Horse-Derived Ceramide Accentuates Glucosylceramide Synthase and Ceramide Synthase 3 by Activating PPARβ/δ and/or PPARγ to Stimulate Ceramide Synthesis
Abstract
1. Introduction
2. Results
2.1. Residual Levels of HC on RHEEs
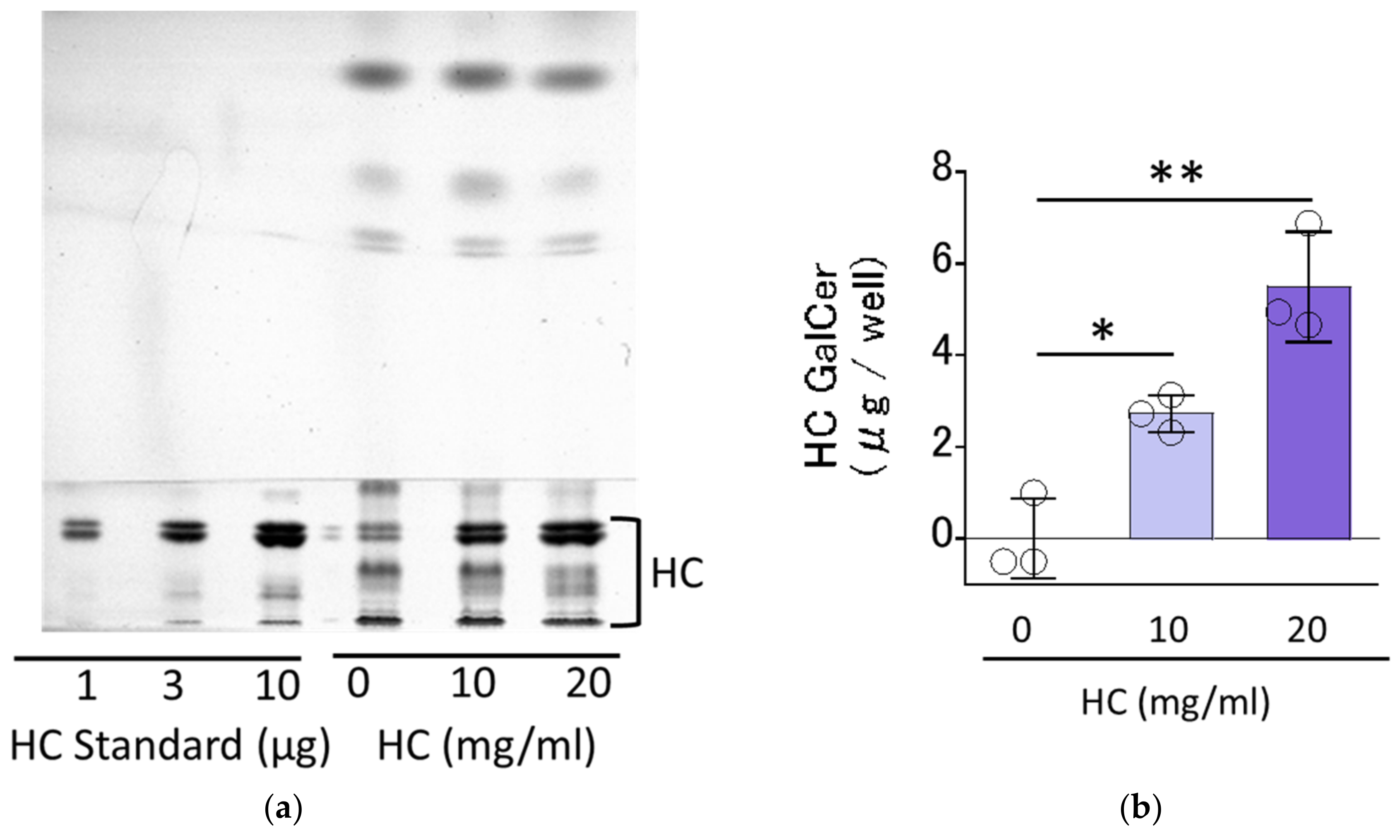
2.2. Effect of HC on SC Ceramide Levels in RHEEs
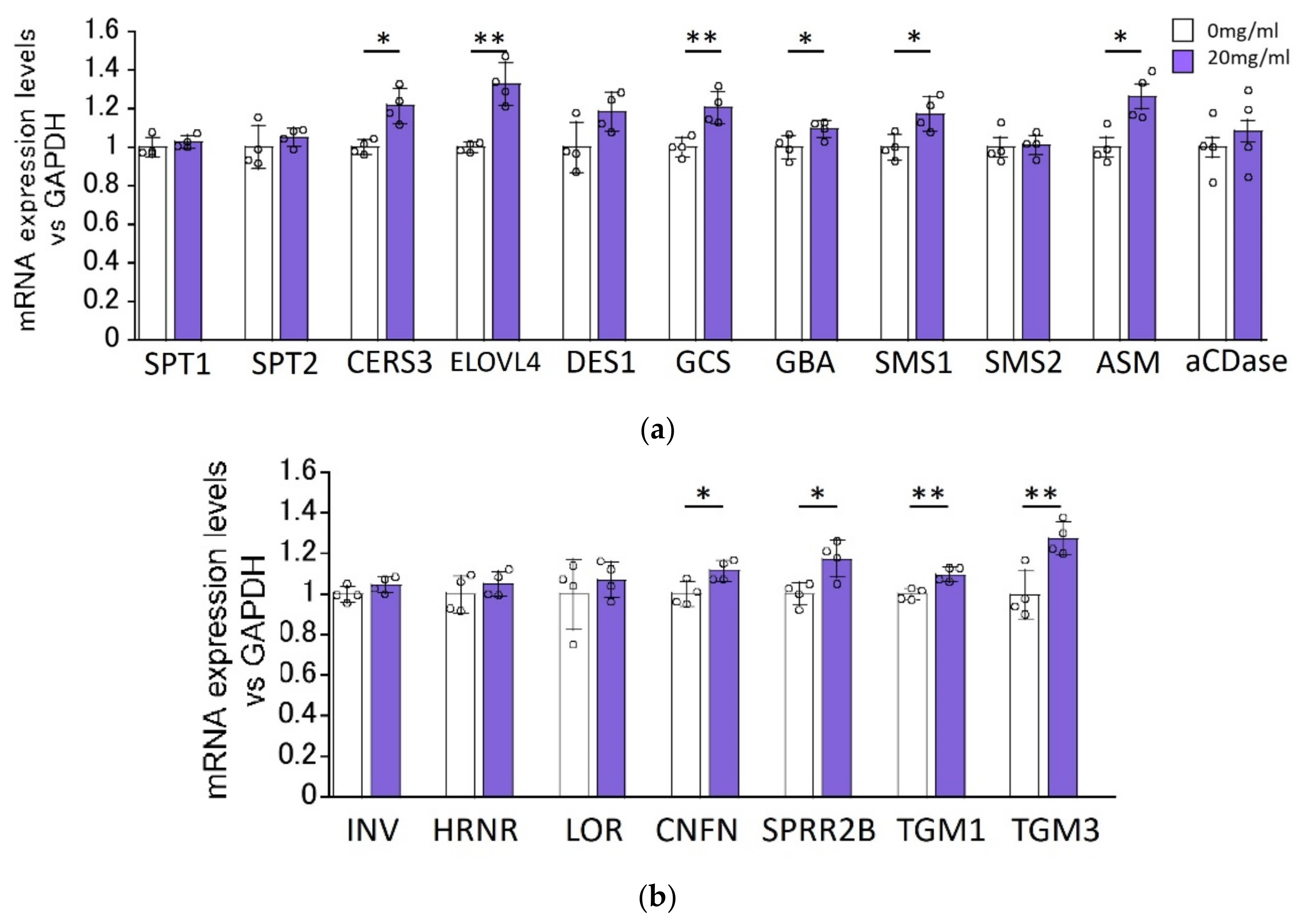
2.3. Effect of HC on the Expression Levels of Ceramide Synthesis- and Keratinization-Related Genes in RHEEs
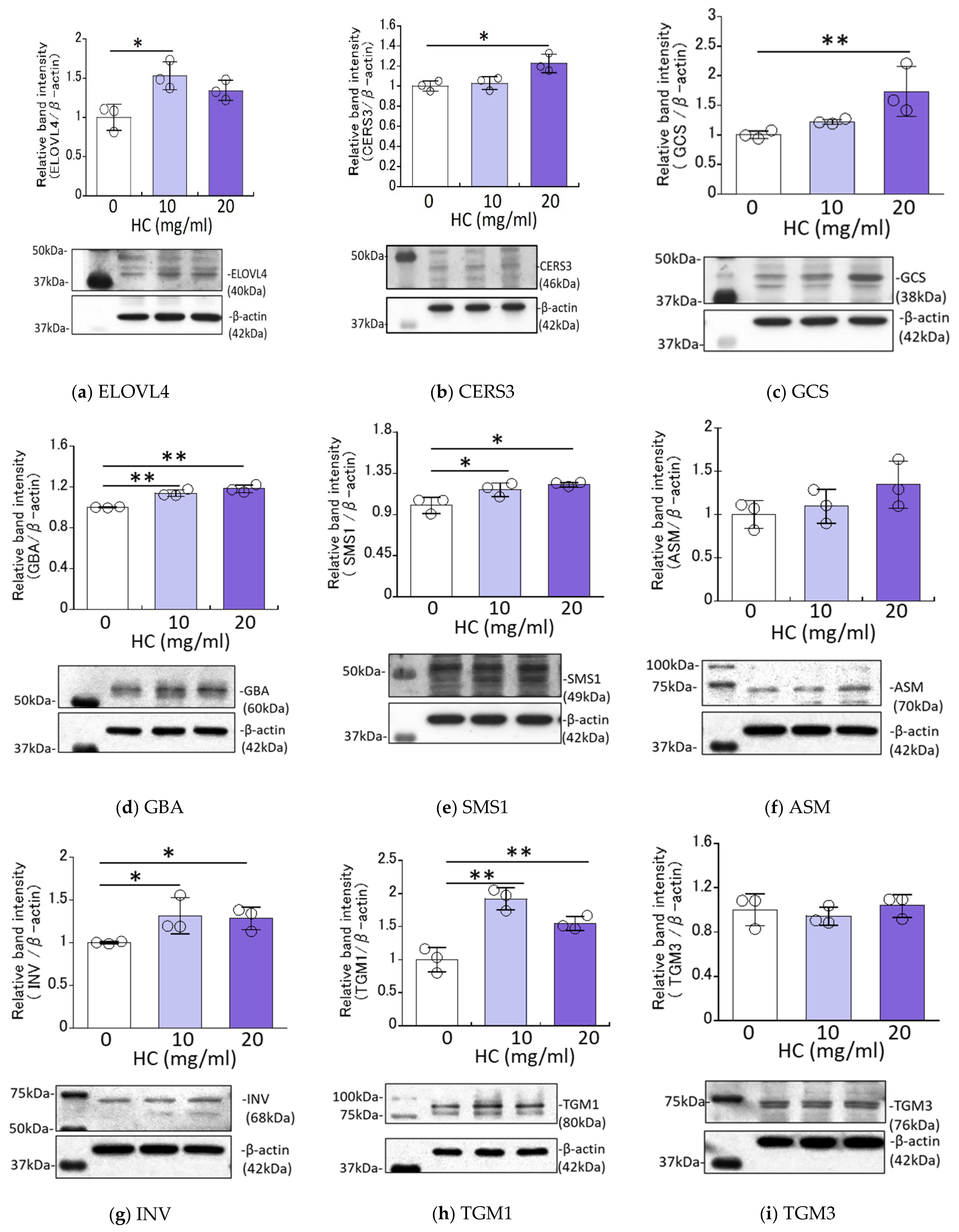
2.4. Effect of HC on the Expression Levels of Ceramide Synthesis- and Keratinization-Related Proteins in RHEEs
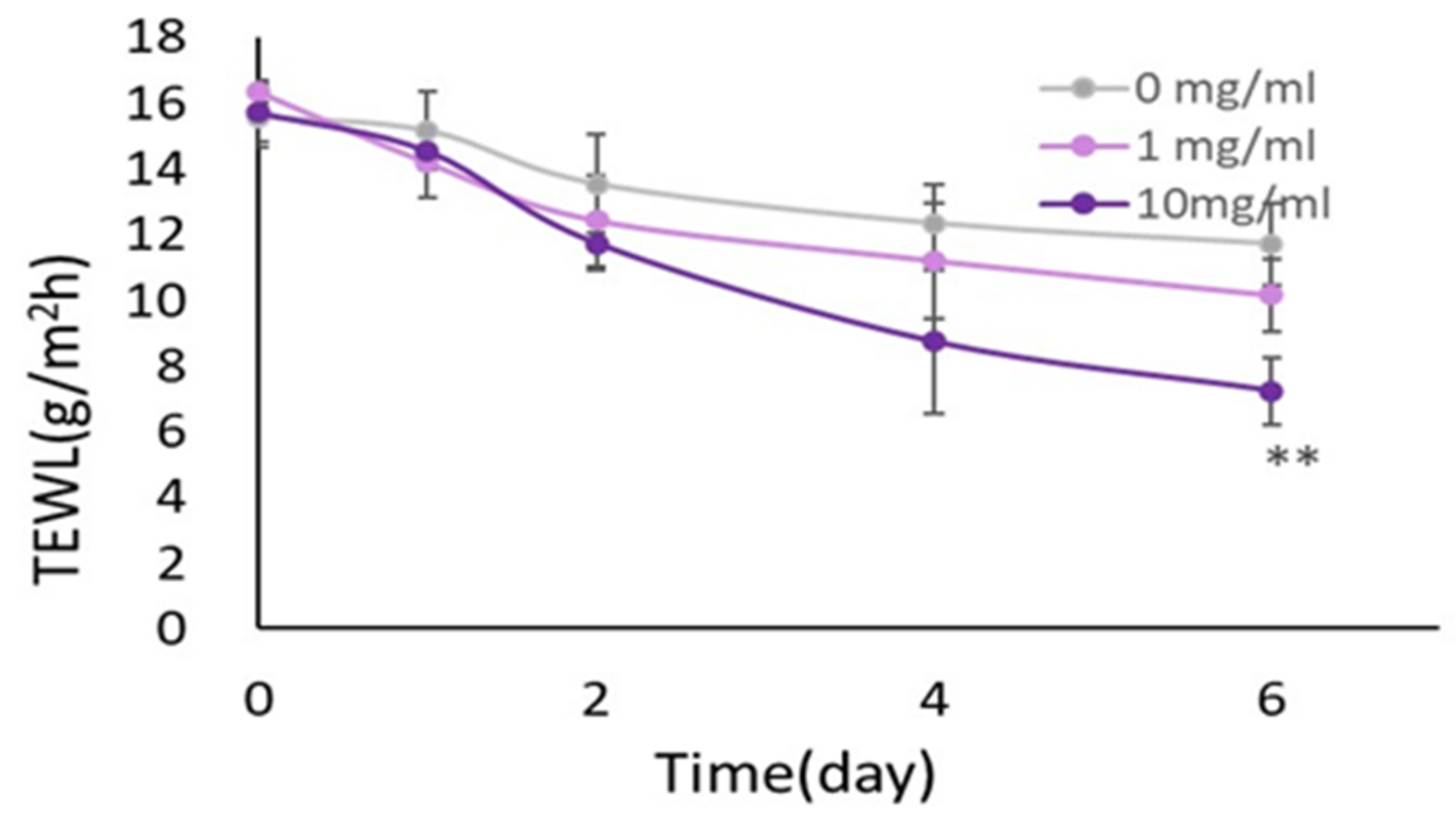
2.5. Improvement of Skin Barrier Function of RHEEs by HC
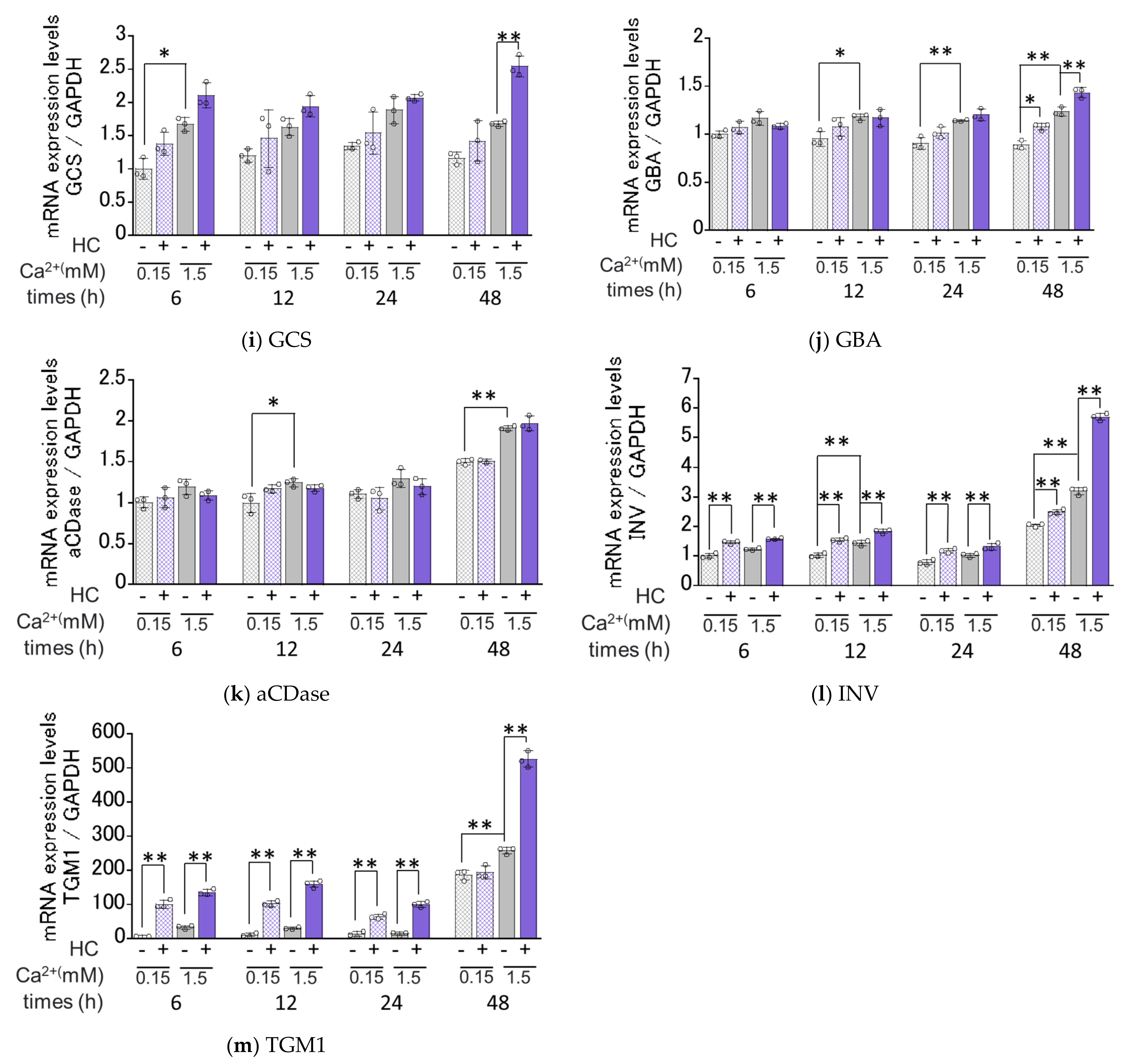
2.6. Effect of HC on the Expression of Ceramide Synthesis- and Keratinization-Related Genes under Proliferating and Differentiating Conditions of NHKs
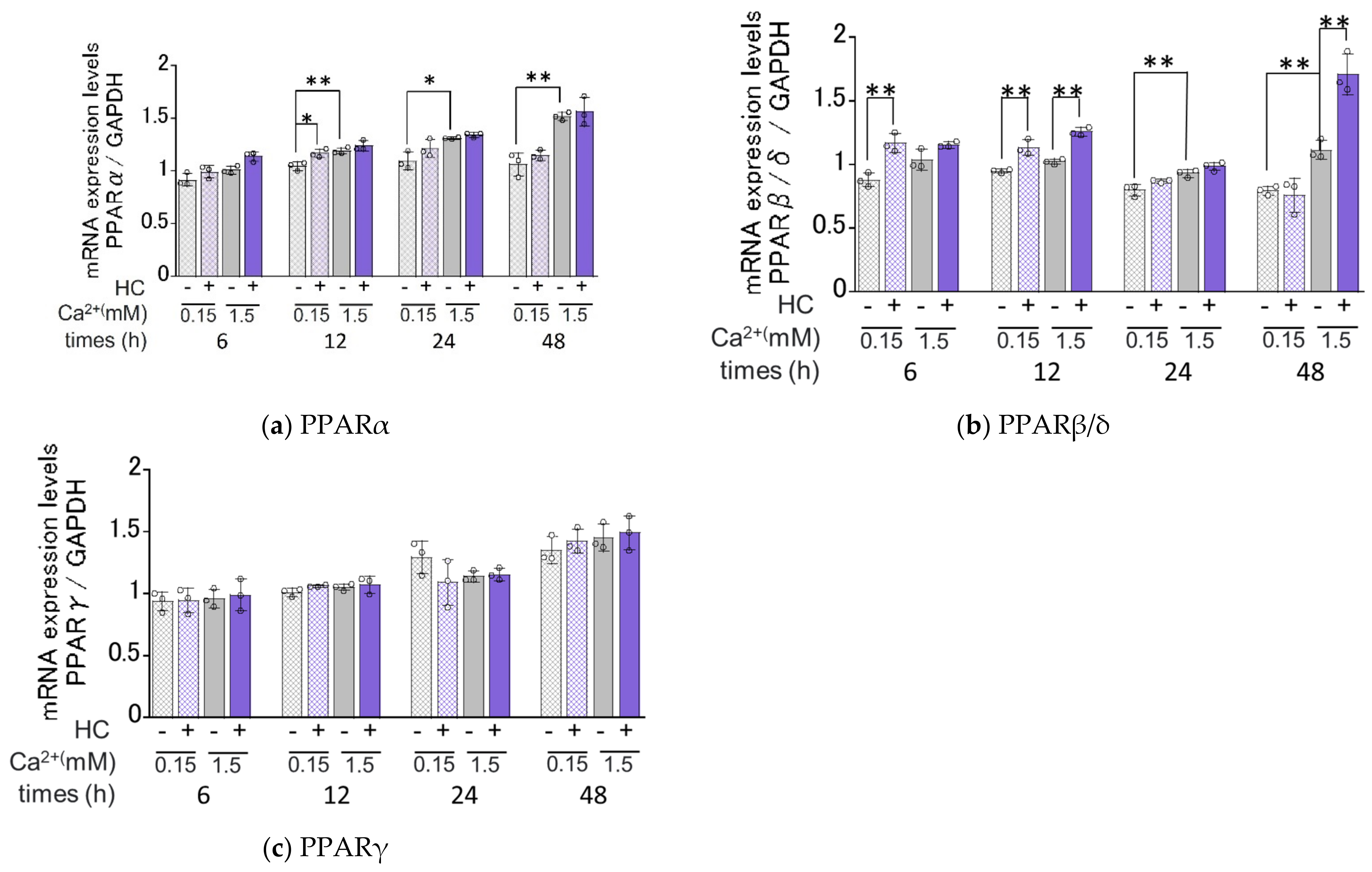
2.7. Effect of HC on the mRNA Expression Levels of PPARs under Proliferating and Differentiating Conditions of NHKs
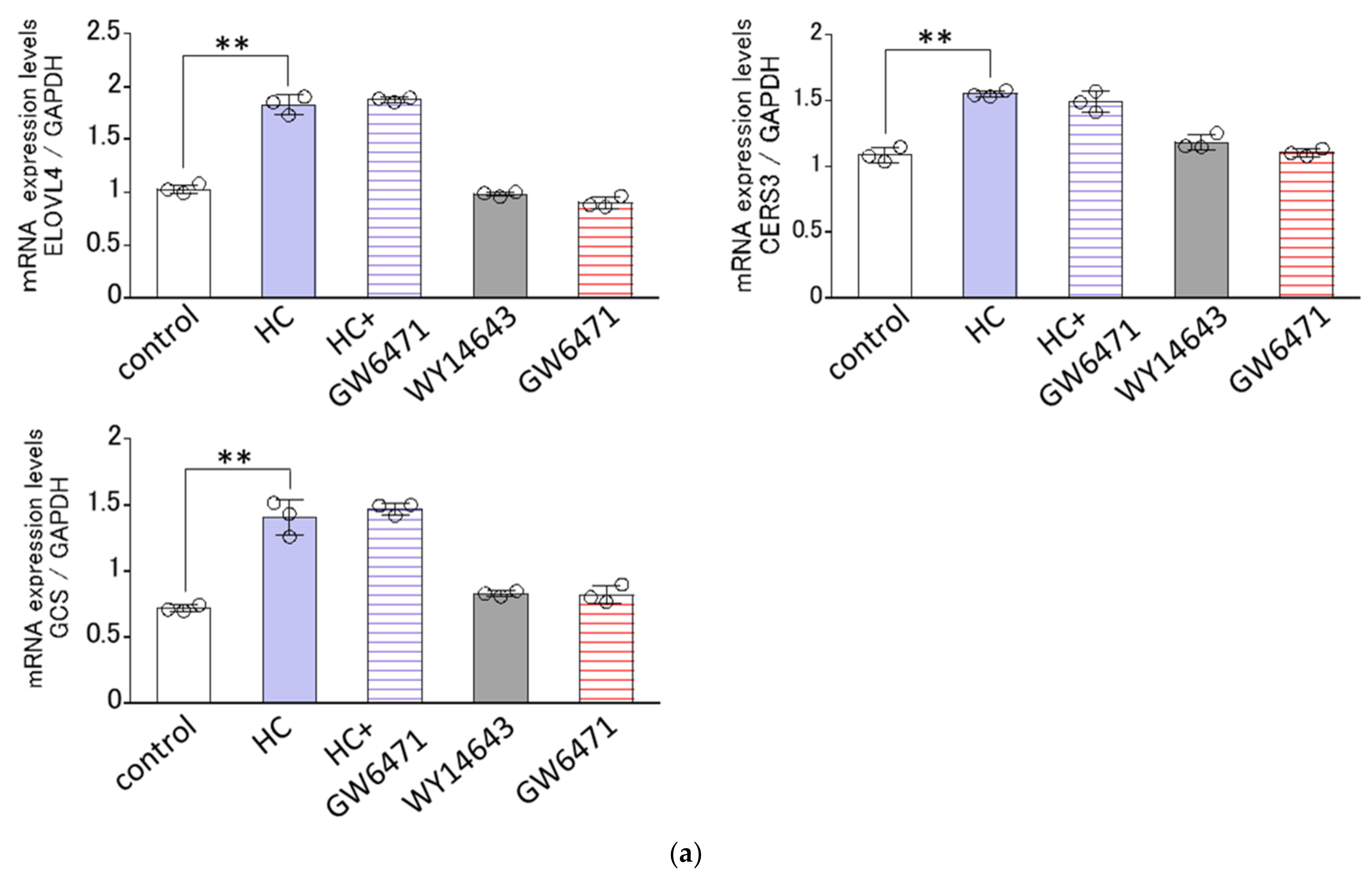
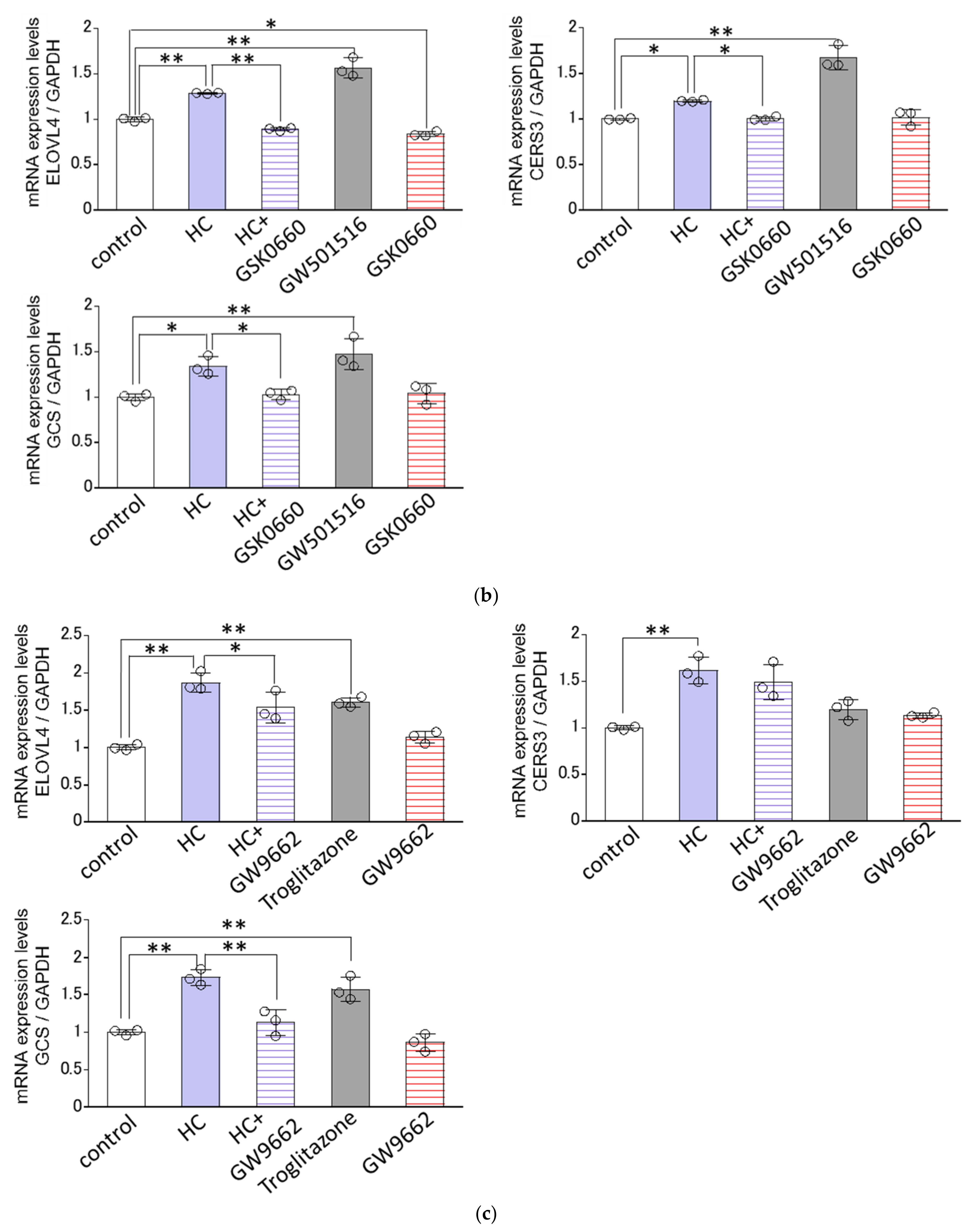
2.8. Effect of Agonists or Antagonists of PPARs on the HC-Stimulated Expression Levels of Ceramide Synthesis-Related Genes under Differentiating Conditions of NHKs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of HC Sample
4.3. Culture of RHEEs and Treatment with HC
4.4. Evaluation of the Residual Level of HC Applied on RHEEs
4.5. Culture of NHKs
4.6. Quantification of Ceramides in the SC of RHEEs
4.7. RNA Extraction and Real-Time RT-PCR
4.8. Western Blotting
4.9. TEWL Measurement in RHEEs
4.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Imokawa, G.; Hattori, M. A possible function of structural lipids in the water-holding properties of the stratum corneum. J. Investig. Dermatol. 1985, 84, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Water and the Stratum Corneum (Volume 1). In Chapter 3: Vitro and In Vivo Models. Bioengineering of Skin; Elsner, P., Berardesca, E., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 23–47. [Google Scholar]
- Akimoto, K.; Yoshikawa, N.; Higaki, Y.; Kawashima, M.; Imokawa, G. Quantitative analysis of stratum corneum lipids in xerosis and asteatotic eczema. J. Dermatol. 1993, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lavrijsen, A.P.; Bouwstra, J.A.; Gooris, G.S.; Weerheim, A.; Boddé, H.E.; Ponec, M. Reduced skin barrier function parallels abnormal stratum corneum lipid organization in patients with lamellar ichthyosis. J. Investig. Dermatol. 1995, 105, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Cutting Edge of the Pathogenesis of Atopic Dermatitis: Sphingomyelin Deacylase, the Enzyme Involved in Its Ceramide Deficiency, Plays a Pivotal Role. Int. J. Mol. Sci. 2021, 22, 1613. [Google Scholar] [CrossRef]
