Fibromyalgia in Pregnancy: Neuro-Endocrine Fluctuations Provide Insight into Pathophysiology and Neuromodulation Treatment
Abstract
1. Introduction
2. Methods
3. Interactions between FM and Pregnancy
4. Estrogen, Progesterone, and Cortisol
5. Prolactin and Immune Mediators
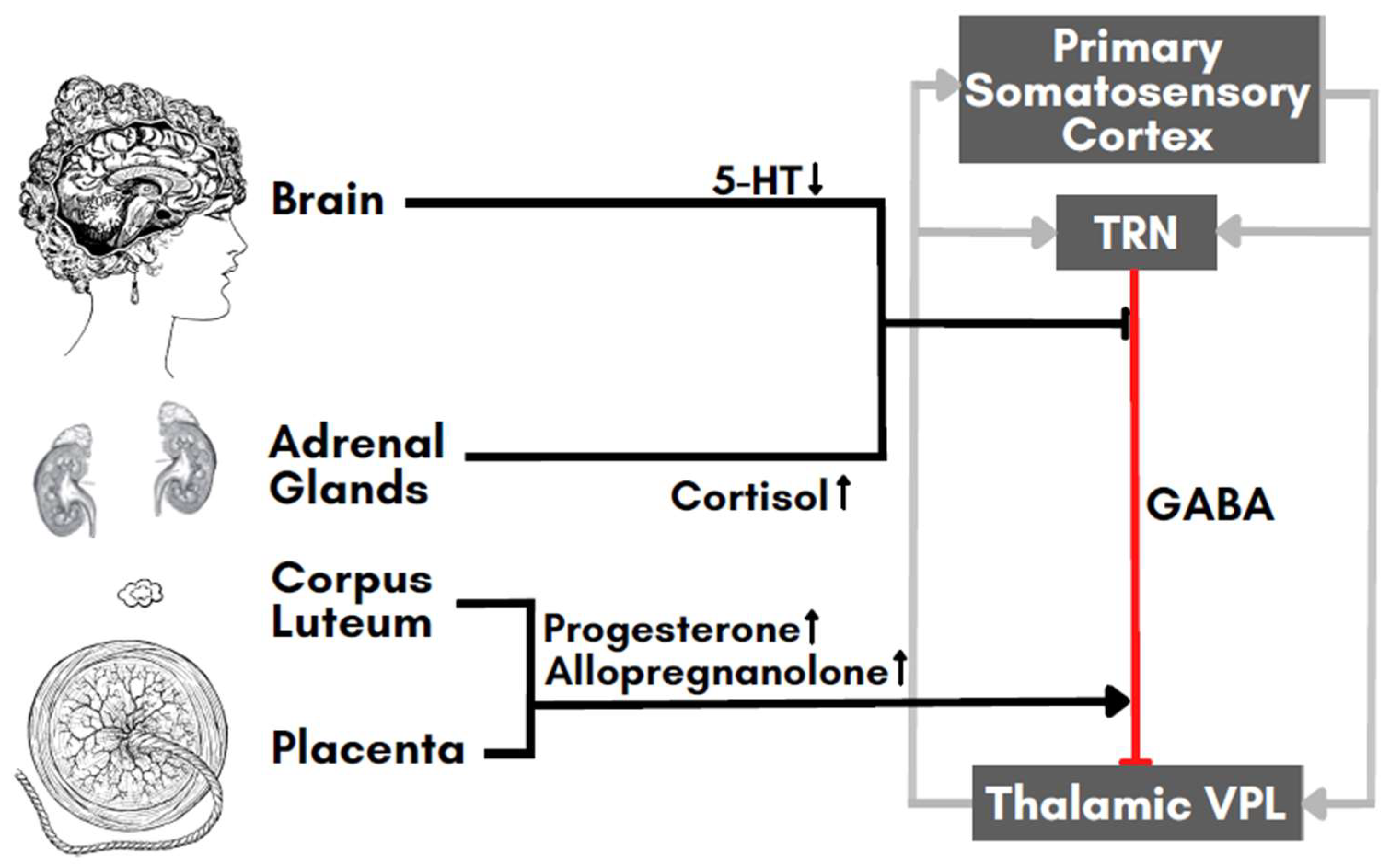
6. Neurotransmitters
7. Perspectives for a Validation of Our Hypothesis
8. Future Interventions for Neuromodulation in Pregnant FM Patients
| Shenoy et al., 2014 [123] | Sreeraj et al., 2016 [124] | Strube et al., 2016 [125] | Palm et al., 2017 [126] | Vigod et al., 2019 [112] | Kurzeck et al., 2021 [118] | |
| Study Type | Case Report | Case Report | Case Report | Uncontrolled | RCT | Open Label |
| N | 1 | 1 | 1 | 3 | 20 | 6 |
| Age | 25 | 23 | 36 | 23, 28, 32 | >18 | 23–43 |
| Dropouts | 0 | 0 | 0 | 0(1) * | 4 ** | 0(2) * |
| Diagnosis | AH | MDD | AH | MDD | MDD | MDD |
| Scale(s) | PSYRATS | HAMD, HAMA | PANSS, AHRS, CDSS, CGI, GAF | HAMD-21, BDI, TMT-A/B | MADRS | HAMD-21, BDI, CGI, TMT-A/B |
| Treatment | Add-On | Mono | Mono | Mono | Mono | Mono |
| tDCS prior to Pregnancy | Yes | No | N/A | N/A | N/A | N/A |
| Weeks in Gestation | 18 | 6 | 32 | 19–31 | 14–32 | 12–33 |
| Parameters | 2 mA, 2 × 20 min | 2 mA, 30 min | 2 mA, 2 × 30 min | 2 mA, 2 × 30 min (2 mA, 30 min) | 2 mA, 30 min | 2 mA, 2 × 30 min (2 mA, 30 min) |
| No. of Sessions | 10 | 10 | 20 | 20 (30) | 15 | 20 (30) |
| Response | Near remission | Remission | 41% improvement (CDSS) | 33.3% remission | 75% vs. 12.5% | 39.3% reduction (HAMD) 57.1% reduction (BDI) 28.6% reduction (CGI) |
| Comments | Add-on tDCS resulted in near remission of auditory hallucination. tDCS was well tolerated and no changes in autonomic function, ventilation rate, or core body temperature were observed. | tDCS was well tolerated without any adverse events. In 3 out of the 10 tDCS sessions, patients experienced transient, mild burning sensation at the target side and fleeting perception of phosphenes during the fade-in phase, which is an anticipated tDCS side effect. | No improvement in auditory hallucinations was recorded. Patients tolerated tDCS well with no reported, noticeable side effects. Fetal examination at 35th gestational week revealed no changes or abnormalities. Delivery of a healthy child occurred with no complications. | Statistically significant changes could be observed. One patient achieved remission. tDCS was well tolerated without adverse events. | No abnormalities or serious pregnancy complications were reported in either group. Percent fractions of 87.5% and 77.8% in the tDCS group and sham group, respectively, were satisfied to extremely satisfied with the treatment and viewed tDCS as an acceptable and alternative treatment option. | Significant changes were observed. tDCS was well tolerated without adverse events. In Phase 1, 33.3% achieved response in HAMD scores; 33.3% showed response and 16.7% remission in BDI scores. In Phase 2, one patient achieved remission for both HAMD and BDI. |
- Perform hormonal-related longitudinal studies in FM patients during different hormonal phases: menses, pregnancy, menopause. Progesterone, prolactin, estrogen, and testosterone should be carefully evaluated and matched with symptom fluctuations.
- Further assess the involvement of cortisol and serotonin through repetitive blood and saliva sampling.
- Assess the feasibility of tDCS in women planning a pregnancy affected by FM.
- Assess home-based, remote tDCS treatment in combination with lifestyle changes, given they have been proven to be successful at reducing the patient’s symptoms [33].
