No Association between Gastrointestinal Rebleeding and DOAC Therapy Resumption: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Data Collection
2.3. Risk of Bias Assessment
2.4. Data Synthesis and Statistical Analysis
2.5. Quality of Evidence
3. Results
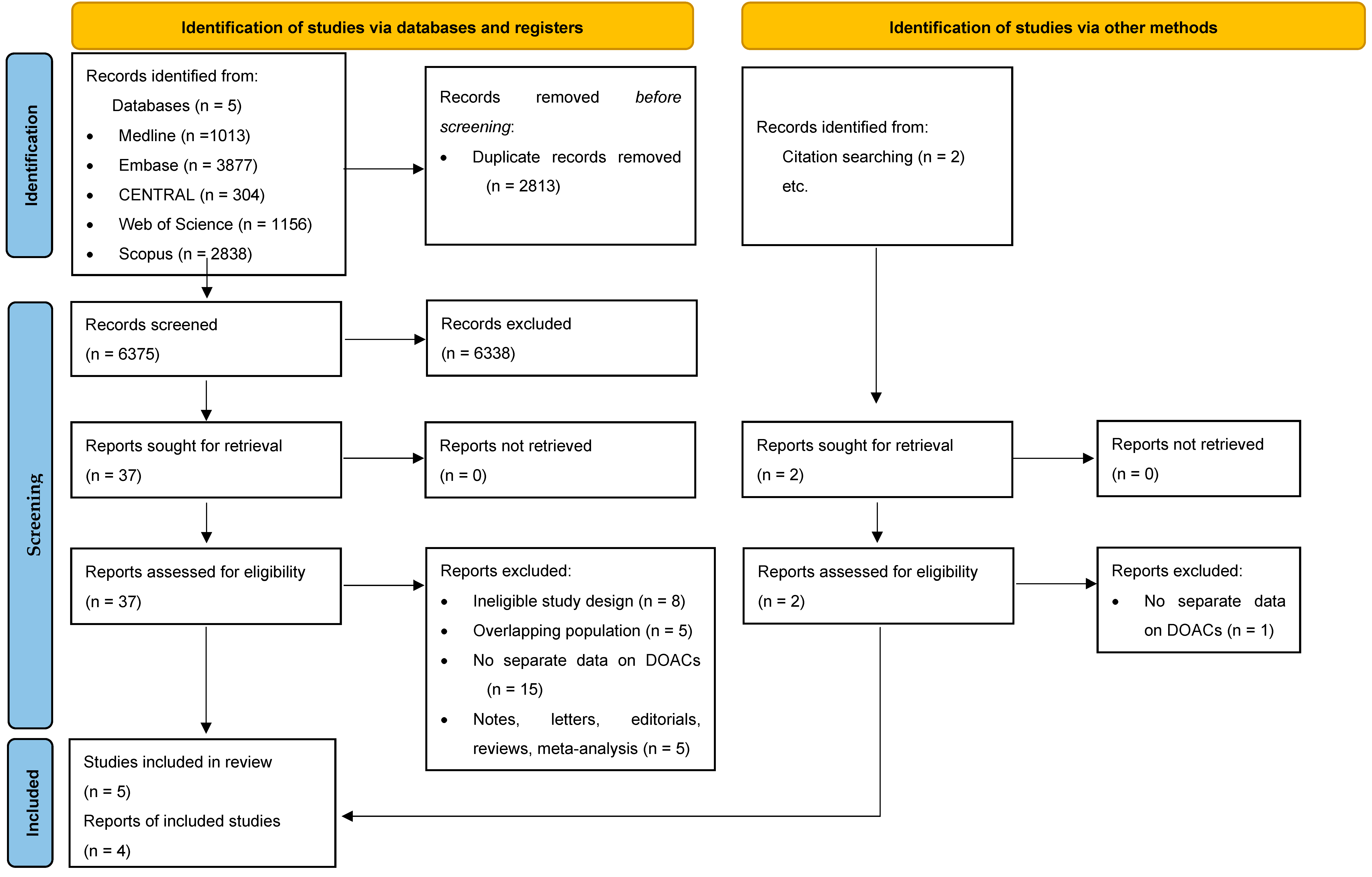
3.1. Search and Selection
3.2. Basic Characteristics of Included Studies
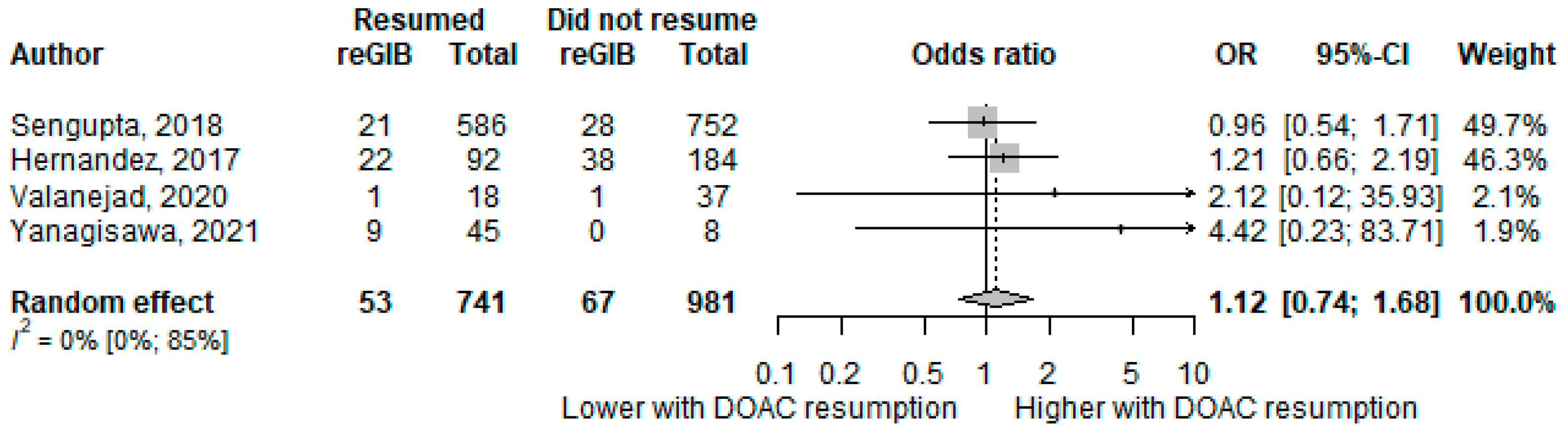
3.3. Gastrointestinal Rebleeding
3.4. Thromboembolic Event
3.5. All-Cause Mortality
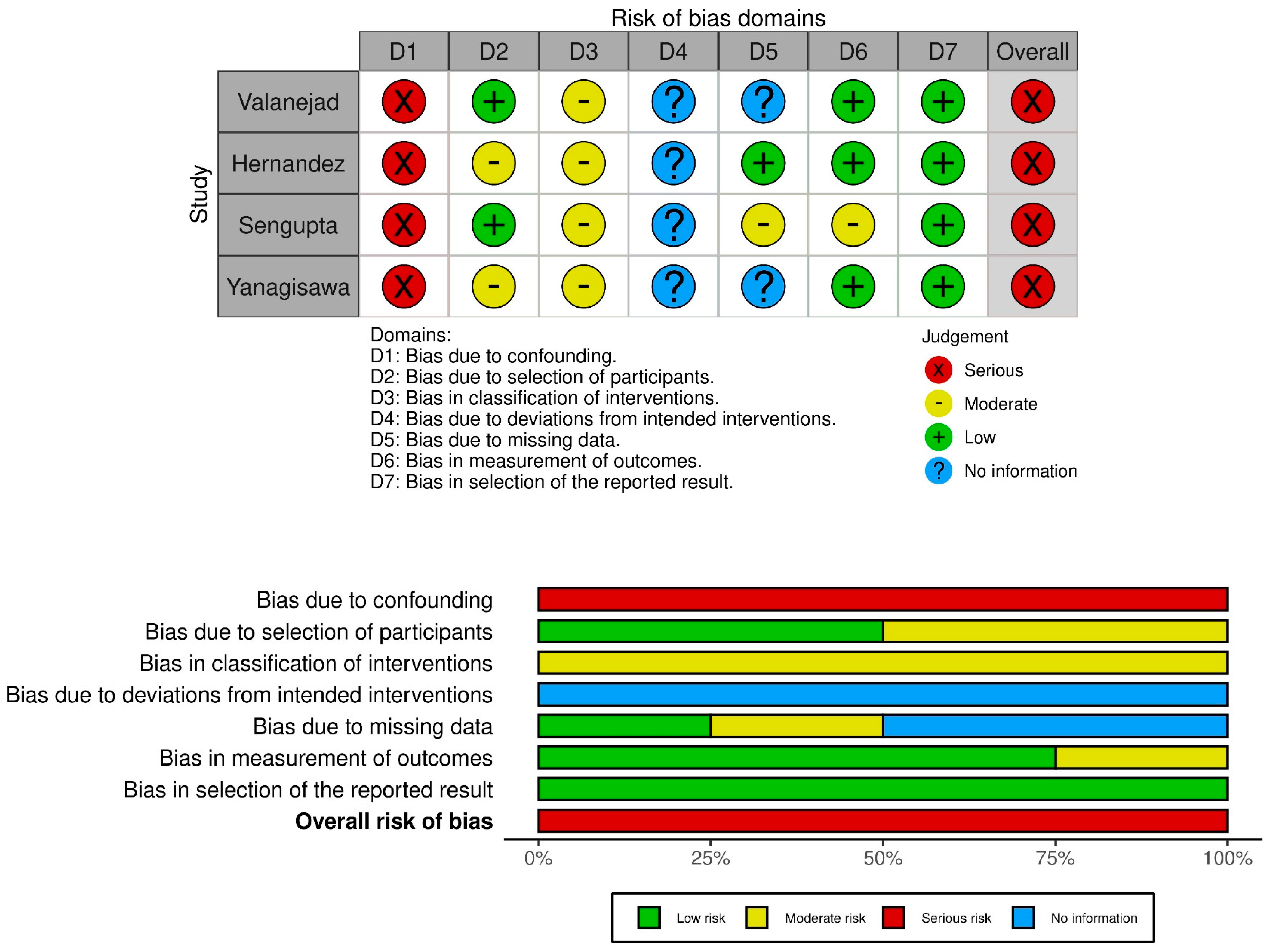
3.6. Risk of Bias Assessment
3.7. Quality of Evidence
4. Discussion
4.1. Recurrent Gastrointestinal Bleeding
4.2. Thromboembolic Complications
4.3. Mortality
4.4. General Considerations
4.5. Strengths and Limitations
4.6. Implications for Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval
References
- Barnes, G.D.; Lucas, E.; Alexander, G.C.; Goldberger, Z.D. National Trends in Ambulatory Oral Anticoagulant Use. Am. J. Med. 2015, 128, 1300–1305.e2. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.H.; Van Hove, M.; Leng, G. Trends in anticoagulant prescribing: A review of local policies in English primary care. BMC Health Serv. Res. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Alexander, G.C.; Nazarian, S.; Segal, J.B.; Wu, A.W. Trends and Variation in Oral Anticoagulant Choice in Patients with Atrial Fibrillation, 2010–2017. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Morrow, D.A.; Murphy, S.A.; Kuder, J.F.; Deenadayalu, N.; Jarolim, P.; Betcher, J.; Shi, M.; et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: An analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 2015, 385, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.L.; Hankey, G.J.; Wojdyla, D.M.; Piccini, J.P.; Lokhnygina, Y.; Patel, M.R.; Breithardt, G.; Singer, D.E.; Becker, R.C.; Hacke, W.; et al. Efficacy and Safety of Rivaroxaban Compared with Warfarin Among Elderly Patients with Nonvalvular Atrial Fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014, 130, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Ave-zum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Wallentin, L.; Ezekowitz, M.D.; Eikelboom, J.; Oldgren, J.; Reilly, P.A.; Brueckmann, M.; Pogue, J.; Alings, M.; Amerena, J.V.; et al. The Long-Term Multicenter Observational Study of Dabigatran Treatment in Patients with Atrial Fibrillation (RELY-ABLE) Study. Circulation 2013, 128, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Noseworthy, P.A.; Yao, X.; Sangaralingham, L.R.; Shah, N.D. Gastrointestinal Safety of Direct Oral Anticoagulants: A Large Population-Based Study. Gastroenterology 2017, 152, 1014–1022.e1. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Dorreen, A.; Martel, M.; Huynh, T.; Barkun, A.N. Risk of Gastrointestinal Bleeding in Patients Taking Non–Vitamin K Antagonist Oral Anticoagulants: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1674–1683.e3. [Google Scholar] [CrossRef]
- Radadiya, D.; Devani, K.; Brahmbhatt, B.; Reddy, C. Major gastrointestinal bleeding risk with direct oral anticoagulants: Does type and dose matter?—A systematic review and network meta-analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, e50–e58. [Google Scholar] [CrossRef] [PubMed]
