Characterization of Carnosine Effect on Human Microglial Cells under Basal Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Propagation and Maintenance of Cells
2.3. Analysis of Cell Viability
2.4. Analysis of Metabolites
2.5. Gene Expression Analysis by Quantitative Real-Time PCR (qRT-PCR)
2.6. Confocal Microscopy Analsysis
2.7. Statistical Analysis
3. Results
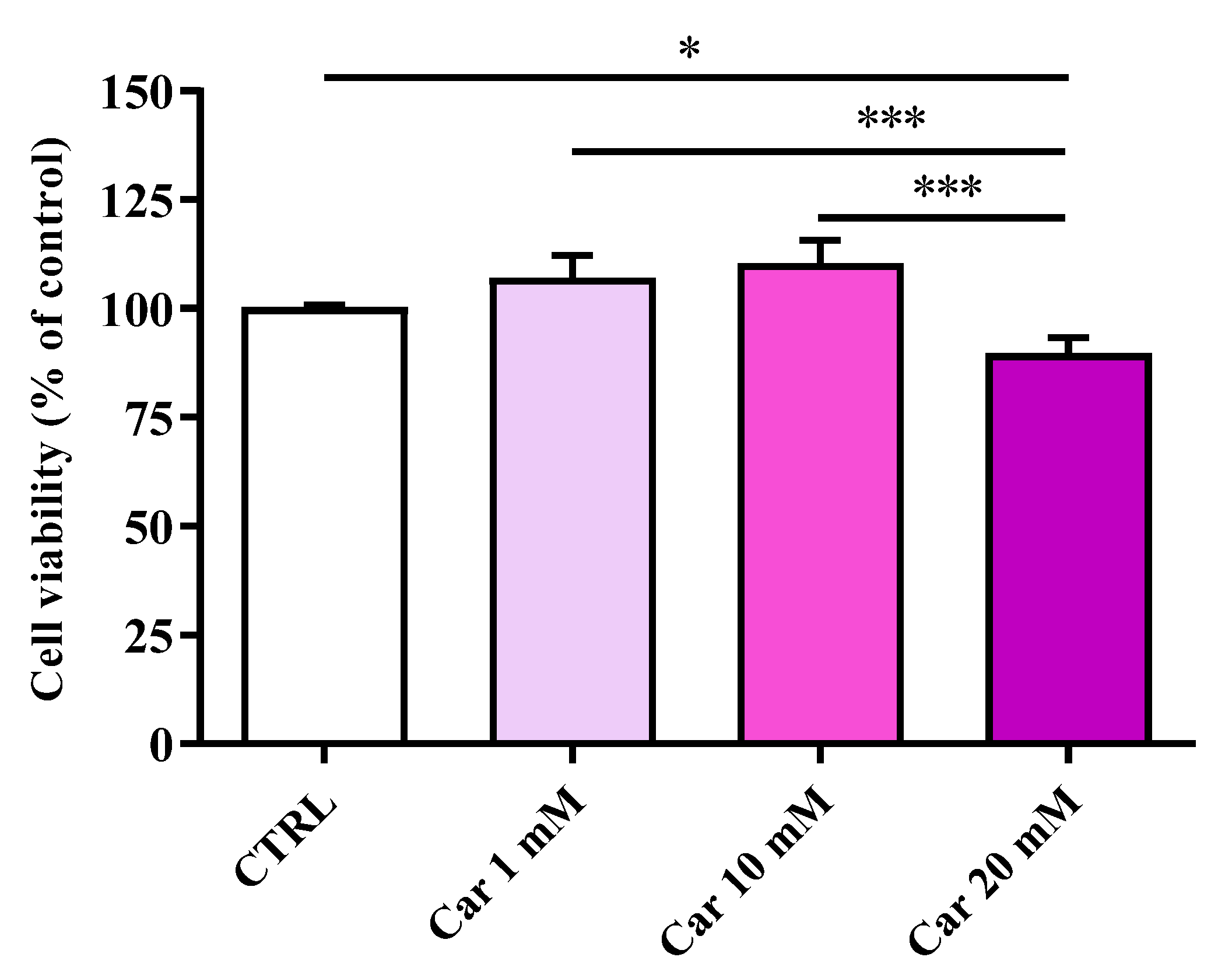
3.1. Carnosine Does Not Affect Cell Viability at Concentrations up to 10 mM
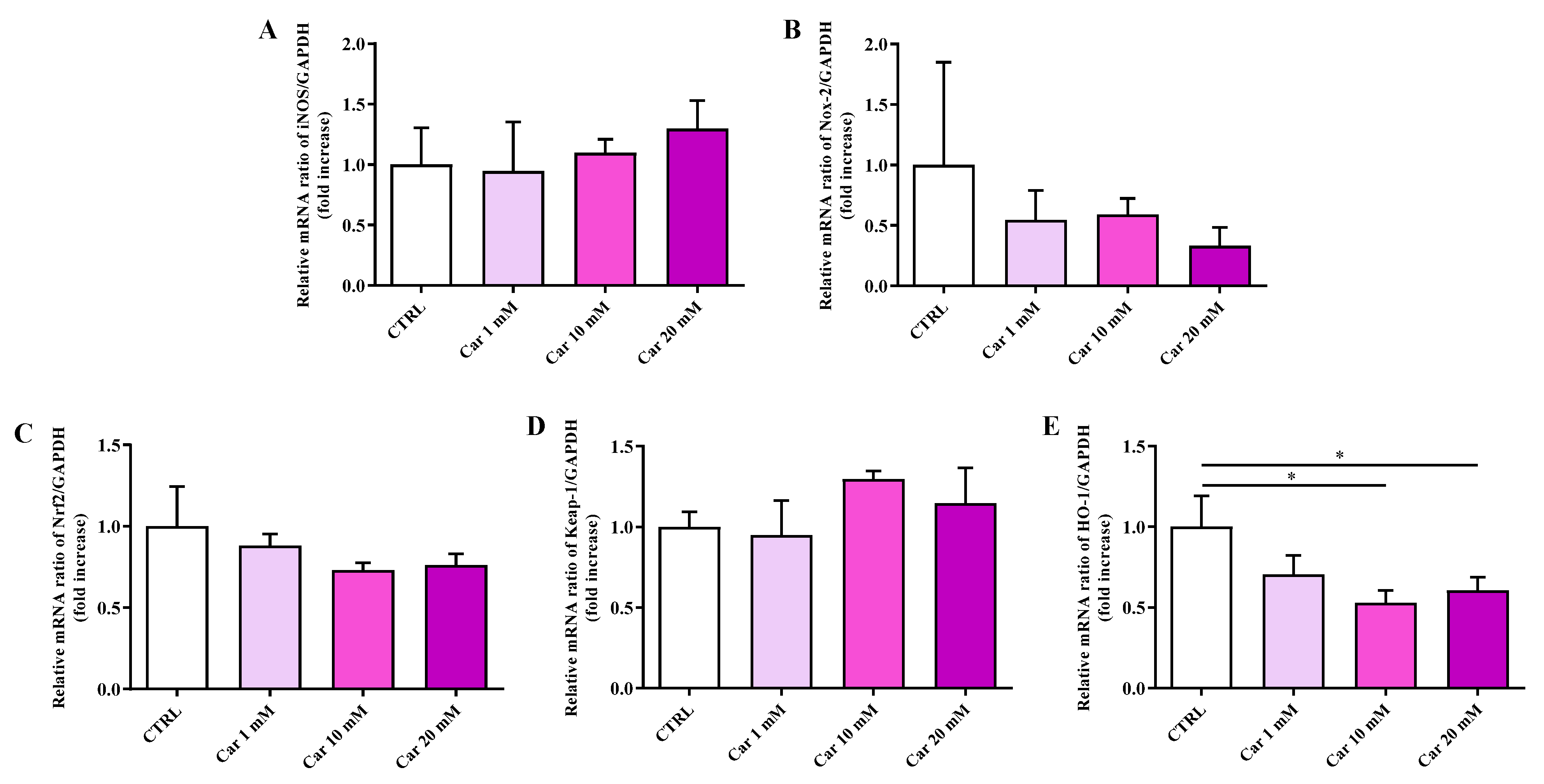
