Role of the Intestinal Microbiota in the Genesis of Major Depression and the Response to Antidepressant Drug Therapy: A Narrative Review
Abstract
1. Introduction
2. Literature Search
3. Types, Symptoms, and Causes of Depression
4. The Role of the Gut-Brain Axis in Depression
4.1. Neurotransmitters
4.2. Short Chain Fatty Acids
4.3. Lipopolysaccharides
4.4. Formyl Peptides
5. Antidepressants Therapeutic Approaches
5.1. SSRIs
5.2. SNRIs
5.3. Tricyclic Antidepressants (TCAs)
5.4. Monoamine Oxidase Inhibitors (MAOIs)
5.5. Atypical Antidepressants
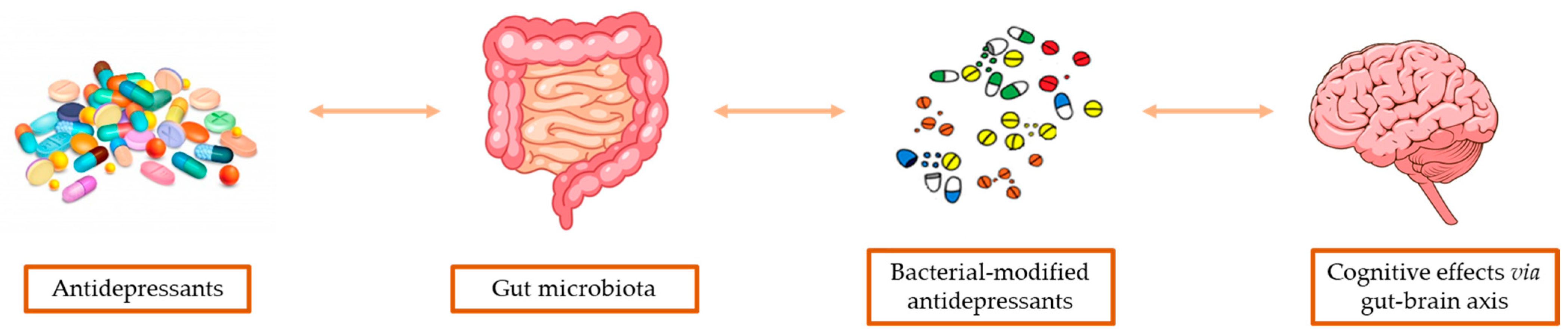
6. Gut-Microbiota Based Pharmacokinetics
7. Modulation of GM by Antidepressants
8. Emerging Therapies Targeting GM
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NICE. National Institute for Health and Care Excellence: Guidelines. In Depression in Adults: Treatment and Management; National Institute for Health and Care Excellence (NICE). NICE: London, UK, 2022. [Google Scholar]
- Wang, H.; Tian, X.; Wang, X.; Wang, Y. Evolution and Emerging Trends in Depression Research From 2004 to 2019: A Literature Visualization Analysis. Front. Psychiatry 2021, 12, 705749. [Google Scholar] [CrossRef]
- Renaud-Charest, O.; Lui, L.M.W.; Eskander, S.; Ceban, F.; Ho, R.; Di Vincenzo, J.D.; Rosenblat, J.D.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. J. Psychiatr. Res. 2021, 144, 129–137. [Google Scholar] [CrossRef]
- Hölzel, L.; Härter, M.; Reese, C.; Kriston, L. Risk factors for chronic depression—A systematic review. J. Affect. Disord. 2011, 129, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Feighner, J.P. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999, 60, 4–11. [Google Scholar]
- Machado-Vieira, R.; Baumann, J.; Wheeler-Castillo, C.; Latov, D.; Henter, I.D.; Salvadore, G.; Zarate, C.A. The Timing of Antidepressant Effects: A Comparison of Diverse Pharmacological and Somatic Treatments. Pharmaceuticals 2010, 3, 19–41. [Google Scholar] [CrossRef]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Kinrys, G.; Gold, A.K.; Pisano, V.D.; Freeman, M.P.; Papakostas, G.I.; Mischoulon, D.; Nierenberg, A.A.; Fava, M. Tachyphylaxis in major depressive disorder: A review of the current state of research. J. Affect. Disord. 2019, 245, 488–497. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- O’Brien, L.; Laporte, A.; Koren, G. Estimating the economic costs of antidepressant discontinuation during pregnancy. Can. J. Psychiatry 2009, 54, 399–408. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.B.; Gautam, B.; Singh, A.; Yadav, N. Chapter 19—Pharmacokinetics and pharmacodynamics analysis of drug candidates. In Bioinformatics; Singh, D.B., Pathak, R.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 305–316. [Google Scholar] [CrossRef]
- Aziz, R.K.; Rizkallah, M.R.; Saad, R.; ElRakaiby, M.T. Translating Pharmacomicrobiomics: Three Actionable Challenges/Prospects in 2020. OMICS 2020, 24, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Griffin, B.T.; Clarke, G.; Hyland, N.P. Drug-gut microbiota interactions: Implications for neuropharmacology. Br. J. Pharmacol. 2018, 175, 4415–4429. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.D.A.; Olivier, B. Translational Studies in the Complex Role of Neurotransmitter Systems in Anxiety and Anxiety Disorders. Adv. Exp. Med. Biol. 2020, 1191, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Zhu, G. Associations Among Monoamine Neurotransmitter Pathways, Personality Traits, and Major Depressive Disorder. Front. Psychiatry 2020, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef]