- Imokawa, G. Lipid abnormalities in atopic dermatitis. J. Am. Acad. Dermatol. 2001, 45, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Role of Ceramide in the Barrier Function of the Stratum Corneum, Implications for the Pathogenesis of Atopic Dermatitis. J. Clin. Exp. Dermatol. Res. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef]
- Nojiri, H.; Ishida, K.; Yao, X.; Liu, W.; Imokawa, G. Amelioration of lactic acid sensations in sensitive skin by stimulating the barrier function and improving the ceramide profile. Arch. Dermatol. Res. 2018, 310, 495–504. [Google Scholar] [CrossRef]
- Ishida, K.T.A.; Bito, K.; Draelos, Z.; Imokawa, G. Treatment with Synthetic Pseudoceramide Improves Atopic Skin, Switching the Ceramide Profile to a Healthy Skin Phenotype. J. Investig. Dermatol. 2020, 140, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Akasaki, S.; Kawamata, A.; Yano, S.; Takaishi, N. Water-retaining function in the stratum corneum and its recovery propertiess by synthetic pseudoceramides. J. Soc. Cosmet. Chem. 1989, 40, 273–285. [Google Scholar]
- Matsuki, H.; Kiyokane, K.; Matsuki, T.; Sato, S.; Imokawa, G. Reevaluation of the Importance of Barrier Dysfunction in the Nonlesional Dry Skin of Atopic Dermatitis Patients through the Use of Two Barrier Creams. Exog. Dermatol. 2004, 3, 293–302. [Google Scholar] [CrossRef]
- Mizutani, H.; Takahashi, M.; Shimizu, M.; Kariya, K.; Sato, H.; Imokawa, G. Usage of pseudoceramide cream in atopic dry skin in comparison with 10% urea cream. Nishinihon J. Dermatol. 2001, 63, 457–461. [Google Scholar] [CrossRef]
- Yamanaka, M.; Ishikawa, O.; Takahashi, A.; Sato, H.; Imokawa, G. Clinical evaluation of Curel medicated cream in patients with atopic dermatitis. Ski. Res. 2001, 43, 278–285. [Google Scholar]
- Nakamura, T.; Satoh, H.; Imokawa, G.; Miachi, Y. Clinical test of skin care products on atopic dry skin. Ski. Res. 2000, 42, 264–269. [Google Scholar]
- Horikawa, T.T.T.; Harada, S.; Chihara, T.; IchihasiI, M. An open clinical trial of glycoceramide-containing cream and lotion on dry skin of atopic dermatitis. Ski. Res. 1998, 40, 415–419. [Google Scholar]
- Imokawa, G.; Akasaki, S.; Kuno, O.; Zama, M.; Kawai, M.; Minematsu, Y. Function of lipids on human skin. J. Disp. Sci. Tech. 1989, 10, 617–641. [Google Scholar] [CrossRef]
