi, C.; Xu, P.; Yang, M.; Shi, Y. Multi-focus image fusion algorithm based on shearlets. Chin. Opt. Lett. 2011, 9, 041001. [Google Scholar] [CrossRef]
- Chen, L.C.; Zhu, Y.; Papandreou, G.; Schroff, F.; Adam, H. Encoder-decoder with atrous separable convolution for semantic image segmentation. In Proceedings of the European Conference on Computer Vision (ECCV) 2018, Munich, Germany, 8–14 September 2018; pp. 801–818. [Google Scholar]
- Zhao, H.; Shi, J.; Qi, X.; Wang, X.; Jia, J. Pyramid scene parsing network. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 2881–2890. [Google Scholar]

| Learning Rate | Training Time | Batch Size | Optimizer |
|---|---|---|---|
| 0.001 | 80 epochs | 8 | Adam |
| Method | Dice | Jaccard |
|---|---|---|
| DeepLabv3+ | 0.750 | 0.605 |
| ST+DeepLabv3+ | 0.784 | 0.648 |
| PSPNet | 0.879 | 0.787 |
| ST+PSPNet | 0.895 | 0.834 |
| U-Net | 0.923 | 0.886 |
| ST+U-Net | 0.935 | 0.890 |
| DU-Net | 0.941 | 0.893 |
| DSU-Net | 0.957 | 0.925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chang, Z.; Zhang, Q.; Li, C.; Miao, F.; Gao, G. Prostate Ultrasound Image Segmentation Based on DSU-Net. Biomedicines 2023, 11, 646. https://doi.org/10.3390/biomedicines11030646
Wang X, Chang Z, Zhang Q, Li C, Miao F, Gao G. Prostate Ultrasound Image Segmentation Based on DSU-Net. Biomedicines. 2023; 11(3):646. https://doi.org/10.3390/biomedicines11030646
Chicago/Turabian StyleWang, Xinyu, Zhengqi Chang, Qingfang Zhang, Cheng Li, Fei Miao, and Gang Gao. 2023. "Prostate Ultrasound Image Segmentation Based on DSU-Net" Biomedicines 11, no. 3: 646. https://doi.org/10.3390/biomedicines11030646
APA StyleWang, X., Chang, Z., Zhang, Q., Li, C., Miao, F., & Gao, G. (2023). Prostate Ultrasound Image Segmentation Based on DSU-Net. Biomedicines, 11(3), 646. https://doi.org/10.3390/biomedicines11030646