- Tapaskar, N.; Pang, A.; Werner, D.A.; Sengupta, N. Resuming Anticoagulation Following Hospitalization for Gastrointestinal Bleeding Is Associated with Reduced Thromboembolic Events and Improved Mortality: Results from a Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2020, 66, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Little, D.; Chai-Adisaksopha, C.; Hillis, C.; Witt, D.; Monreal, M.; Crowther, M.; Siegal, D. Resumption of anticoagulant therapy after anticoagulant-related gastrointestinal bleeding: A systematic review and meta-analysis. Thromb. Res. 2019, 175, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Veitch, A.M.; Radaelli, F.; Alikhan, R.; Dumonceau, J.-M.; Eaton, D.; Jerrome, J.; Lester, W.; Nylander, D.; Thoufeeq, M.; Vanbiervliet, G.; et al. Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update. Endoscopy 2021, 53, 947–969. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Chiu, P.; Chan, F.K.L.; Lau, J.Y.; Goh, K.-L.; Ho, L.H.; Jung, H.-Y.; Sollano, J.D.; Gotoda, T.; Reddy, N.; et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: An update 2018. Gut 2018, 67, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, G.F.; Mahaffey, K.W.; Cuker, A.; Dobesh, P.P.; Doherty, J.U.; Eikelboom, J.W.; Florido, R.; Gluckman, T.J.; Hucker, W.J.; Mehran, R.; et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants. J. Am. Coll. Cardiol. 2020, 76, 594–622. [Google Scholar] [CrossRef]