- Imokawa, G.; Yada, Y.; Higuchi, K.; Okuda, M.; Ohashi, Y.; Kawamata, A. Pseudo-acylceramide with linoleic acid produces selective recovery of diminished cutaneous barrier function in essential fatty acid-deficient rats and has an inhibitory effect on epidermal hyperplasia. J. Clin. Investig. 1994, 94, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Surfactant-induced depletion of ceramides and other intercellular lipids: Implication for the mechanism leading to dehydration of the stratum corneum. Exog. Dermatol. 2004, 3, 81–98. [Google Scholar] [CrossRef]
- Imokawa, G.; Kuno, H.; Kawai, M. Stratum corneum lipids serve as a bound-water modulator. J. Investig. Dermatol. 1991, 96, 845–851. [Google Scholar] [CrossRef]
- Ledeen, R.W. Biology of gangliosides: Neuritogenic and neuronotrophic properties. J. Neurosci. Res. 1984, 12, 147–159. [Google Scholar] [CrossRef]
- Hamanaka, S.; Yamaguchi, Y.; Yamamoto, T.; Asagami, C. Occurrence of galactosylceramide in pig epidermal cells. Biochim. Biophys. Acta 1988, 961, 374–377. [Google Scholar]
- Hamanaka, S.; Takemoto, T.; Hamanaka, Y.; Asagami, C.; Suzuki, M.; Suzuki, A.; Otsuka, F. Structure determination of glycosphingolipids of cultured human keratinocytes. Biochim. Biophys. Acta 1993, 1167, 1–8. [Google Scholar] [CrossRef]
- Uchida, Y.; Iwamori, M.; Nagai, Y. Activation of keratinization of keratinocytes from fetal rat skin with N-(O-linoleoyl) omega-hydroxy fatty acyl sphingosyl glucose (lipokeratinogenoside) as a marker of epidermis. Biochem. Biophys. Res. Commun. 1990, 170, 162–168. [Google Scholar] [CrossRef]
- Hara, M.; Uchida, Y.; Haratake, A.; Mimura, K.; Hamanaka, S. Galactocerebroside and not glucocerebroside or ceramide stimulate epidermal beta-glucocerebrosidase activity. J. Dermatol. Sci. 1998, 16, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kage, M.; Itaya, Y.; Horikoshi, J.; Tokudome, Y. Effect of galactosylceramide on stratum corneum intercellular lipid synthesis in a three-dimensional cultured human epidermis model. Biochem. Biophys. Rep. 2021, 28, 101135. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Sawada, E.; Imokawa, G. A reconstructed human epidermal keratinization culture model to characterize ceramide metabolism in the stratum corneum. Arch. Dermatol. Res. 2012, 304, 563–577. [Google Scholar] [CrossRef]
- Sawada, E.; Yoshida, N.; Sugiura, A.; Imokawa, G. Th1 cytokines accentuate but Th2 cytokines attenuate ceramide production in the stratum corneum of human epidermal equivalents: An implication for the disrupted barrier mechanism in atopic dermatitis. J. Dermatol. Sci. 2012, 68, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Shimoda, H.; Takarada, T.; Imokawa, G. Strawberry seed extract and its major component, tiliroside, promote ceramide synthesis in the stratum corneum of human epidermal equivalents. PLoS ONE 2018, 13, e0205061. [Google Scholar] [CrossRef]