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kia, S.; Choy, E. Update on Treatment Guideline in Fibromyalgia Syndrome with Focus on Pharmacology. Biomedicines 2017, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Boomershine, C.S. Fibromyalgia: The prototypical central sensitivity syndrome. Curr. Rheumatol. Rev. 2015, 11, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Alciati, A.; Nucera, V.; Masala, I.F.; Giallanza, M.; La Corte, L.; Giorgi, V.; Sarzi-Puttini, P.; Atzeni, F. One year in review 2021: Fibromyalgia. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 130), 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.I.; Goldenberg, D.L.; Pope, H.G., Jr.; Keck, P.E., Jr.; Schlesinger, L. Comorbidity of fibromyalgia with medical and psychiatric disorders. Am. J. Med. 1992, 92, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, S.; Di Giannuario, A.; Di Giovannandrea, R.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender differences in pain and its relief. Ann. Ist. Super. Sanita 2016, 52, 184–189. [Google Scholar] [CrossRef]
- Arout, C.A.; Sofuoglu, M.; Bastian, L.A.; Rosenheck, R.A. Gender Differences in the Prevalence of Fibromyalgia and in Concomitant Medical and Psychiatric Disorders: A National Veterans Health Administration Study. J. Womens Health 2018, 27, 1035–1044. [Google Scholar] [CrossRef]
- Clauw, D.J. Fibromyalgia: An overview. Am. J. Med. 2009, 122, S3–S13. [Google Scholar] [CrossRef]
- McNally, J.D.; Matheson, D.A.; Bakowsky, V.S. The epidemiology of self-reported fibromyalgia in Canada. Chronic Dis. Can. 2006, 27, 9–16. [Google Scholar]
- McLeod, J.D. Juvenile fibromyalgia syndrome and improved recognition by pediatric primary care providers. J. Pediatr. Health Care 2014, 28, e9–e18. [Google Scholar] [CrossRef]
- Rossi, M.F.; Tumminello, A.; Marconi, M.; Gualano, M.R.; Santoro, P.E.; Malorni, W.; Moscato, U. Sex and gender differences in migraines: A narrative review. Neurol. Sci. 2022, 43, 5729–5734. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Staud, R. Cytokine and immune system abnormalities in fibromyalgia and other central sensitivity syndromes. Curr. Rheumatol. Rev. 2015, 11, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Goebel, A.; Krock, E.; Gentry, C.; Israel, M.R.; Jurczak, A.; Urbina, C.M.; Sandor, K.; Vastani, N.; Maurer, M.; Cuhadar, U.; et al. Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M. Estrogens and neuroprotection. Trends Endocrinol. Metab. 2002, 13, 229–230. [Google Scholar] [CrossRef]
- Roof, R.L.; Hall, E.D. Gender differences in acute CNS trauma and stroke: Neuroprotective effects of estrogen and progesterone. J. Neurotrauma 2000, 17, 367–388. [Google Scholar] [CrossRef]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in brain. Front. Neuroendocr. 2008, 29, 313–339. [Google Scholar] [CrossRef]
- Brinton, R.D. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: Recent insights and remaining challenges. Learn. Mem. 2001, 8, 121–133. [Google Scholar] [CrossRef]
- Wiesenfeld-Hallin, Z. Sex differences in pain perception. Gend. Med. 2005, 2, 137–145. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Sorda, G. Relationship of female sex hormones with pain perception: Focus on estrogens. Pain Manag. 2011, 1, 229–238. [Google Scholar] [CrossRef]
- Haus, E.; Smolensky, M.H. Biologic rhythms in the immune system. Chronobiol. Int. 1999, 16, 581–622. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Gregus, A.M.; Levine, I.S.; Eddinger, K.A.; Yaksh, T.L.; Buczynski, M.W. Sex differences in neuroimmune and glial mechanisms of pain. Pain 2021, 162, 2186–2200. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Meester, I.; Rivera-Silva, G.F.; Gonzalez-Salazar, F. Immune System Sex Differences May Bridge the Gap Between Sex and Gender in Fibromyalgia. Front. Neurosci. 2019, 13, 1414. [Google Scholar] [CrossRef]
- Demori, I.; Giordano, G.; Mucci, V.; Losacco, S.; Marinelli, L.; Massobrio, P.; Blanchini, F.; Burlando, B. Thalamocortical bistable switch as a theoretical model of fibromyalgia pathogenesis inferred from a literature survey. J. Comput. Neurosci. 2022, 50, 471–484. [Google Scholar] [CrossRef]
