Simultaneous Quantification of Ivacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis Patients’ Plasma by a Novel LC–MS/MS Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Calibration Curve, Quality Control, and Stock Solution Preparation
2.3. Human Samples
2.4. Sample Preparation
2.5. Chromatographic Conditions
2.6. MS/MS Conditions
2.7. Method Validation
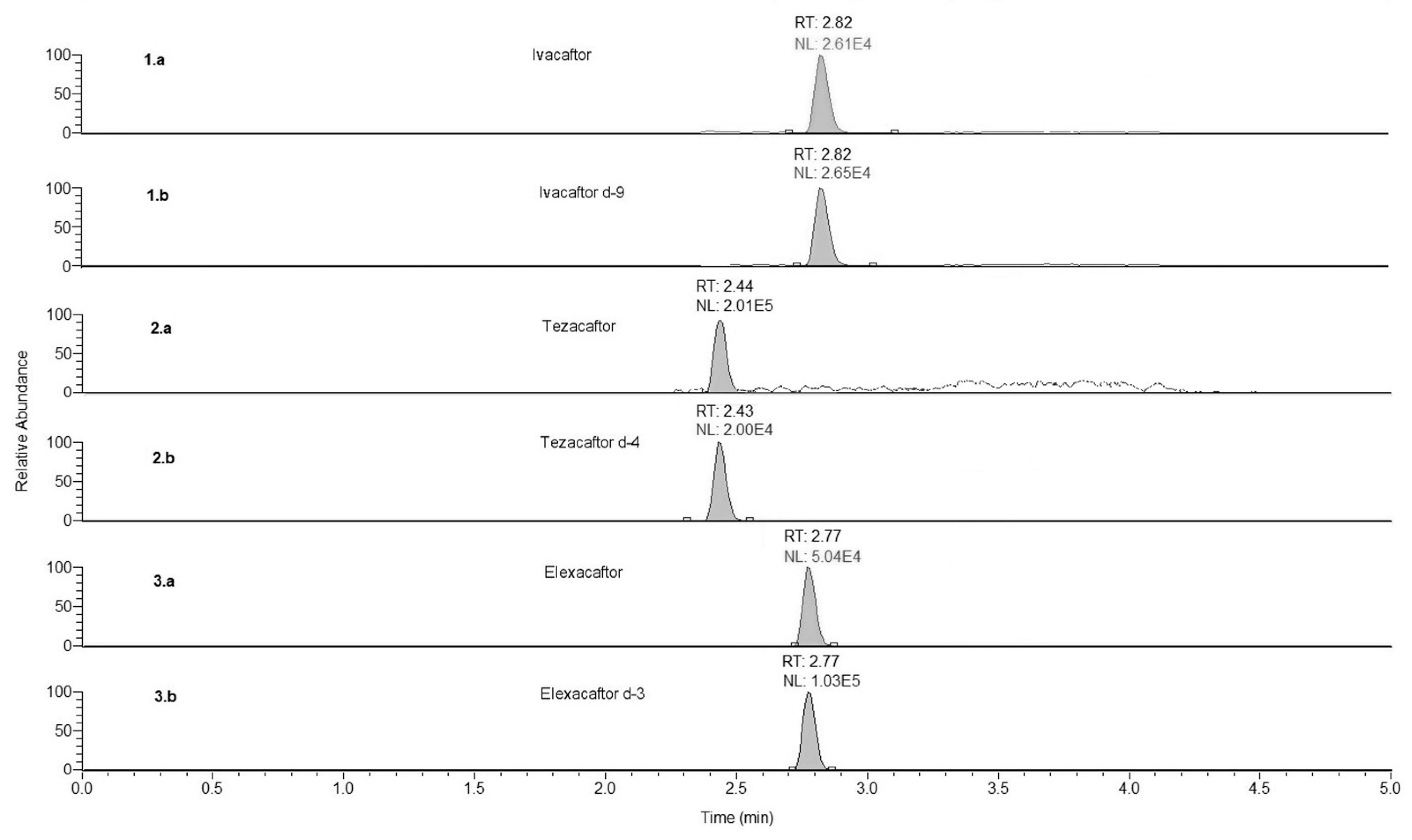
2.7.1. Selectivity
2.7.2. Carry-Over
2.7.3. Matrix Effects and Extraction Recovery
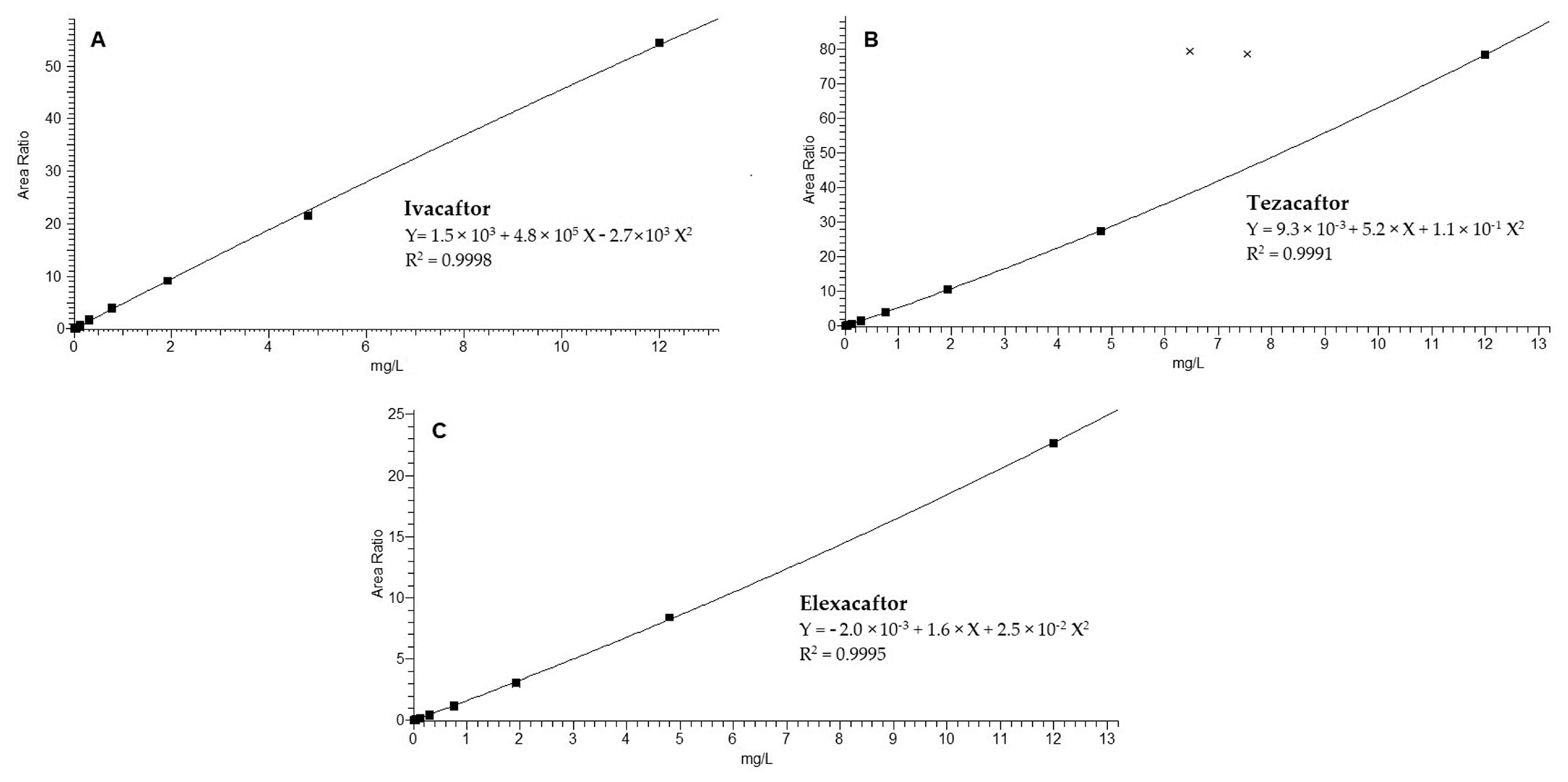
2.7.4. Linearity
2.7.5. Precision, Accuracy, and LLOQ
2.7.6. Stability
2.8. Statistical Analyses
3. Results
3.1. Method Development
3.2. Method Validation
3.3. Analysis of Patients’ Samples
3.4. Correlation Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elborn, J.S. Cystic Fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Assael, B.M. Cystic Fibrosis: A Clinical View. Cell Mol. Life Sci. 2017, 74, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Gentzsch, M.; Mall, M.A. Ion Channel Modulators in Cystic Fibrosis. Chest 2018, 154, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Sondo, E.; Cresta, F.; Pastorino, C.; Tomati, V.; Capurro, V.; Pesce, E.; Lena, M.; Iacomino, M.; Baffico, A.M.; Coviello, D.; et al. The L467F-F508del Complex Allele Hampers Pharmacological Rescue of Mutant CFTR by Elexacaftor/Tezacaftor/Ivacaftor in Cystic Fibrosis Patients: The Value of the Ex Vivo Nasal Epithelial Model to Address Non-Responders to CFTR-Modulating Drugs. Int. J. Mol. Sci. 2022, 23, 3175. [Google Scholar] [CrossRef] [PubMed]
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic Fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef]