- aan het Rot, M.; Mathew, S.J.; Charney, D.S. Neurobiological mechanisms in major depressive disorder. CMAJ 2009, 180, 305–313. [Google Scholar] [CrossRef]
- Ho, H.Y.; Chin-Hung Chen, V.; Tzang, B.S.; Hsieh, C.C.; Wang, W.K.; Weng, Y.P.; Hsu, Y.T.; Hsaio, H.P.; Weng, J.C.; Chen, Y.L. Circulating cytokines as predictors of depression in patients with breast cancer. J. Psychiatr. Res. 2021, 136, 306–311. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021, 9, 649103. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Tsai, C.F.; Tu, P.C.; Wang, Y.P.; Chu, C.J.; Huang, Y.H.; Lin, H.C.; Hou, M.C.; Lee, F.Y.; Liu, P.Y.; Lu, C.L. Altered cognitive control network is related to psychometric and biochemical profiles in covert hepatic encephalopathy. Sci. Rep. 2019, 9, 6580. [Google Scholar] [CrossRef]
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.L.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in anti-inflammatory gut bacteria are associated with deprsession in a sample of young adults. Brain Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, J.R.; Roach, J.; Azcarate-Peril, M.A.; Thompson, A.L. Maternal precarity and HPA axis functioning shape infant gut microbiota and HPA axis development in humans. PLoS ONE 2021, 16, e0251782. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The Contribution of Gut Microbiota-Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021, 15, 616883. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Rai, S.N. The Link between Obesity, Microbiota Dysbiosis, and Neurodegenerative Pathogenesis. Diseases 2021, 9, 45. [Google Scholar] [CrossRef]
- Ho, J.T.; Chan, G.C.; Li, J.C. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol. 2015, 16, 21. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Mundula, T.; Nannini, G.; Amedei, A. Microbiota shaping—The effects of probiotics, prebiotics, and fecal microbiota transplant on cognitive functions: A systematic review. World J. Gastroenterol. 2021, 27, 6715–6732. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014, 817, 115–133. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology in the microbiome-gut-brain axis: How bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013, 9, e1003726. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.J.K.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef]
- Gaudichon, C.; Calvez, J. Determinants of amino acid bioavailability from ingested protein in relation to gut health. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, Y.; Zhong, Q.; Bai, S.J.; Zhou, C.J.; Tian, T.; Chen, J.J. Associations Between Disordered Microbial Metabolites and Changes of Neurotransmitters in Depressed Mice. Front. Cell. Infect. Microbiol. 2022, 12, 906303. [Google Scholar] [CrossRef] [PubMed]
- Baier, J.; Gänsbauer, M.; Giessler, C.; Arnold, H.; Muske, M.; Schleicher, U.; Lukassen, S.; Ekici, A.; Rauh, M.; Daniel, C.; et al. Arginase impedes the resolution of colitis by altering the microbiome and metabolome. J. Clin. Investig. 2020, 130, 5703–5720. [Google Scholar] [CrossRef]
- Xiong, L.; Teng, J.L.; Botelho, M.G.; Lo, R.C.; Lau, S.K.; Woo, P.C. Arginine Metabolism in Bacterial Pathogenesis and Cancer Therapy. Int. J. Mol. Sci. 2016, 17, 363. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, A.S.; Abdelgawad, M.A.; Mathew, B. Exploration of the Detailed Structure-Activity Relationships of Isatin and Their Isomers As Monoamine Oxidase Inhibitors. ACS Omega 2022, 7, 16244–16259. [Google Scholar] [CrossRef] [PubMed]