- Jones, E.G. Anatomy of cerebral cortex: Columnar input-output organization. In The Organization of the Cerebral Cortex; Schmitt, F.O., Worden, F.G., Adelmann, G., Dennis, S.G., Eds.; MIT Press: Cambridge, MA, USA, 1981; pp. 199–235. [Google Scholar]
- Li, C.; Lei, Y.; Tian, Y.; Xu, S.; Shen, X.; Wu, H.; Bao, S.; Wang, F. The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain. Mol. Pain 2019, 15, 1744806919847366. [Google Scholar] [CrossRef]
- Foerster, B.R.; Petrou, M.; Edden, R.A.; Sundgren, P.C.; Schmidt-Wilcke, T.; Lowe, S.E.; Harte, S.E.; Clauw, D.J.; Harris, R.E. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis. Rheum. 2012, 64, 579–583. [Google Scholar] [CrossRef]
- Sluka, K.A.; Clauw, D.J. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef]
- Pomares, F.B.; Roy, S.; Funck, T.; Feier, N.A.; Thiel, A.; Fitzcharles, M.A.; Schweinhardt, P. Upregulation of cortical GABAA receptor concentration in fibromyalgia. Pain 2020, 161, 74–82. [Google Scholar] [CrossRef]
- Becker, S.; Schweinhardt, P. Dysfunctional neurotransmitter systems in fibromyalgia, their role in central stress circuitry and pharmacological actions on these systems. Pain Res. Treat. 2012, 2012, 741746. [Google Scholar] [CrossRef]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef] [PubMed]
- Demori, I.; Molinari, E.; Rapallo, F.; Mucci, V.; Marinelli, L.; Losacco, S.; Burlando, B. Online Questionnaire with Fibromyalgia Patients Reveals Correlations among Type of Pain, Psychological Alterations, and Effectiveness of Non-Pharmacological Therapies. Healthcare 2022, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Sackeim, H.A.; Aaronson, S.T.; Carpenter, L.L.; Hutton, T.M.; Mina, M.; Pages, K.; Verdoliva, S.; West, W.S. Clinical outcomes in a large registry of patients with major depressive disorder treated with Transcranial Magnetic Stimulation. J. Affect. Disord. 2020, 277, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kedzior, K.K.; Azorina, V.; Reitz, S.K. More female patients and fewer stimuli per session are associated with the short-term antidepressant properties of repetitive transcranial magnetic stimulation (rTMS): A meta-analysis of 54 sham-controlled studies published between 1997-2013. Neuropsychiatr. Dis. Treat. 2014, 10, 727–756. [Google Scholar] [CrossRef]
- Zuo, X.N.; Kelly, C.; Di Martino, A.; Mennes, M.; Margulies, D.S.; Bangaru, S.; Grzadzinski, R.; Evans, A.C.; Zang, Y.F.; Castellanos, F.X.; et al. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J. Neurosci. 2010, 30, 15034–15043. [Google Scholar] [CrossRef]
- Scheinost, D.; Finn, E.S.; Tokoglu, F.; Shen, X.; Papademetris, X.; Hampson, M.; Constable, R.T. Sex differences in normal age trajectories of functional brain networks. Hum. Brain Mapp. 2015, 36, 1524–1535. [Google Scholar] [CrossRef]
- Ruigrok, A.N.; Salimi-Khorshidi, G.; Lai, M.C.; Baron-Cohen, S.; Lombardo, M.V.; Tait, R.J.; Suckling, J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014, 39, 34–50. [Google Scholar] [CrossRef]
- Cosgrove, K.P.; Mazure, C.M.; Staley, J.K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry 2007, 62, 847–855. [Google Scholar] [CrossRef]
- Luders, E.; Narr, K.L.; Thompson, P.M.; Rex, D.E.; Jancke, L.; Steinmetz, H.; Toga, A.W. Gender differences in cortical complexity. Nat. Neurosci. 2004, 7, 799–800. [Google Scholar] [CrossRef]
- Smith, M.J.; Adams, L.F.; Schmidt, P.J.; Rubinow, D.R.; Wassermann, E.M. Effects of ovarian hormones on human cortical excitability. Ann. Neurol. 2002, 51, 599–603. [Google Scholar] [CrossRef]
- Inghilleri, M.; Conte, A.; Curra, A.; Frasca, V.; Lorenzano, C.; Berardelli, A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin. Neurophysiol. 2004, 115, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Keel, J.C.; Greenberg, B.D.; Adams, L.F.; Schmidt, P.J.; Rubinow, D.A.; Wassermann, E.M. Menstrual cycle effects on cortical excitability. Neurology 1999, 53, 2069–2072. [Google Scholar] [CrossRef]