- Takeda, S.; Terazawa, S.; Shimoda, H.; Imokawa, G. β-Sitosterol 3-O-D-glucoside increases ceramide levels in the stratum corneum via the up-regulated expression of ceramide synthase-3 and glucosylceramide synthase in a reconstructed human epidermal keratinization model. PLoS ONE 2021, 16, e0248150. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, A.; Nomura, T.; Mizuno, A.; Imokawa, G. Reevaluation of the non-lesional dry skin in atopic dermatitis by acute barrier disruption: An abnormal permeability barrier homeostasis with defective processing to generate ceramide. Arch. Dermatol. Res. 2014, 306, 427–440. [Google Scholar] [CrossRef]
- Peng, J.; Chen, B.; Shen, Z.; Deng, H.; Liu, D.; Xie, X.; Gan, X.; Xu, X.; Huang, Z.; Chen, J. DNA promoter hypermethylation contributes to down-regulation of galactocerebrosidase gene in lung and head and neck cancers. Int. J. Clin. Exp. Pathol. 2015, 8, 11042–11050. [Google Scholar] [PubMed]
- Ogretmen, B.; Pettus, B.J.; Rossi, M.J.; Wood, R.; Usta, J.; Szulc, Z.; Bielawska, A.; Obeid, L.M.; Hannun, Y.A. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J. Biol. Chem. 2002, 277, 12960–12969. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Imokawa, G. The ceramide deficiency induced by IL-4 in the stratum corneum can be abrogated by rosiglitazone in human epidermal equivalents. J. Dermatol. Sci. 2016, 84, e42. [Google Scholar] [CrossRef]
- Mizutani, Y.; Sun, H.; Ohno, Y.; Sassa, T.; Wakashima, T.; Obara, M.; Yuyama, K.; Kihara, A.; Igarashi, Y. Cooperative Synthesis of Ultra Long-Chain Fatty Acid and Ceramide during Keratinocyte Differentiation. PLoS ONE 2013, 8, e67317. [Google Scholar] [CrossRef]
- Schmuth, M.; Haqq, C.M.; Cairns, W.J.; Holder, J.C.; Dorsam, S.; Chang, S.; Lau, P.; Fowler, A.J.; Chuang, G.; Moser, A.H.; et al. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J. Investig. Dermatol. 2004, 122, 971–983. [Google Scholar] [CrossRef]
- Shirakura, Y.; Kikuchi, K.; Matsumura, K.; Mukai, K.; Mitsutake, S.; Igarashi, Y. 4,8-Sphingadienine and 4-hydroxy-8-sphingenine activate ceramide production in the skin. Lipids Health Dis. 2012, 11, 108. [Google Scholar] [CrossRef]