- Irsfeld, M.; Spadafore, M.; Prüß, B.M. β-phenylethylamine, a small molecule with a large impact. WebmedCentral 2013, 4, 4409. [Google Scholar] [PubMed]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Ivković, S.; Mitić, M.; Adžić, M. Tryptophan metabolites in depression: Modulation by gut microbiota. Front. Behav. Neurosci. 2022, 16, 987697. [Google Scholar] [CrossRef] [PubMed]
- Jaglin, M.; Rhimi, M.; Philippe, C.; Pons, N.; Bruneau, A.; Goustard, B.; Daugé, V.; Maguin, E.; Naudon, L.; Rabot, S. Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Front. Neurosci. 2018, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Brydges, C.R.; Bhattacharyya, S.; Dehkordi, S.M.; Milaneschi, Y.; Penninx, B.; Jansen, R.; Kristal, B.S.; Han, X.; Arnold, M.; Kastenmüller, G.; et al. Metabolomic and inflammatory signatures of symptom dimensions in major depression. Brain Behav. Immun. 2022, 102, 42–52. [Google Scholar] [CrossRef]
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.J.; Xie, J.; Chen, J.J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef]
- Dudek, K.A.; Dion-Albert, L.; Lebel, M.; LeClair, K.; Labrecque, S.; Tuck, E.; Ferrer Perez, C.; Golden, S.A.; Tamminga, C.; Turecki, G.; et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc. Natl. Acad. Sci. USA 2020, 117, 3326–3336. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, O.Y.; Song, J. Alleviation of Depression by Glucagon-Like Peptide 1 Through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front. Pharmacol. 2020, 11, 1270. [Google Scholar] [CrossRef]
- Detka, J.; Głombik, K. Insights into a possible role of glucagon-like peptide-1 receptor agonists in the treatment of depression. Pharmacol. Rep. 2021, 73, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Mohr, A.E.; Crawford, M.; Jasbi, P.; Fessler, S.; Sweazea, K.L. Lipopolysaccharide and the gut microbiota: Considering structural variation. FEBS Lett. 2022, 596, 849–875. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, P.P.; Hu, K.L.; Li, L.N.; Yu, X.; Lu, Y.; Chang, H.S. Antidepressant-Like Effect and Mechanism of Action of Honokiol on the Mouse Lipopolysaccharide (LPS) Depression Model. Molecules 2019, 24, 2035. [Google Scholar] [CrossRef]
- Tang, X.H.; Zhang, G.F.; Xu, N.; Duan, G.F.; Jia, M.; Liu, R.; Zhou, Z.Q.; Yang, J.J. Extrasynaptic CaMKIIα is involved in the antidepressant effects of ketamine by downregulating GluN2B receptors in an LPS-induced depression model. J. Neuroinflammation 2020, 17, 181. [Google Scholar] [CrossRef]
- Zhu, J.; Li, L.; Ding, J.; Huang, J.; Shao, A.; Tang, B. The Role of Formyl Peptide Receptors in Neurological Diseases via Regulating Inflammation. Front. Cell. Neurosci. 2021, 15, 753832. [Google Scholar] [CrossRef] [PubMed]
- Gallo, I.; Rattazzi, L.; Piras, G.; Gobbetti, T.; Panza, E.; Perretti, M.; Dalley, J.W.; D’Acquisto, F. Formyl peptide receptor as a novel therapeutic target for anxiety-related disorders. PLoS ONE 2014, 9, e114626. [Google Scholar] [CrossRef] [PubMed]