- Bunai, T.; Hirosawa, T.; Kikuchi, M.; Fukai, M.; Yokokura, M.; Ito, S.; Takata, Y.; Terada, T.; Ouchi, Y. tDCS-induced modulation of GABA concentration and dopamine release in the human brain: A combination study of magnetic resonance spectroscopy and positron emission tomography. Brain Stimul. 2021, 14, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Saa’d, S.; Many, A.; Jacob, G.; Ablin, J.N. High prevalence of fibromyalgia symptoms among healthy full-term pregnant women. Rheumatol. Int. 2013, 33, 1555–1560. [Google Scholar] [CrossRef]
- Koné, M.C.; Kambiré, N.A.; Kouakou, K.; Ahoua, Y. Fibromyalgia of Women Who Gave Birth and Pregnancy Outcome Parameters. Open J. Epidemiol. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Zioni, T.; Buskila, D.; Aricha-Tamir, B.; Wiznitzer, A.; Sheiner, E. Pregnancy outcome in patients with fibromyalgia syndrome. J. Matern Fetal Neonatal. Med. 2011, 24, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Genc, H.; Atasever, M.; Duyur Cakit, B.; Seval, M.; Koc, A. The Effects of Fibromyalgia Syndrome on Physical Function and Psychological Status of Pregnant Females. Arch. Rheumatol. 2017, 32, 129–140. [Google Scholar] [CrossRef]
- Ostensen, M.; Rugelsjoen, A.; Wigers, S.H. The effect of reproductive events and alterations of sex hormone levels on the symptoms of fibromyalgia. Scand. J. Rheumatol. 1997, 26, 355–360. [Google Scholar] [CrossRef]
- Backstrom, T.; Haage, D.; Lofgren, M.; Johansson, I.M.; Stromberg, J.; Nyberg, S.; Andreen, L.; Ossewaarde, L.; van Wingen, G.A.; Turkmen, S.; et al. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience 2011, 191, 46–54. [Google Scholar] [CrossRef]
- Alonso, C.; Loevinger, B.L.; Muller, D.; Coe, C.L. Menstrual cycle influences on pain and emotion in women with fibromyalgia. J. Psychosom. Res. 2004, 57, 451–458. [Google Scholar] [CrossRef]
- Gur, A.; Cevik, R.; Sarac, A.J.; Colpan, L.; Em, S. Hypothalamic-pituitary-gonadal axis and cortisol in young women with primary fibromyalgia: The potential roles of depression, fatigue, and sleep disturbance in the occurrence of hypocortisolism. Ann. Rheum. Dis. 2004, 63, 1504–1506. [Google Scholar] [CrossRef]
- Dunnett, A.J.; Roy, D.; Stewart, A.; McPartland, J.M. The diagnosis of fibromyalgia in women may be influenced by menstrual cycle phase. J. Bodyw. Mov. Ther. 2007, 11, 99–105. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Sadana, N.; Chen, X. Estrogen receptors in pain modulation: Cellular signaling. Biol. Sex Differ. 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.M.; Bachiocco, V.; Costantino, A.; Stefani, R.; Ceccarelli, I.; Bertaccini, A.; Meriggiola, M.C. Cross-sex hormone administration changes pain in transsexual women and men. Pain 2007, 132 (Suppl. 1), S60–S67. [Google Scholar] [CrossRef] [PubMed]
- Vacca, V.; Marinelli, S.; Pieroni, L.; Urbani, A.; Luvisetto, S.; Pavone, F. 17beta-estradiol counteracts neuropathic pain: A behavioural, immunohistochemical, and proteomic investigation on sex-related differences in mice. Sci. Rep. 2016, 6, 18980. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.N.; McFarland, K.; Olsson, R.; Burstein, E.S. Estrogen Receptor Beta Selective Agonists as Agents to Treat Chemotherapeutic-Induced Neuropathic Pain. ACS Chem. Neurosci. 2016, 7, 1180–1187. [Google Scholar] [CrossRef]
- Schertzinger, M.; Wesson-Sides, K.; Parkitny, L.; Younger, J. Daily Fluctuations of Progesterone and Testosterone Are Associated With Fibromyalgia Pain Severity. J. Pain 2018, 19, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.J.; Cooper, M.A.; Simmons, R.D.; Weir, C.J.; Belelli, D. Neurosteroids: Endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S48–S58. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obs. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef]