- Chon, S.H.; Tannahill, R.; Yao, X.; Southall, M.D.; Pappas, A. Keratinocyte differentiation and upregulation of ceramide synthesis induced by an oat lipid extract via the activation of PPAR pathways. Exp. Dermatol. 2015, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Jiang, Y.J. The mechanisms by which lipids coordinately regulate the formation of the protein and lipid domains of the stratum corneum: Role of fatty acids, oxysterols, cholesterol sulfate and ceramides as signaling molecules. Dermatoendocrinol 2011, 3, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, S.; Hara, M.; Nishio, H.; Otsuka, F.; Suzuki, A.; Uchida, Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J. Investig. Dermatol. 2002, 119, 416–423. [Google Scholar] [CrossRef]
- Uchida, Y.; Hara, M.; Nishio, H.; Sidransky, E.; Inoue, S.; Otsuka, F.; Suzuki, A.; Elias, P.M.; Holleran, W.M.; Hamanaka, S. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J. Lipid Res. 2000, 41, 2071–2082. [Google Scholar] [CrossRef]
- Tokudome, Y.; Jinno, M.; Todo, H.; Kon, T.; Sugibayashi, K.; Hashimoto, F. Increase in ceramide level after application of various sizes of sphingomyelin liposomes to a cultured human skin model. Ski. Pharm. Physiol. 2011, 24, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nakagawa, H.; Matsuo, N.; Nomura, T.; Takizawa, M.; Imokawa, G. Biosynthesis of acylceramide in murine epidermis: Characterization by inhibition of glucosylation and deglucosylation, and by substrate specificity. J. Investig. Dermatol. 2004, 122, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nakagawa, H.; Yaginuma, T.; Takema, Y.; Imokawa, G. An accumulation of glucosylceramide in the stratum corneum due to attenuated activity of beta-glucocerebrosidase is associated with the early phase of UVB-induced alteration in cutaneous barrier function. Arch. Dermatol. Res. 2005, 297, 18–25. [Google Scholar] [CrossRef]
- Takagi, Y.; Kriehuber, E.; Imokawa, G.; Elias, P.M.; Holleran, W.M. Beta-glucocerebrosidase activity in mammalian stratum corneum. J. Lipid Res. 1999, 40, 861–869. [Google Scholar] [CrossRef]
- Holleran, W.M.; Takagi, Y.; Menon, G.K.; Jackson, S.M.; Lee, J.M.; Feingold, K.R.; Elias, P.M. Permeability barrier requirements regulate epidermal beta-glucocerebrosidase. J. Lipid Res. 1994, 35, 905–912. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Canals, D.; Hannun, Y.A. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal 2009, 21, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, K.; Hurwitz, R.; Zenk, T.; Desnick, R.J.; Ferlinz, K.; Schuchman, E.H.; Sandhoff, K. Purification, characterization, and biosynthesis of human acid ceramidase. J. Biol. Chem. 1995, 270, 11098–11102. [Google Scholar] [CrossRef]