- Castagnola, E.; Cangemi, G.; Mesini, A.; Castellani, C.; Martelli, A.; Cattaneo, D.; Mattioli, F. Pharmacokinetics and Pharmacodynamics of Antibiotics in Cystic Fibrosis: A Narrative Review. Int. J. Antimicrob. Agents 2021, 58, 106381. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2020, 10, 1662. [Google Scholar] [CrossRef]
- Pettit, R.S.; Fellner, C. CFTR Modulators for the Treatment of Cystic Fibrosis. Pharm. Ther. 2014, 39, 500. [Google Scholar]
- de Boeck, K.; Amaral, M.D. Progress in Therapies for Cystic Fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Guhr Lee, T.N.; Cholon, D.M.; Quinney, N.L.; Gentzsch, M.; Esther, C.R. Accumulation and Persistence of Ivacaftor in Airway Epithelia with Prolonged Treatment. J. Cyst. Fibros. 2020, 19, 746–751. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Mall, M.A.; Ramsey, B.W.; McKone, E.F.; Tullis, E.; Marigowda, G.; McKee, C.M.; Waltz, D.; Moskowitz, S.M.; Savage, J.; et al. Clinical Development of Triple-Combination CFTR Modulators for Cystic Fibrosis Patients with One or Two F508del Alleles. ERJ Open Res. 2019, 5, 00082–02019. [Google Scholar] [CrossRef]
- Lommatzsch, S.T.; Taylor-Cousar, J.L. The Combination of Tezacaftor and Ivacaftor in the Treatment of Patients with Cystic Fibrosis: Clinical Evidence and Future Prospects in Cystic Fibrosis Therapy. Ther. Adv. Respir. Dis. 2019, 13, 1753466619844424. [Google Scholar] [CrossRef]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef]
- van der Meer, R.; Wilms, E.B.; Heijerman, H.G.M. CFTR Modulators: Does One Dose Fit All? J. Pers. Med. 2021, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Kaftrio | European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kaftrio (accessed on 12 January 2023).