- Haldeman-Englert, C.; Turley, R.; Novick, T. Health Encyclopedia. Available online: https://www.urmc.rochester.edu/encyclopedia/content.aspx?ContentTypeID=167&ContentID=progesterone (accessed on 15 December 2022).
- Solano, M.E.; Arck, P.C. Steroids, Pregnancy and Fetal Development. Front. Immunol. 2019, 10, 3017. [Google Scholar] [CrossRef]
- Carr, B.R.; Parker, C.R., Jr.; Madden, J.D.; MacDonald, P.C.; Porter, J.C. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am. J. Obs. Gynecol. 1981, 139, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.; Perani, C.V.; Nixon, M.; Constancia, M.; Sandovici, I.; Zazara, D.E.; Leone, G.; Zhang, M.Z.; Arck, P.C.; Solano, M.E. Sex-specific regulation of stress-induced fetal glucocorticoid surge by the mouse placenta. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E109–E120. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.J. Neuroactive steroids and stress axis regulation: Pregnancy and beyond. J. Steroid. Biochem. Mol. Biol. 2016, 160, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.J.; McKay, A.J.; Ochedalski, T.; Piastowska, A.; Rebas, E.; Lachowicz, A.; Russell, J.A. Central opioid inhibition of neuroendocrine stress responses in pregnancy in the rat is induced by the neurosteroid allopregnanolone. J. Neurosci. 2009, 29, 6449–6460. [Google Scholar] [CrossRef]
- Groeneweg, F.L.; Karst, H.; de Kloet, E.R.; Joels, M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011, 209, 153–167. [Google Scholar] [CrossRef]
- Shen, H.; Gong, Q.H.; Aoki, C.; Yuan, M.; Ruderman, Y.; Dattilo, M.; Williams, K.; Smith, S.S. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat. Neurosci. 2007, 10, 469–477. [Google Scholar] [CrossRef]
- Fries, E.; Hesse, J.; Hellhammer, J.; Hellhammer, D.H. A new view on hypocortisolism. Psychoneuroendocrinology 2005, 30, 1010–1016. [Google Scholar] [CrossRef]
- Griep, E.N.; Boersma, J.W.; Lentjes, E.G.; Prins, A.P.; van der Korst, J.K.; de Kloet, E.R. Function of the hypothalamic-pituitary-adrenal axis in patients with fibromyalgia and low back pain. J. Rheumatol. 1998, 25, 1374–1381. [Google Scholar]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef]
- Barnea, E.R.; Fares, F.; Shahar, K. Stimulatory effect of prolactin on human placental progesterone secretion at term in vitro: Possible inhibitory effect on oestradiol secretion. Placenta 1989, 10, 37–43. [Google Scholar] [CrossRef]
- Levine, S.; Muneyyirci-Delale, O. Stress-Induced Hyperprolactinemia: Pathophysiology and Clinical Approach. Obs. Gynecol. Int. 2018, 2018, 9253083. [Google Scholar] [CrossRef]
- Buskila, D.; Sukenik, S.; Shoenfeld, Y. The possible role of prolactin in autoimmunity. Am. J. Reprod. Immunol. 1991, 26, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Swart, J.M.; Grattan, D.R.; Ladyman, S.R.; Brown, R.S.E. Changes in maternal motivation across reproductive states in mice: A role for prolactin receptor activation on GABA neurons. Horm. Behav. 2021, 135, 105041. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, V.; Apud, J.A.; Gudelsky, G.A.; Cocchi, D.; Masotto, C.; Casanueva, F.; Racagni, G.; Muller, E.E. Prolactin in cerebrospinal fluid increases the synthesis and release of hypothalamic gamma-aminobutyric acid. J. Endocrinol. 1985, 106, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef]
- Maddipati, K.R. Non-inflammatory Physiology of “Inflammatory” Mediators—Unalamation, a New Paradigm. Front. Immunol. 2020, 11, 580117. [Google Scholar] [CrossRef]
- Jarmund, A.H.; Giskeodegard, G.F.; Ryssdal, M.; Steinkjer, B.; Stokkeland, L.M.T.; Madssen, T.S.; Stafne, S.N.; Stridsklev, S.; Moholdt, T.; Heimstad, R.; et al. Cytokine Patterns in Maternal Serum From First Trimester to Term and Beyond. Front. Immunol. 2021, 12, 752660. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Stelzer, I.A.; Arck, P.C. Immunity and the Endocrine System. Encycl. Immunobiol. 2016, 5, 73–85. [Google Scholar]