- Trojan, E.; Bryniarska, N.; Leśkiewicz, M.; Regulska, M.; Chamera, K.; Szuster-Głuszczak, M.; Leopoldo, M.; Lacivita, E.; Basta-Kaim, A. The Contribution of Formyl Peptide Receptor Dysfunction to the Course of Neuroinflammation: A Potential Role in the Brain Pathology. Curr. Neuropharmacol. 2020, 18, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Tylek, K.; Trojan, E.; Regulska, M.; Lacivita, E.; Leopoldo, M.; Basta-Kaim, A. Formyl peptide receptor 2, as an important target for ligands triggering the inflammatory response regulation: A link to brain pathology. Pharmacol. Rep. 2021, 73, 1004–1019. [Google Scholar] [CrossRef]
- Timonen, M.; Liukkonen, T. Management of depression in adults. BMJ 2008, 336, 435–439. [Google Scholar] [CrossRef]
- O’Donnell, J.M.; Shelton, R.C. Drug Therapy of Depression and Anxiety Disorders. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12th ed.; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Taylor, C.; Fricker, A.D.; Devi, L.A.; Gomes, I. Mechanisms of action of antidepressants: From neurotransmitter systems to signaling pathways. Cell. Signal. 2005, 17, 549–557. [Google Scholar] [CrossRef]
- Jha, M.K.; Rush, A.J.; Trivedi, M.H. When Discontinuing SSRI Antidepressants Is a Challenge: Management Tips. Am. J. Psychiatry 2018, 175, 1176–1184. [Google Scholar] [CrossRef]
- Richelson, E. Pharmacology of antidepressants—Characteristics of the ideal drug. Mayo Clin. Proc. 1994, 69, 1069–1081. [Google Scholar] [CrossRef]
- Dunner, D.L. Combining antidepressants. Shanghai Arch. Psychiatry 2014, 26, 363–364. [Google Scholar] [CrossRef]
- Ruberto, V.L.; Jha, M.K.; Murrough, J.W. Pharmacological Treatments for Patients with Treatment-Resistant Depression. Pharmaceuticals 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Belzeaux, R.; Bergon, A.; Jeanjean, V.; Loriod, B.; Formisano-Tréziny, C.; Verrier, L.; Loundou, A.; Baumstarck-Barrau, K.; Boyer, L.; Gall, V.; et al. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl. Psychiatry 2012, 2, e185. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015, 23, 1–21. [Google Scholar] [CrossRef]
- Gautam, S.; Jain, A.; Gautam, M.; Vahia, V.N.; Grover, S. Clinical Practice Guidelines for the management of Depression. Indian J. Psychiatry 2017, 59, S34–S50. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Adams, S.M.; Miller, K.E.; Zylstra, R.G. Pharmacologic management of adult depression. Am. Fam. Physician 2008, 77, 785–792. [Google Scholar]
- Wada, K.; Yamada, N.; Hamamura, T.; Suzuki, H.; Nakano, Y.; Kuroda, S. Add-on polytherapy with antidepressants and its significance in inpatients with major depression. Psychiatry Clin. Neurosci. 1999, 53, 557–562. [Google Scholar] [CrossRef]
- Gabriel, M.; Sharma, V. Antidepressant discontinuation syndrome. CMAJ 2017, 189, E747. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Serotonin norepinephrine reuptake inhibitors: A pharmacological comparison. Innov. Clin. Neurosci. 2014, 11, 37–42. [Google Scholar]
- Perry, R.; Cassagnol, M. Desvenlafaxine: A new serotonin-norepinephrine reuptake inhibitor for the treatment of adults with major depressive disorder. Clin. Ther. 2009, 31, 1374–1404. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: Focus on modulation of CNS monoamine neurotransmitter release. Pharmacol. Ther. 2014, 143, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Riederer, P.F. A review of the mechanisms and role of monoamine oxidase inhibitors in Parkinson’s disease. Neurology 2004, 63, S32–S35. [Google Scholar] [CrossRef]
- Costa, R.; Oliveira, N.G.; Dinis-Oliveira, R.J. Pharmacokinetic and pharmacodynamic of bupropion: Integrative overview of relevant clinical and forensic aspects. Drug Metab. Rev. 2019, 51, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Anttila, S.A.; Leinonen, E.V. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001, 7, 249–264. [Google Scholar] [CrossRef]