- Choong, E.; Sauty, A.; Koutsokera, A.; Blanchon, S.; André, P.; Decosterd, L. Therapeutic Drug Monitoring of Ivacaftor, Lumacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis: Where Are We Now? Pharmaceutics 2022, 14, 1674. [Google Scholar] [CrossRef] [PubMed]
- Bioanalytical Method Validation—Scientific Guideline | European Medicines Agency. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation-scientific-guideline (accessed on 12 January 2023).
- Lynch, K.L. CLSI C62-A: A New Standard for Clinical Mass Spectrometry. Clin. Chem. 2016, 62, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Liou, T.G.; Adler, F.R.; Fitzsimmons, S.C.; Cahill, B.C.; Hibbs, J.R.; Marshall, B.C. Predictive 5-Year Survivorship Model of Cystic Fibrosis. Am. J. Epidemiol. 2001, 153, 345–352. [Google Scholar] [CrossRef]
- de Aquino, C.S.B.; Rodrigues, J.C.; da Silva-Filho, L.V.R.F. Routine Spirometry in Cystic Fibrosis Patients: Impact on Pulmonary Exacerbation Diagnosis and FEV1 Decline. J. Bras. Pneumol. 2022, 48, E20210237. [Google Scholar] [CrossRef]
- Vermeulen, F.; Lebecque, P.; de Boeck, K.; Leal, T. Biological Variability of the Sweat Chloride in Diagnostic Sweat Tests: A Retrospective Analysis. J. Cyst. Fibros. 2017, 16, 30–35. [Google Scholar] [CrossRef]
- Il Test Del Sudore. Raccomandazioni per Una Corretta Esecuzione Ed Interpretazione Dei Risultati—SIFC. Available online: https://www.sifc.it/documento/il-test-del-sudore-raccomandazioni-per-una-corretta-esecuzione-ed-interpretazione-dei-risultati/ (accessed on 12 January 2023).