- Sando, G.N.; Howard, E.J.; Madison, K.C. Induction of ceramide glucosyltransferase activity in cultured human keratinocytes. Correlation with culture differentiation. J. Biol. Chem. 1996, 271, 22044–22051. [Google Scholar] [CrossRef]
- Watanabe, R.; Wu, K.; Paul, P.; Marks, D.L.; Kobayashi, T.; Pittelkow, M.R.; Pagano, R.E. Up-regulation of glucosylceramide synthase expression and activity during human keratinocyte differentiation. J. Biol. Chem. 1998, 273, 9651–9655. [Google Scholar] [CrossRef]
- Chujor, C.S.; Feingold, K.R.; Elias, P.M.; Holleran, W.M. Glucosylceramide synthase activity in murine epidermis: Quantitation, localization, regulation, and requirement for barrier homeostasis. J. Lipid Res. 1998, 39, 277–285. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Ternes, P.; Holthuis, J.C. The multigenic sphingomyelin synthase family. J. Biol. Chem. 2006, 281, 29421–29425. [Google Scholar] [CrossRef]
- Michel, C.; van Echten-Deckert, G.; Rother, J.; Sandhoff, K.; Wang, E.; Merrill, A.H., Jr. Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J. Biol. Chem. 1997, 272, 22432–22437. [Google Scholar] [CrossRef]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef]
- Kihara, A. Very long-chain fatty acids: Elongation, physiology and related disorders. J. Biochem. 2012, 152, 387–395. [Google Scholar] [CrossRef]
- Hanada, K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 2003, 1632, 16–30. [Google Scholar] [CrossRef]
- Arana, L.; Gangoiti, P.; Ouro, A.; Trueba, M.; Gómez-Muñoz, A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Hauke, R.; Batra, S.K. Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin. Pharm. 2008, 83, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Mao-Qiang, M.; Fowler, A.J.; Schmuth, M.; Lau, P.; Chang, S.; Brown, B.E.; Moser, A.H.; Michalik, L.; Desvergne, B.; Wahli, W.; et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J. Investig. Dermatol. 2004, 123, 305–312. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsuda, H.; Kubo, M.; Morikawa, T.; Nishida, N.; Yoshikawa, M. Potent anti-obese principle from Rosa canina: Structural requirements and mode of action of trans-tiliroside. Bioorg. Med. Chem. Lett 2007, 17, 3059–3064. [Google Scholar] [CrossRef]
- Escher, P.; Wahli, W. Peroxisome proliferator-activated receptors: Insight into multiple cellular functions. Mutat Res. 2000, 448, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Wahli, W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology 1998, 139, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Barish, G.; Lu, B.; Evans, R.M.; Crumrine, D.; Schmuth, M.; Elias, P.M.; Feingold, K.R. PPARδ activation promotes stratum corneum formation and epidermal permeability barrier development during late gestation. J. Investig. Dermatol. 2010, 130, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Rivier, M.; Castiel, I.; Safonova, I.; Ailhaud, G.; Michel, S. Peroxisome proliferator-activated receptor-alpha enhances lipid metabolism in a skin equivalent model. J. Investig. Dermatol. 2000, 114, 681–687. [Google Scholar] [CrossRef]