- Cusack, B.; Nelson, A.; Richelson, E. Binding of antidepressants to human brain receptors: Focus on newer generation compounds. Psychopharmacology 1994, 114, 559–565. [Google Scholar] [CrossRef]
- Owens, M.J.; Morgan, W.N.; Plott, S.J.; Nemeroff, C.B. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J. Pharmacol. Exp. Ther. 1997, 283, 1305–1322. [Google Scholar]
- Gerace, E.; Polenzani, L.; Magnani, M.; Zianni, E.; Stocca, G.; Gardoni, F.; Pellegrini-Giampietro, D.E.; Corradetti, R. Antidepressant-induced increase in GluA2 expression does not translate in changes of AMPA receptor-mediated synaptic transmission at CA3/CA1 synapses in rats. Neuropharmacology 2023, 223, 109307. [Google Scholar] [CrossRef]
- Haria, M.; Fitton, A.; McTavish, D. Trazodone. A review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders. Drugs Aging 1994, 4, 331–355. [Google Scholar] [CrossRef]
- Cruz, M.P. Vilazodone HCl (Viibryd): A Serotonin Partial Agonist and Reuptake Inhibitor For the Treatment of Major Depressive Disorder. P&T 2012, 37, 28–31. [Google Scholar]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, T.S.; Turnbaugh, P.J. Tiny Gatekeepers: Microbial Control of Host Drug Transporters. Clin. Pharmacol. Ther. 2022, 112, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Klünemann, M.; Andrejev, S.; Blasche, S.; Mateus, A.; Phapale, P.; Devendran, S.; Vappiani, J.; Simon, B.; Scott, T.A.; Kafkia, E.; et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 2021, 597, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal drug transporters: An overview. Adv. Drug Deliv. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef]
- Drozdzik, M.; Czekawy, I.; Oswald, S.; Drozdzik, A. Intestinal drug transporters in pathological states: An overview. Pharmacol. Rep. 2020, 72, 1173–1194. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Geier, A.; Oude Elferink, R.P. ABC of oral bioavailability: Transporters as gatekeepers in the gut. Gut 2003, 52, 1788–1795. [Google Scholar] [CrossRef]
- Shin, H.J.; Anzai, N.; Enomoto, A.; He, X.; Kim, D.K.; Endou, H.; Kanai, Y. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology 2007, 45, 1046–1055. [Google Scholar] [CrossRef]
- Cario, E. P-glycoprotein multidrug transporter in inflammatory bowel diseases: More questions than answers. World J. Gastroenterol. 2017, 23, 1513–1520. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef]
- Jansen, J.; Jansen, K.; Neven, E.; Poesen, R.; Othman, A.; van Mil, A.; Sluijter, J.; Sastre Torano, J.; Zaal, E.A.; Berkers, C.R.; et al. Remote sensing and signaling in kidney proximal tubules stimulates gut microbiome-derived organic anion secretion. Proc. Natl. Acad. Sci. USA 2019, 116, 16105–16110. [Google Scholar] [CrossRef]
- Adkins, C.E.; Mittapalli, R.K.; Manda, V.K.; Nounou, M.I.; Mohammad, A.S.; Terrell, T.B.; Bohn, K.A.; Yasemin, C.; Grothe, T.R.; Lockman, J.A.; et al. P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Front. Pharmacol. 2013, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The Impact of Gut Microbiota Disorders on the Blood-Brain Barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, Y.F.; Nacheva, P.M. Biodegradability of fluoxetine, mefenamic acid, and metoprolol using different microbial consortiums. Environ. Sci. Pollut. Res. Int. 2017, 24, 6779–6793. [Google Scholar] [CrossRef]