- Halvorsen, S.; Storey, R.F.; Rocca, B.; Sibbing, D.; Berg, J.T.; Grove, E.L.; Weiss, T.W.; Collet, J.-P.; Andreotti, F.; Gulba, D.C.; et al. Management of antithrombotic therapy after bleeding in patients with coronary artery disease and/or atrial fibrillation: Expert consensus paper of the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J. 2016, 38, 1455–1462. [Google Scholar] [CrossRef]
- Candeloro, M.; van Es, N.; Cantor, N.; Schulman, S.; Carrier, M.; Ageno, W.; Aibar, J.; Donadini, M.P.; Bavalia, R.; Arsenault, M.; et al. Recurrent bleeding and thrombotic events after resumption of oral anticoagulants following gastrointestinal bleeding: Communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J. Thromb. Haemost. 2021, 19, 2618–2628. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.; Chandler, J.; Welch, V.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N.; Haenszel, W. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. Gynecol. Oncol. 1959, 22, 719–748. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2015, 7, 55–79. [Google Scholar] [CrossRef]
- Paule, R.; Mandel, J. Consensus Values and Weighting Factors. J. Res. Natl. Bur. Stand. 1982, 87, 377. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.1.1; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 9 January 2023).
- Schwarzer, G. Meta: General Package for Meta-Analysis, Version 5.2.0; Springer: Berlin/Heidelberg, Germany, 2022; Available online: https://github.com/guido-s/meta (accessed on 9 January 2023).
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3; (updated February 2022); Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 9 January 2023).
- GRADEPro Guideline Development Tool. Available online: www.gradepro.org (accessed on 7 January 2023).