- Man, M.Q.; Choi, E.H.; Schmuth, M.; Crumrine, D.; Uchida, Y.; Elias, P.M.; Holleran, W.M.; Feingold, K.R. Basis for improved permeability barrier homeostasis induced by PPAR and LXR activators: Liposensors stimulate lipid synthesis, lamellar body secretion, and post-secretory lipid processing. J. Investig. Dermatol. 2006, 126, 386–392. [Google Scholar] [CrossRef]
- Man, M.Q.; Barish, G.D.; Schmuth, M.; Crumrine, D.; Barak, Y.; Chang, S.; Jiang, Y.; Evans, R.M.; Elias, P.M.; Feingold, K.R. Deficiency of PPARbeta/delta in the epidermis results in defective cutaneous permeability barrier homeostasis and increased inflammation. J. Investig. Dermatol. 2008, 128, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Motta, S.; Monti, M.; Sesana, S.; Caputo, R.; Carelli, S.; Ghidoni, R. Ceramide composition of the psoriatic scale. Biochim. Biophys. Acta 1993, 1182, 147–151. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Sato, H.; Naoe, A.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; et al. Comprehensive quantification of ceramide species in human stratum corneum. J. Lipid Res. 2009, 50, 1708–1719. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Shimizu, E.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; Kitahara, T.; et al. Characterization of overall ceramide species in human stratum corneum. J. Lipid Res. 2008, 49, 1466–1476. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Taira, N.; Tsuboi, T.; Yoshioka, M.; Masaki, H.; Muraoka, O. 3-O-Laurylglyceryl ascorbate reinforces skin barrier function through not only the reduction of oxidative stress but also the activation of ceramide synthesis. Int. J. Cosmet Sci. 2017, 39, 49–55. [Google Scholar] [CrossRef]


| Primer Name | Sequence | |
|---|---|---|
| SPT1 | Forward Reverse | 5′-GCGCGCTACTTGGAGAAAGA-3′ 5′-TGTTCCACCGTGACCACAAC-3′ |
| SPT2 | Forward Reverse | 5′-AGCCGCCAAAGTCCTTGAG-3′ 5′-GAAGGTGAAGGTCGGAGTCAACG-3′ |
| ELOVL4 | Forward Reverse | 5′-CATGTGTATCATCACTGTACG-3′ 5′-AAAGGAATTCAACTGGGCTC-3′ |
| CERS3 | Forward Reverse | 5′-ACATTCCACAAGGCAACCATTG-3′ 5′-CTCTTGATTCCGCCGACTCC-3′ |
| DES1 | Forward Reverse | 5′-GCGTTTGGCAGTTGCATTAA-3′ 5′-CATTGTGGGCAATCTCATGAA-3′ |
| GCS | Forward Reverse | 5′-ATGTGTCATTGCCTGGCATG-3′ 5′-CCAGGCGACTGCATAATCAAG-3′ |