- Uceyler, N.; Hauser, W.; Sommer, C. Systematic review with meta-analysis: Cytokines in fibromyalgia syndrome. BMC Musculoskelet. Disord. 2011, 12, 245. [Google Scholar] [CrossRef]
- Al-Nimer, M.S.M.; Mohammad, T.A.M.; Alsakeni, R.A. Serum levels of serotonin as a biomarker of newly diagnosed fibromyalgia in women: Its relation to the platelet indices. J. Res. Med. Sci. 2018, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Sci. World J. 2012, 2012, 640389. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, P.G.; Balden, E.; Tomkins, G.; Santoro, J.; Kroenke, K.; Jackson, J.L. Treatment of fibromyalgia with antidepressants: A meta-analysis. J. Gen. Intern. Med. 2000, 15, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.J. Neurobiological mechanisms of acupuncture for some common illnesses: A clinician’s perspective. J. Acupunct. Meridian Stud. 2014, 7, 105–114. [Google Scholar] [CrossRef]
- Zhao, Z.Q. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 2008, 85, 355–375. [Google Scholar] [CrossRef]
- Goldenberg, D.L.; Clauw, D.J.; Palmer, R.E.; Clair, A.G. Opioid Use in Fibromyalgia: A Cautionary Tale. Mayo Clin. Proc. 2016, 91, 640–648. [Google Scholar] [CrossRef]
- Munsch, T.; Freichel, M.; Flockerzi, V.; Pape, H.C. Contribution of transient receptor potential channels to the control of GABA release from dendrites. Proc. Natl. Acad. Sci. USA 2003, 100, 16065–16070. [Google Scholar] [CrossRef]
- Eaton, S.A.; Salt, T.E. Modulatory effects of serotonin on excitatory amino acid responses and sensory synaptic transmission in the ventrobasal thalamus. Neuroscience 1989, 33, 285–292. [Google Scholar] [CrossRef]
- Smolen, A.; Smolen, T.N.; Han, P.C. Alterations in regional brain GABA concentration and turnover during pregnancy. Pharm. Biochem. Behav. 1993, 44, 63–69. [Google Scholar] [CrossRef]
- Maguire, J.; Mody, I. GABA(A)R plasticity during pregnancy: Relevance to postpartum depression. Neuron 2008, 59, 207–213. [Google Scholar] [CrossRef]
- Atasever, M.; Kalem, M.N.; Sonmez, C.; Seval, M.M.; Yuce, T.; Aker, S.S.; Koc, A.; Genc, H. Lower serotonin level and higher rate of fibromyalgia syndrome with advancing pregnancy. J. Matern. Fetal Neonatal Med. 2017, 30, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, F.; Valensise, H.; Sacco, M.; Allegri, M. Chronic pain management in pregnancy and lactation. Minerva Anestesiol 2014, 80, 211–224. [Google Scholar] [PubMed]
- Concas, A.; Mostallino, M.C.; Porcu, P.; Follesa, P.; Barbaccia, M.L.; Trabucchi, M.; Purdy, R.H.; Grisenti, P.; Biggio, G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc. Natl. Acad. Sci. USA 1998, 95, 13284–13289. [Google Scholar] [CrossRef] [PubMed]
- Follesa, P.; Serra, M.; Cagetti, E.; Pisu, M.G.; Porta, S.; Floris, S.; Massa, F.; Sanna, E.; Biggio, G. Allopregnanolone synthesis in cerebellar granule cells: Roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol. Pharm. 2000, 57, 1262–1270. [Google Scholar]
- Walker, K.A.; Ficek, B.N.; Westbrook, R. Understanding the Role of Systemic Inflammation in Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 3340–3342. [Google Scholar] [CrossRef]
- Rossi, S.; Studer, V.; Motta, C.; De Chiara, V.; Barbieri, F.; Bernardi, G.; Centonze, D. Inflammation inhibits GABA transmission in multiple sclerosis. Mult. Scler. 2012, 18, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA imbalance following traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Saitow, F.; Murano, M.; Suzuki, H. Modulatory effects of serotonin on GABAergic synaptic transmission and membrane properties in the deep cerebellar nuclei. J. Neurophysiol. 2009, 101, 1361–1374. [Google Scholar] [CrossRef]