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Gu, H.; Liu, G.; Wang, J.; Aubry, A.F.; Arnold, M.E. Selecting the Correct Weighting Factors for Linear and Quadratic Calibration Curves with Least-Squares Regression Algorithm in Bioanalytical LC-MS/MS Assays and Impacts of Using Incorrect Weighting Factors on Curve Stability, Data Quality, and Assay Performance. Anal. Chem. 2014, 86, 8959–8966. [Google Scholar] [CrossRef]
- Gross, A.S. Best Practice in Therapeutic Drug Monitoring. Br. J. Clin. Pharmacol. 2001, 52 (Suppl. 1), 5–9. [Google Scholar] [CrossRef]
- Holford, N.H.G.; Buclin, T. Safe and Effective Variability-a Criterion for Dose Individualization. Ther. Drug Monit. 2012, 34, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Vonk, S.E.M.; van der Meer-Vos, M.; Bos, L.D.J.; Neerincx, A.H.; Majoor, C.J.; Maitland-van der Zee, A.H.; Mathôt, R.A.A.; Kemper, E.M. Quantitative Method for the Analysis of Ivacaftor, Hydroxymethyl Ivacaftor, Ivacaftor Carboxylate, Lumacaftor, and Tezacaftor in Plasma and Sputum Using Liquid Chromatography With Tandem Mass Spectrometry and Its Clinical Applicability. Ther. Drug Monit. 2021, 43, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Habler, K.; Kalla, A.S.; Rychlik, M.; Bruegel, M.; Teupser, D.; Nährig, S.; Vogeser, M.; Paal, M. Isotope Dilution LC-MS/MS Quantification of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators Ivacaftor, Lumacaftor, Tezacaftor, Elexacaftor, and Their Major Metabolites in Human Serum. Clin. Chem. Lab. Med. 2021, 60, 82–91. [Google Scholar] [CrossRef]
- Satya, M.; Sakuntala, V.; Rao, A.L.; Carey, M.W. Simultaneous Estimation of Ivacaftor and Tezacaftor in Rat Plasma by Liquid Chromatography Coupled with Tandem-Mass-Spectrometry: Application to Pharmacokinetic Studies. Thai J. Pharm. Sci. TJPS 2021, 45, 451–460. [Google Scholar]
- Ryan, K.J.; Guimbellot, J.S.; Dowell, A.E.; Reed-Walker, K.D.; Kerstner-Wood, C.D.; Anderson, J.D.; Liu, Z.; Acosta, E.P. Quantitation of Cystic Fibrosis Triple Combination Therapy, Elexacaftor/Tezacaftor/Ivacaftor, in Human Plasma and Cellular Lysate. J. Chromatogr. B 2022, 1213, 123518. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Qiu, F.; Schneider-Futschik, E.K. Multiple Reaction Monitoring Mass Spectrometry for the Drug Monitoring of Ivacaftor, Tezacaftor, and Elexacaftor Treatment Response in Cystic Fibrosis: A High-Throughput Method. ACS Pharmacol. Transl. Sci. 2020, 3, 987–996. [Google Scholar] [CrossRef]
- Schneider, E.K.; Reyes-Ortega, F.; Wilson, J.W.; Kotsimbos, T.; Keating, D.; Li, J.; Velkov, T. Development of HPLC and LC-MS/MS Methods for the Analysis of Ivacaftor, Its Major Metabolites and Lumacaftor in Plasma and Sputum of Cystic Fibrosis Patients Treated with ORKAMBI or KALYDECO. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1038, 57–62. [Google Scholar] [CrossRef]
- Schneider, E.K.; Reyes-Ortega, F.; Li, J.; Velkov, T. Optimized LC-MS/MS Method for the High-Throughput Analysis of Clinical Samples of Ivacaftor, Its Major Metabolites, and Lumacaftor in Biological Fluids of Cystic Fibrosis Patients. J. Vis. Exp. 2017, 2017, e56084. [Google Scholar] [CrossRef]