- Dowd, J.; Sengupta, N. 341 Antithrombotic Cessation Following Hospitalization for Diverticular Hemorrhage Is Associated with an Increased Risk of Follow Up Ischemic and Thromboembolic Events. Gastroenterology 2020, 158, S-59–S-60. [Google Scholar] [CrossRef]
- Sengupta, N.; Jones, B.A. Risk Factors for Rebleeding and Thromboembolism after Hospitalization for Gastrointestinal Bleeding in Patients on Novel Oral Anticoagulants. Gastroenterology 2017, 152, S112. [Google Scholar] [CrossRef]
- Tapaskar, N.; Ham, S.A.; Micic, D.; Sengupta, N. Restarting Warfarin vs Direct Oral Anticoagulants After Major Gastrointestinal Bleeding and Associated Outcomes in Atrial Fibrillation: A Cohort Study. Clin. Gastroenterol. Hepatol. 2020, 20, 381–389.e9. [Google Scholar] [CrossRef] [PubMed]
- Tapaskar, N.; Pang, A.; Sengupta, N. 578 Resuming Anticoagulation Following Hospitalization for Gastrointestinal Bleeding Is Associated with Reduced Thromboembolic Events and Improved Mortality: Results from a Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, S331–S332. [Google Scholar] [CrossRef]
- Nigam, N.; Patel, P.; Sengupta, N. Mortality benefit in resuming novel oral anticoagulants after hospitalization for gastrointestinal bleeding. Am. J. Gastroenterol. 2016, 111, S403–S404. [Google Scholar] [CrossRef]
- Rajan, D.; Bonde, A.N.; Olesen, J.B.; Lamberts, M.; Lip, G.Y.H.; Pedersen, C.T.; Gislason, G.; Staerk, L. P1256Resumption of anticoagulant treatment in patients with atrial fibrillation following gastrointestinal bleeding: A nationwide cohort study. Eur. Heart J. 2019, 40, ehz748.0214. [Google Scholar] [CrossRef]
- Sengupta, N.; Marshall, A.L.; Jones, B.A.; Ham, S.; Tapper, E.B. Rebleeding vs Thromboembolism After Hospitalization for Gastrointestinal Bleeding in Patients on Direct Oral Anticoagulants. Clin. Gastroenterol. Hepatol. 2018, 16, 1893–1900.e2. [Google Scholar] [CrossRef]
- Valanejad, S.M.; Davis, K.A.; Nisly, S.A. Outcomes Associated with Resuming Direct Oral Anticoagulant Therapy Following Admission for a Gastrointestinal Bleed. Ann. Pharmacother. 2020, 54, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Abe, K.; Amano, H.; Komatsuda, S.; Honda, T.; Manabe, D.; Yamamoto, H.; Kozuma, K.; Kodashima, S.; Asaoka, Y.; et al. Thrombotic events and rebleeding after hemorrhage in patients taking direct oral anticoagulants for non-valvular atrial fibrillation. PLoS ONE 2021, 16, e0260585. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Zhang, Y.; Brooks, M.; Chin, P.K.; Saba, S. Anticoagulation Use and Clinical Outcomes After Major Bleeding on Dabigatran or Warfarin in Atrial Fibrillation. Stroke 2017, 48, 159–166. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S.; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A consensus report from the bleeding academic research consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Qureshi, W.; Mittal, C.; Patsias, I.; Garikapati, K.; Kuchipudi, A.; Cheema, G.; Elbatta, M.; Alirhayim, Z.; Khalid, F. Restarting Anticoagulation and Outcomes After Major Gastrointestinal Bleeding in Atrial Fibrillation. Am. J. Cardiol. 2014, 113, 662–668. [Google Scholar] [CrossRef]