- Rudroff, T.; Workman, C.D.; Fietsam, A.C.; Kamholz, J. Response Variability in Transcranial Direct Current Stimulation: Why Sex Matters. Front. Psychiatry 2020, 11, 585. [Google Scholar] [CrossRef]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef]
- Kim, S.C.; Landon, J.E.; Solomon, D.H. Clinical characteristics and medication uses among fibromyalgia patients newly prescribed amitriptyline, duloxetine, gabapentin, or pregabalin. Arthritis Care Res. 2013, 65, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Fitelson, E.; Kim, S.; Baker, A.S.; Leight, K. Treatment of postpartum depression: Clinical, psychological and pharmacological options. Int. J. Womens Health 2010, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gentile, S.; Fusco, M.L. Managing fibromyalgia syndrome in pregnancy no bridges between USA and EU. Arch. Womens Ment. Health 2019, 22, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.F.; Nelson, S.B. Synaptic plasticity: Taming the beast. Nat. Neurosci. 2000, 3, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Carcea, I.; Froemke, R.C. Cortical plasticity, excitatory-inhibitory balance, and sensory perception. Prog. Brain Res. 2013, 207, 65–90. [Google Scholar] [CrossRef]
- Hendry, S.H.; Schwark, H.D.; Jones, E.G.; Yan, J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J. Neurosci. 1987, 7, 1503–1519. [Google Scholar] [CrossRef]
- Wang, M. Neurosteroids and GABA-A Receptor Function. Front. Endocrinol. 2011, 2, 44. [Google Scholar] [CrossRef]
- Chaieb, L.; Antal, A.; Paulus, W. Gender-specific modulation of short-term neuroplasticity in the visual cortex induced by transcranial direct current stimulation. Vis. Neurosci. 2008, 25, 77–81. [Google Scholar] [CrossRef]
- Dedoncker, J.; Brunoni, A.R.; Baeken, C.; Vanderhasselt, M.A. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimul. 2016, 9, 501–517. [Google Scholar] [CrossRef]
- Vigod, S.N.; Murphy, K.E.; Dennis, C.L.; Oberlander, T.F.; Ray, J.G.; Daskalakis, Z.J.; Blumberger, D.M. Transcranial direct current stimulation (tDCS) for depression in pregnancy: A pilot randomized controlled trial. Brain Stimul. 2019, 12, 1475–1483. [Google Scholar] [CrossRef]
- Raimundo, R.J.S.; Uribe, C.E.; Brasil-Neto, J.P. Lack of clinically detectable acute changes on autonomic or thermoregulatory functions in healthy subjects after transcranial direct current stimulation (tDCS). Brain Stimul. 2012, 5, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Gimenes, R.; Valle, A.C.; Ferreira, M.J.; Rocha, R.R.; Natalle, L.; Bravo, R.; Rigonatti, S.P.; Freedman, S.D.; Nitsche, M.A.; et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006, 54, 3988–3998. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Kumpf, U.; Behler, N.; Wulf, L.; Kirsch, B.; Worsching, J.; Keeser, D.; Hasan, A.; Padberg, F. Home Use, Remotely Supervised, and Remotely Controlled Transcranial Direct Current Stimulation: A Systematic Review of the Available Evidence. Neuromodulation 2018, 21, 323–333. [Google Scholar] [CrossRef]
- Merzagora, A.C.; Foffani, G.; Panyavin, I.; Mordillo-Mateos, L.; Aguilar, J.; Onaral, B.; Oliviero, A. Prefrontal hemodynamic changes produced by anodal direct current stimulation. Neuroimage 2010, 49, 2304–2310. [Google Scholar] [CrossRef]
- Kurzeck, A.K.; Dechantsreiter, E.; Wilkening, A.; Kumpf, U.; Nenov-Matt, T.; Padberg, F.; Palm, U. Transcranial Direct Current Stimulation (tDCS) for Depression during Pregnancy: Results from an Open-Label Pilot Study. Brain Sci. 2021, 11, 947. [Google Scholar] [CrossRef]
- Konstantinou, P.; Kassianos, A.P.; Georgiou, G.; Panayides, A.; Papageorgiou, A.; Almas, I.; Wozniak, G.; Karekla, M. Barriers, facilitators, and interventions for medication adherence across chronic conditions with the highest non-adherence rates: A scoping review with recommendations for intervention development. Transl. Behav. Med. 2020, 10, 1390–1398. [Google Scholar] [CrossRef]