- Mehta, Z.; Kamal, K.M.; Miller, R.; Covvey, J.R.; Giannetti, V. Adherence to Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators: Analysis of a National Specialty Pharmacy Database. J. Drug. Assess. 2021, 10, 62–67. [Google Scholar] [CrossRef] [PubMed]
| Males (n = 28) | Females (n = 34) | |||
|---|---|---|---|---|
| Age (Yrs) | Weight (Kg) | Age (Yrs) | Weight (Kg) | |
| Mean | 30.4 | 66.8 | 30.4 | 53.5 |
| SD | 10.6 | 10.9 | 16.5 | 7.99 |
| Median | 32.4 | 66.6 | 23.9 | 52.5 |
| Min–Max | 13–51 | 38.0–84.0 | 12–75 | 40.5–79.8 |
| INTER-DAY | |||||||||
| Ivacaftor | Tezacaftor | Elexacaftor | |||||||
| Nominal value (mg/L) | CV% | Accuracy % | Nominal value (mg/L) | CV% | Accuracy % | Nominal value (mg/L) | CV% | Accuracy % | |
| LLOQ | 0.008 | 13% | 115% | 0.008 | 13% | 109% | 0.008 | 5% | 107% |
| QC low | 0.04 | 11% | 114% | 0.04 | 10% | 99% | 0.04 | 4% | 106% |
| QC medium | 0.41 | 9% | 91% | 0.41 | 4% | 98% | 0.41 | 4% | 98% |
| QC high | 5 | 9% | 92% | 5 | 2% | 92% | 5 | 8% | 107% |
| INTRA-DAY | |||||||||
| Ivacaftor | Tezacaftor | Elexacaftor | |||||||
| Nominal value (mg/L) | CV% | Accuracy % | Nominal value (mg/L) | CV% | Accuracy % | Nominal value (mg/L) | CV% | Accuracy % | |
| LLOQ | 0.008 | 14% | 115% | 0.008 | 9% | 105% | 0.008 | 7% | 105% |
| QC low | 0.04 | 3% | 110% | 0.04 | 7% | 104% | 0.04 | 3% | 99% |
| QC medium | 0.41 | 8% | 110% | 0.41 | 5% | 99% | 0.41 | 4% | 98% |
| QC high | 5 | 9% | 91% | 5 | 2% | 106% | 5 | 3% | 108% |
| TEZ (mg/L) | ELX (mg/L) | IVA (mg/L) | |
|---|---|---|---|
| Children | 2.68 ± 0.92 | 4.39 ± 1.59 | 0.71 ± 0.20 |
| Young Adults | 2.87 ± 1.38 | 4.70 ± 2.26 | 0.84 ± 0.52 |
| Adults | 3.40 ± 1.40 | 5.68 ± 2.07 | 0.90 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigliasco, F.; Cafaro, A.; Stella, M.; Baiardi, G.; Barco, S.; Pedemonte, N.; D’Orsi, C.; Cresta, F.; Casciaro, R.; Castellani, C.; et al. Simultaneous Quantification of Ivacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis Patients’ Plasma by a Novel LC–MS/MS Method. Biomedicines 2023, 11, 628. https://doi.org/10.3390/biomedicines11020628
Pigliasco F, Cafaro A, Stella M, Baiardi G, Barco S, Pedemonte N, D’Orsi C, Cresta F, Casciaro R, Castellani C, et al. Simultaneous Quantification of Ivacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis Patients’ Plasma by a Novel LC–MS/MS Method. Biomedicines. 2023; 11(2):628. https://doi.org/10.3390/biomedicines11020628
Chicago/Turabian StylePigliasco, Federica, Alessia Cafaro, Manuela Stella, Giammarco Baiardi, Sebastiano Barco, Nicoletta Pedemonte, Claudia D’Orsi, Federico Cresta, Rosaria Casciaro, Carlo Castellani, and et al. 2023. "Simultaneous Quantification of Ivacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis Patients’ Plasma by a Novel LC–MS/MS Method" Biomedicines 11, no. 2: 628. https://doi.org/10.3390/biomedicines11020628
APA StylePigliasco, F., Cafaro, A., Stella, M., Baiardi, G., Barco, S., Pedemonte, N., D’Orsi, C., Cresta, F., Casciaro, R., Castellani, C., Calevo, M. G., Mattioli, F., & Cangemi, G. (2023). Simultaneous Quantification of Ivacaftor, Tezacaftor, and Elexacaftor in Cystic Fibrosis Patients’ Plasma by a Novel LC–MS/MS Method. Biomedicines, 11(2), 628. https://doi.org/10.3390/biomedicines11020628