| GBA | Forward Reverse | 5′-TGGCATTGCTGTACATTGG-3′ 5′-CGTTCTTCTGACTGGCAACC-3′ |
| SMS1 | Forward Reverse | 5′-CAACATTGGCGTAGACAT-3′ 5′-TAGGAGGTACTCGTTCGTG-3′ |
| SMS2 | Forward Reverse | 5′-ACTACTCTACCTGTGCCTGG-3′ 5′-AGCAGCCAGCAGATTAAATG-3′ |
| ASM | Forward Reverse | 5′-TGGCTCTATGAAGCGATGG-3′ 5′-AGGCCGATGTAGGTAGTTGC-3′ |
| aCDase | Forward Reverse | 5′-CCTTCTTCCTTGATGATCGC-3′ 5′-GTGGAGTTCACCATGGTTCG-3′ |
| INV | Forward Reverse | 5′-GGCCACCCAAACATAAATAACCAC-3′ 5′-CACCTAGCGGACCCGAAATAAGT-3′ |
| HRNR | Forward Reverse | 5′-AGGACAGGGCTATAGTCAGCA-3′ 5′-CCGAAGCGTGATGGGAGG-3′ |
| LOR | Forward Reverse | 5′-GAGTTGGAGGTGTTTTCCAGGG-3′ 5′-GCAGAACTAGATGCAGCCGGA-3′ |
| CNFN | Forward Reverse | 5′-ACACAGGTCTCACGGACTG-3′ 5′-CAGCACTCGC CAAAGTCGT-3′ |
| SPRR2B | Forward Reverse | 5′-GCCTGGGAACCATCAGAGAA-3′ 5′-TCATGCCCAGGTGAAAGACA-3′ |
| TGM1 | Forward Reverse | 5′-GCTCGAAGGCTCTGGGTTACA-3′ 5′-TCGCACAGGCACAAACGAC-3′ |
| TGM3 | Forward Reverse | 5′-TCACACAGACAAGTTCTCCA-3′ 5′-ATTGGAGAGTGGAAACACAG-3′ |
| PPARα | Forward Reverse | 5′-GGTGGACACGGAAAGCCCAC-3′ 5′-GGACCACAGGATAAGTCACC-3′ |
| PPARβ/δ | Forward Reverse | 5′-CACACGGCGCCCTTTG-3′ 5′-CCTTCTCTGCCTGCCACAA-3′ |
| PPARγ | Forward Reverse | 5′-ATTCTGGCCCACCAACTTTG-3′ 5′-TCCATTACGGAGAGATCCACG-3′ |
| GAPDH | Forward Reverse | 5′-GAAGGTGAAGGTCGGAGTCAACG-3′ 5′-AGTCCTTCCACGATAACCAAAGTTG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igarashi, T.; Yanagi, H.; Yagi, M.; Ichihashi, M.; Imokawa, G. Horse-Derived Ceramide Accentuates Glucosylceramide Synthase and Ceramide Synthase 3 by Activating PPARβ/δ and/or PPARγ to Stimulate Ceramide Synthesis. Biomedicines 2023, 11, 548. https://doi.org/10.3390/biomedicines11020548
Igarashi T, Yanagi H, Yagi M, Ichihashi M, Imokawa G. Horse-Derived Ceramide Accentuates Glucosylceramide Synthase and Ceramide Synthase 3 by Activating PPARβ/δ and/or PPARγ to Stimulate Ceramide Synthesis. Biomedicines. 2023; 11(2):548. https://doi.org/10.3390/biomedicines11020548
Chicago/Turabian StyleIgarashi, Tami, Hiroki Yanagi, Masayuki Yagi, Masamitsu Ichihashi, and Genji Imokawa. 2023. "Horse-Derived Ceramide Accentuates Glucosylceramide Synthase and Ceramide Synthase 3 by Activating PPARβ/δ and/or PPARγ to Stimulate Ceramide Synthesis" Biomedicines 11, no. 2: 548. https://doi.org/10.3390/biomedicines11020548
APA StyleIgarashi, T., Yanagi, H., Yagi, M., Ichihashi, M., & Imokawa, G. (2023). Horse-Derived Ceramide Accentuates Glucosylceramide Synthase and Ceramide Synthase 3 by Activating PPARβ/δ and/or PPARγ to Stimulate Ceramide Synthesis. Biomedicines, 11(2), 548. https://doi.org/10.3390/biomedicines11020548