- Kurzeck, A.K.; Kirsch, B.; Weidinger, E.; Padberg, F.; Palm, U. Transcranial Direct Current Stimulation (tDCS) for Depression during Pregnancy: Scientific Evidence and What Is Being Said in the Media-A Systematic Review. Brain Sci. 2018, 8, 155. [Google Scholar] [CrossRef]
- Braz Ade, S.; de Paula, A.P.; Diniz Mde, F.; de Almeida, R.N. Non-pharmacological therapy and complementary and alternative medicine in fibromyalgia. Rev. Bras. Reumatol. 2011, 51, 269–282. [Google Scholar]
- Petzinger, G.M.; Fisher, B.E.; McEwen, S.; Beeler, J.A.; Walsh, J.P.; Jakowec, M.W. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013, 12, 716–726. [Google Scholar] [CrossRef]
- Shenoy, S.; Bose, A.; Chhabra, H.; Dinakaran, D.; Agarwal, S.M.; Shivakumar, V.; Narayanaswamy, J.C.; Sivakumar, P.T.; Venkatasubramanian, G. Transcranial direct current stimulation (tDCS) for auditory verbal hallucinations in schizophrenia during pregnancy: A case report. Brain Stimul. 2015, 8, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Sreeraj, V.S.; Bose, A.; Shanbhag, V.; Narayanaswamy, J.C.; Venkatasubramanian, G.; Benegal, V. Monotherapy With tDCS for Treatment of Depressive Episode During Pregnancy: A Case Report. Brain Stimul. 2016, 9, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Strube, W.; Kirsch, B.; Padberg, F.; Hasan, A.; Palm, U. Transcranial Direct Current Stimulation as Monotherapy for the Treatment of Auditory Hallucinations During Pregnancy: A Case Report. J. Clin. Psychopharmacol. 2016, 36, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Kirsch, B.; Leitner, B.; Popovic, D.; Padberg, F. P017 Transcranial direct current stimulation (tDCS) for the treatment of depression during pregnancy: A pilot study. Clin. Neurophysiol. 2017, 128, e17–e18. [Google Scholar] [CrossRef]
- Donahue, M.J.; Near, J.; Blicher, J.U.; Jezzard, P. Baseline GABA concentration and fMRI response. Neuroimage 2010, 53, 392–398. [Google Scholar] [CrossRef]
- Kiemes, A.; Davies, C.; Kempton, M.J.; Lukow, P.B.; Bennallick, C.; Stone, J.M.; Modinos, G. GABA, Glutamate and Neural Activity: A Systematic Review With Meta-Analysis of Multimodal (1)H-MRS-fMRI Studies. Front. Psychiatry 2021, 12, 644315. [Google Scholar] [CrossRef]
| Pregnancy Trimesters | ||||
|---|---|---|---|---|
| Hormone | Menstrual Cycle (min and max) | First | Second | Third |
| Progesterone (ng/mL) | 2–25 | 8–48 | 32–80 | 99–342 |
| 17β-estradiol (pg/mL) | 30–400 | 188–2497 | 1278–7192 | 6137–3460 |
| Prolactin (ng/mL) | < 20 | 36–213 | 110–330 | 137–372 |
| Cortisol (µg/dL) | 10–20 (CAR) | 7–19 | 10–42 | 12–50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucci, V.; Demori, I.; Browne, C.J.; Deblieck, C.; Burlando, B. Fibromyalgia in Pregnancy: Neuro-Endocrine Fluctuations Provide Insight into Pathophysiology and Neuromodulation Treatment. Biomedicines 2023, 11, 615. https://doi.org/10.3390/biomedicines11020615
Mucci V, Demori I, Browne CJ, Deblieck C, Burlando B. Fibromyalgia in Pregnancy: Neuro-Endocrine Fluctuations Provide Insight into Pathophysiology and Neuromodulation Treatment. Biomedicines. 2023; 11(2):615. https://doi.org/10.3390/biomedicines11020615
Chicago/Turabian StyleMucci, Viviana, Ilaria Demori, Cherylea J. Browne, Choi Deblieck, and Bruno Burlando. 2023. "Fibromyalgia in Pregnancy: Neuro-Endocrine Fluctuations Provide Insight into Pathophysiology and Neuromodulation Treatment" Biomedicines 11, no. 2: 615. https://doi.org/10.3390/biomedicines11020615
APA StyleMucci, V., Demori, I., Browne, C. J., Deblieck, C., & Burlando, B. (2023). Fibromyalgia in Pregnancy: Neuro-Endocrine Fluctuations Provide Insight into Pathophysiology and Neuromodulation Treatment. Biomedicines, 11(2), 615. https://doi.org/10.3390/biomedicines11020615