- Yu, J.B.; Zhao, Z.X.; Peng, R.; Pan, L.B.; Fu, J.; Ma, S.R.; Han, P.; Cong, L.; Zhang, Z.W.; Sun, L.X.; et al. Gut Microbiota-Based Pharmacokinetics and the Antidepressant Mechanism of Paeoniflorin. Front. Pharmacol. 2019, 10, 268. [Google Scholar] [CrossRef]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef]
- Rucklidge, J.J. Could yeast infections impair recovery from mental illness? A case study using micronutrients and olive leaf extract for the treatment of ADHD and depression. Adv. Mind. Body Med. 2013, 27, 14–18. [Google Scholar]
- Jiang, H.Y.; Pan, L.Y.; Zhang, X.; Zhang, Z.; Zhou, Y.Y.; Ruan, B. Altered gut bacterial-fungal interkingdom networks in patients with current depressive episode. Brain Behav. 2020, 10, e01677. [Google Scholar] [CrossRef]
- Uittamo, J.; Siikala, E.; Kaihovaara, P.; Salaspuro, M.; Rautemaa, R. Chronic candidosis and oral cancer in APECED-patients: Production of carcinogenic acetaldehyde from glucose and ethanol by Candida albicans. Int. J. Cancer 2009, 124, 754–756. [Google Scholar] [CrossRef]
- Jamal, M.; Ameno, K.; Tanaka, N.; Ito, A.; Takakura, A.; Kumihashi, M.; Kinoshita, H. Ethanol and Acetaldehyde After Intraperitoneal Administration to Aldh2-Knockout Mice-Reflection in Blood and Brain Levels. Neurochem. Res. 2016, 41, 1029–1034. [Google Scholar] [CrossRef]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Marmiroli, N. Tricyclic antidepressants inhibit Candida albicans growth and biofilm formation. Int. J. Antimicrob. Agents 2018, 52, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Marmiroli, N. Antimicrobial Properties of Antidepressants and Antipsychotics-Possibilities and Implications. Pharmaceuticals 2021, 14, 915. [Google Scholar] [CrossRef] [PubMed]
- Foletto, V.S.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Franco, L.N.; de Paula, B.R.; Hörner, R. Repositioning of antidepressant drugs and synergistic effect with ciprofloxacin against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2021, 37, 53. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, X.; Li, G.; Gao, J.; Liang, Y. The change of gut microbiota in MDD patients under SSRIs treatment. Sci. Rep. 2021, 11, 14918. [Google Scholar] [CrossRef] [PubMed]
- Skillington, O.; Mills, S.; Gupta, A.; Mayer, E.A.; Gill, C.I.R.; Del Rio, D.; O’Riordan, K.J.; Cryan, J.F.; Ross, R.P.; Stanton, C. The contrasting human gut microbiota in early and late life and implications for host health and disease. Nutr. Healthy Aging 2021, 6, 157–178. [Google Scholar] [CrossRef]
- Mundula, T.; Russo, E.; Curini, L.; Giudici, F.; Piccioni, A.; Franceschi, F.; Amedei, A. Chronic Systemic Low-Grade Inflammation and Modern Lifestyle: The Dark Role of Gut Microbiota on Related Diseases with a Focus on COVID-19 Pandemic. Curr. Med. Chem. 2022, 29, 5370–5396. [Google Scholar] [CrossRef]
- Lee, S.H.; Paz-Filho, G.; Mastronardi, C.; Licinio, J.; Wong, M.L. Is increased antidepressant exposure a contributory factor to the obesity pandemic? Transl. Psychiatry 2016, 6, e759. [Google Scholar] [CrossRef]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L.; Nie, K. Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson’s Disease. Front. Neurol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Betrapally, N.S.; Hylemon, P.B.; White, M.B.; Gillevet, P.M.; Unser, A.B.; Fagan, A.; Daita, K.; Heuman, D.M.; Zhou, H.; et al. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci. Rep. 2016, 6, 26800. [Google Scholar] [CrossRef]