- Witt, D.M.; Delate, T.; Garcia, D.A.; Clark, N.P.; Hylek, E.M.; Ageno, W.; Dentali, F.; Crowther, M.A. Risk of Thromboembolism, Recurrent Hemorrhage, and Death After Warfarin Therapy Interruption for Gastrointestinal Tract Bleeding. Arch. Intern. Med. 2012, 172, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Wallvik, N.; Eriksson, J.; Hoijer, J.; Bottai, M.; Holmstrom, M.; Schulman, S. Optimal timing of vitamin K antagonist resumption after upper gastrointestinal bleeding. A risk modelling analysis. Thromb. Haemost. 2017, 117, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Siau, K.; Hannah, J.L.; Hodson, J.; Widlak, M.; Bhala, N.; Iqbal, T.H. Stopping antithrombotic therapy after acute upper gastrointestinal bleeding is associated with reduced survival. Postgrad. Med. J. 2018, 94, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Pannach, S.; Goetze, J.; Marten, S.; Schreier, T.; Tittl, L.; Beyer-Westendorf, J. Management and outcome of gastrointestinal bleeding in patients taking oral anticoagulants or antiplatelet drugs. J. Gastroenterol. 2017, 52, 1211–1220. [Google Scholar] [CrossRef]
- Turcato, G.; Bonora, A.; Zorzi, E.; Zaboli, A.; Zannoni, M.; Ricci, G.; Pfeifer, N.; Maccagnani, A.; Tenci, A. Thirty-day mortality in atrial fibrillation patients with gastrointestinal bleeding in the emergency department: Differences between direct oral anticoagulant and warfarin users. Intern. Emerg. Med. 2019, 15, 311–318. [Google Scholar] [CrossRef]
- Chai-Adisaksopha, C.; Hillis, C.; Isayama, T.; Lim, W.; Iorio, A.; Crowther, M. Mortality outcomes in patients receiving direct oral anticoagulants: A systematic review and meta-analysis of randomized controlled trials. J. Thromb. Haemost. 2015, 13, 2012–2020. [Google Scholar] [CrossRef]
- Rücker, V.; Heuschmann, P.U.; O’Flaherty, M.; Weingärtner, M.; Hess, M.; Sedlak, C.; Schwab, S.; Kolominsky-Rabas, P.L. Twenty-Year Time Trends in Long-Term Case-Fatality and Recurrence Rates After Ischemic Stroke Stratified by Etiology. Stroke 2020, 51, 2778–2785. [Google Scholar] [CrossRef]
- Yamaguchi, D.; Tominaga, N.; Miyahara, K.; Tsuruoka, N.; Sakata, Y.; Takeuchi, Y.; Matsunaga, T.; Hidaka, H.; Akutagawa, T.; Noda, T.; et al. Safety and Efficacy of the Noncessation Method of Antithrombotic Agents after Emergency Endoscopic Hemostasis in Patients with Nonvariceal Upper Gastrointestinal Bleeding: A Multicenter Pilot Study. Can. J. Gastroenterol. Hepatol. 2021, 2021, 6672440. [Google Scholar] [CrossRef]
- National Library of Medicine. Non-Warfarin Oral AntiCoagulant Resumption after Gastrointestinal Bleeding in Atrial Fibrillation Patients (NOAC-GAP). Available online: https://clinicaltrials.gov/ct2/show/NCT03785080 (accessed on 7 January 2023).
| Study | Design | Anticoagulant | Indication | Number of Patients | Age (Median, IQR) | Female (%) | Major GIB (%) | Intervention | Outcomes | Follow-Up Length (Months) | ||
| Resumed | Did Not Resume | Resumed | Did Not Resume | |||||||||
| Sengupta (2018) [37] | Retrospective cohort | Apixaban (n = 51, 4%) Rivaroxaban (n = 608, 45%) Dabigatran (n = 679, 51%) | AF | 586 | 752 | 78 (70–83) | 79 (71–84) | 687 (51) | n/a | Resumption during follow-up (median 40 days; IQR: 17-88) | 90-day/6 months hospital readmission with GIB or thromboembolic complications | 6 |
| Valanejad (2020) [38] | Retrospective cohort | Apixaban (n = 18, 31.6%) Rivaroxaban (n = 34, 59.6%) Dabigatran (n = 5, 8.8%) | AF, DVT, PE | 37 | 18 | 75 (68–79) | 74.5 (71.3–82.5) | 31 (56) | 37 (67) a | Resume in ≤7 days from admission | 90-day hospital readmission with GIB, 12 months mortality | 12 |