- Dethloff, F.; Vargas, F.; Elijah, E.; Quinn, R.; Park, D.I.; Herzog, D.P.; Müller, M.B.; Gentry, E.C.; Knight, R.; Gonzalez, A.; et al. Paroxetine Administration Affects Microbiota and Bile Acid Levels in Mice. Front. Psychiatry 2020, 11, 518. [Google Scholar] [CrossRef]
- McMillin, M.; DeMorrow, S. Effects of bile acids on neurological function and disease. FASEB J. 2016, 30, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Bile Acid Receptors and Gastrointestinal Functions. Liver Res. 2019, 3, 31–39. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, K.; Hu, J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Del Toro-Barbosa, M.; Hurtado-Romero, A.; Garcia-Amezquita, L.E.; García-Cayuela, T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients 2020, 12, 3896. [Google Scholar] [CrossRef]
| Classification | Mechanism of Action | Half-Life | Side Effects/Toxicity |
|---|---|---|---|
| SSRIs (Citalopram, Escitalopram, Fluoxetine, Fluvoxamine, Paroxetine, Sertraline) | SERT inhibition, enhancement of serotonergic transmission | 1–4 days | Cognitive impairment, nausea, prolonged QT interval, serotonin syndrome, sexual dysfunction, suicidal thoughts, xerostomia |
| SNRIs (Desvenlafaxine, Duloxetine, Levomilnacipran, Milnacipran, Venlafaxine) | SERT and NET inhibition | 8–14 hours | Constipation, high diastolic blood pressure, nausea, serotonin syndrome, sexual dysfunction, suicidal thoughts |
| TCAs (Amitriptyline, Amoxapine, Clomipramine, Desipramine, Doxepin, Imipramine, Maprotiline, Nortriptyline, Protriptyline, Trimipramine) | Inhibition of SERT, NET, α1, α2, M1 and H1 receptors. | 1–3 days | Blurred vision, constipation, orthostatic hypertension, seizures, sexual disfunction, suicidal thoughts, tachycardia, weight gain |
| MAOIs (Isocarboxazid, Phenelzine, Selegiline, Tranylcypromine) | MAOA and MAOB inhibition, increase of 5-HT, histamine, DA, NE and epinephrine levels | 2–12 hours | Headache, insomnia, serotonin syndrome, sexual dysfunction, weight gain |
| Atypical Antidepressants (Bupropion, Mirtazapine, Trazodone, Vilazodone, Vortioxetine) | DAT inhibition, antagonism of α1, 5-HT2 and 5-HT3 receptors | 1–3 days | Abnormal bleeding, agitation, dry mouth, headache, insomnia, nausea, seizures, sexual dysfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundula, T.; Baldi, S.; Gerace, E.; Amedei, A. Role of the Intestinal Microbiota in the Genesis of Major Depression and the Response to Antidepressant Drug Therapy: A Narrative Review. Biomedicines 2023, 11, 550. https://doi.org/10.3390/biomedicines11020550
Mundula T, Baldi S, Gerace E, Amedei A. Role of the Intestinal Microbiota in the Genesis of Major Depression and the Response to Antidepressant Drug Therapy: A Narrative Review. Biomedicines. 2023; 11(2):550. https://doi.org/10.3390/biomedicines11020550
Chicago/Turabian StyleMundula, Tiziana, Simone Baldi, Elisabetta Gerace, and Amedeo Amedei. 2023. "Role of the Intestinal Microbiota in the Genesis of Major Depression and the Response to Antidepressant Drug Therapy: A Narrative Review" Biomedicines 11, no. 2: 550. https://doi.org/10.3390/biomedicines11020550
APA StyleMundula, T., Baldi, S., Gerace, E., & Amedei, A. (2023). Role of the Intestinal Microbiota in the Genesis of Major Depression and the Response to Antidepressant Drug Therapy: A Narrative Review. Biomedicines, 11(2), 550. https://doi.org/10.3390/biomedicines11020550