| Yanagisawa (2021) * [39] | Retrospective Cohort | Apixaban (n = 11, 20.8%) Rivaroxaban (n = 27, 50.9%) Dabigatran (n = 13, 24.5%) Edoxaban (n = 2, 3.8%) | AF | 45 | 8 | 77 | 79.5 | 23 (43.3) | 26 (46) b | Resumption during follow-up (90% in 14 days) | Recurrent GIB or MACCE during follow-up | 28 (10–44) d |
| Hernandez (2017) * [40] | Retrospective cohort | Dabigatran (n = 276) (Warfarin) | AF | 92 | 184 | 79.64 (8.67) c | 81.9 (7.63) c | 225 (67.3) | 276 (100) a | Resume in 3 months | Recurrent GIB during follow-up * | 12 |
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | DOACs | No Therapy | Relative (95% CI) | Absolute (95% CI) | ||
| Recurrent bleeding (assessed with: event) | ||||||||||||
| 4 | observational studies | very serious a | not serious b | not serious | serious c | none | 53/741 (7.2%) | 67/981 (6.8%) | OR 1.09 (0.72 to 1.64) | 6 more per 1000 (from 18 fewer to 39 more) | ⨁◯◯◯ Very low | CRITICAL |
| All-cause mortality (follow-up: mean 12; assessed with: event) | ||||||||||||
| 1 | observational studies | serious d | not serious | not serious | very serious c | none | 4/18 (22.2%) | 4/37 (10.8%) | OR 2.36 (0.52 to 10.78) | 114 more per 1000 (from 49 fewer to 458 more) | ⨁◯◯◯ Very low | IMPORTANT |
| Thromboembolic event (follow-up: mean 3 months; assessed with: event) | ||||||||||||
| 1 | observational studies | very serious e | not serious | not serious | not serious | none | 16/586 (2.7%) | 17/752 (2.3%) | OR 1.21 (0.61 to 2.42) | 5 more per 1000 (from 9 fewer to 30 more) | ⨁◯◯◯ Very low | CRITICAL |
| Majer adverse cardiac and cerebrovascular events (MACCE) | ||||||||||||
| 1 | observational studies | very serious e | not serious | not serious | very serious c | none | 1/45 (2.2%) | 3/8 (37.5%) | OR 0.037 (0.003 to 0.430) | 353 fewer per 1000 (from 373 fewer to 170 fewer) | ⨁◯◯◯ Very low | CRITICAL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pálinkás, D.; Teutsch, B.; Gagyi, E.B.; Engh, M.A.; Kalló, P.; Veres, D.S.; Földvári-Nagy, L.; Hosszúfalusi, N.; Hegyi, P.; Erőss, B. No Association between Gastrointestinal Rebleeding and DOAC Therapy Resumption: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 554. https://doi.org/10.3390/biomedicines11020554
Pálinkás D, Teutsch B, Gagyi EB, Engh MA, Kalló P, Veres DS, Földvári-Nagy L, Hosszúfalusi N, Hegyi P, Erőss B. No Association between Gastrointestinal Rebleeding and DOAC Therapy Resumption: A Systematic Review and Meta-Analysis. Biomedicines. 2023; 11(2):554. https://doi.org/10.3390/biomedicines11020554
Chicago/Turabian StylePálinkás, Dániel, Brigitta Teutsch, Endre Botond Gagyi, Marie Anne Engh, Patrícia Kalló, Dániel S. Veres, László Földvári-Nagy, Nóra Hosszúfalusi, Péter Hegyi, and Bálint Erőss. 2023. "No Association between Gastrointestinal Rebleeding and DOAC Therapy Resumption: A Systematic Review and Meta-Analysis" Biomedicines 11, no. 2: 554. https://doi.org/10.3390/biomedicines11020554
APA StylePálinkás, D., Teutsch, B., Gagyi, E. B., Engh, M. A., Kalló, P., Veres, D. S., Földvári-Nagy, L., Hosszúfalusi, N., Hegyi, P., & Erőss, B. (2023). No Association between Gastrointestinal Rebleeding and DOAC Therapy Resumption: A Systematic Review and Meta-Analysis. Biomedicines, 11(2), 554. https://doi.org/10.3390/biomedicines11020554