3.2. Carnosine at 20 mM Increases IL-6 and Decreases HO-1 mRNA Expression Levels
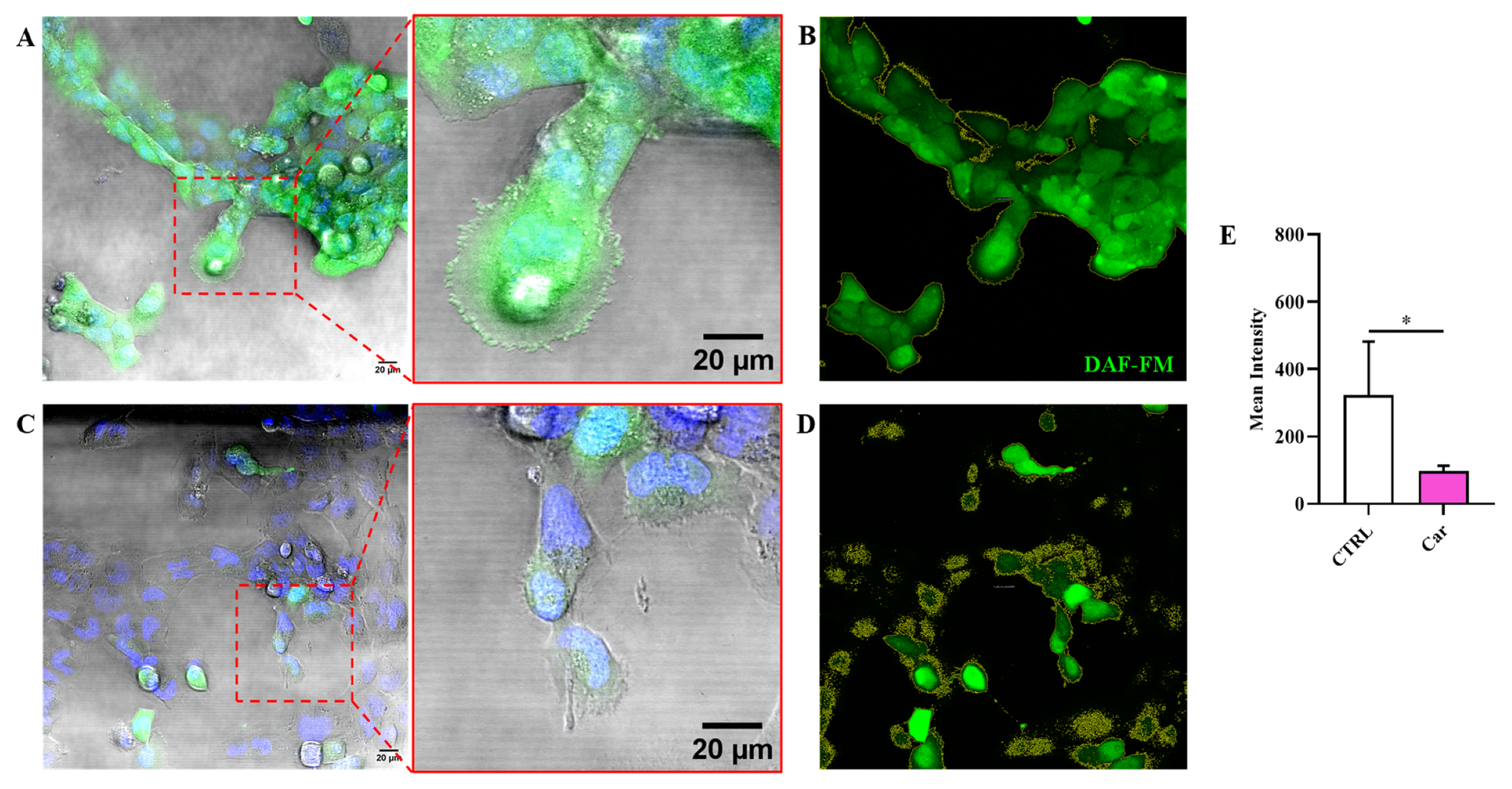
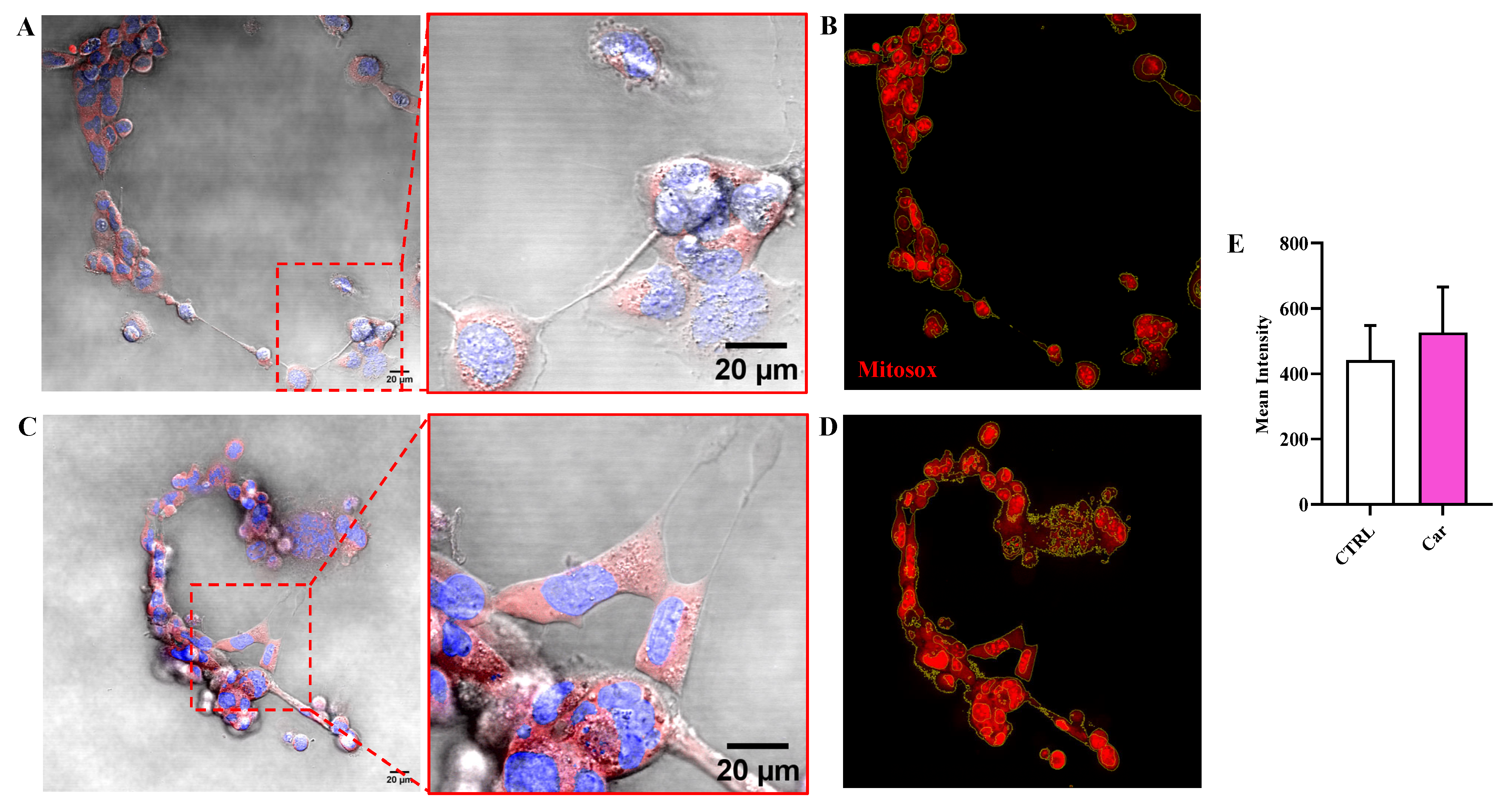
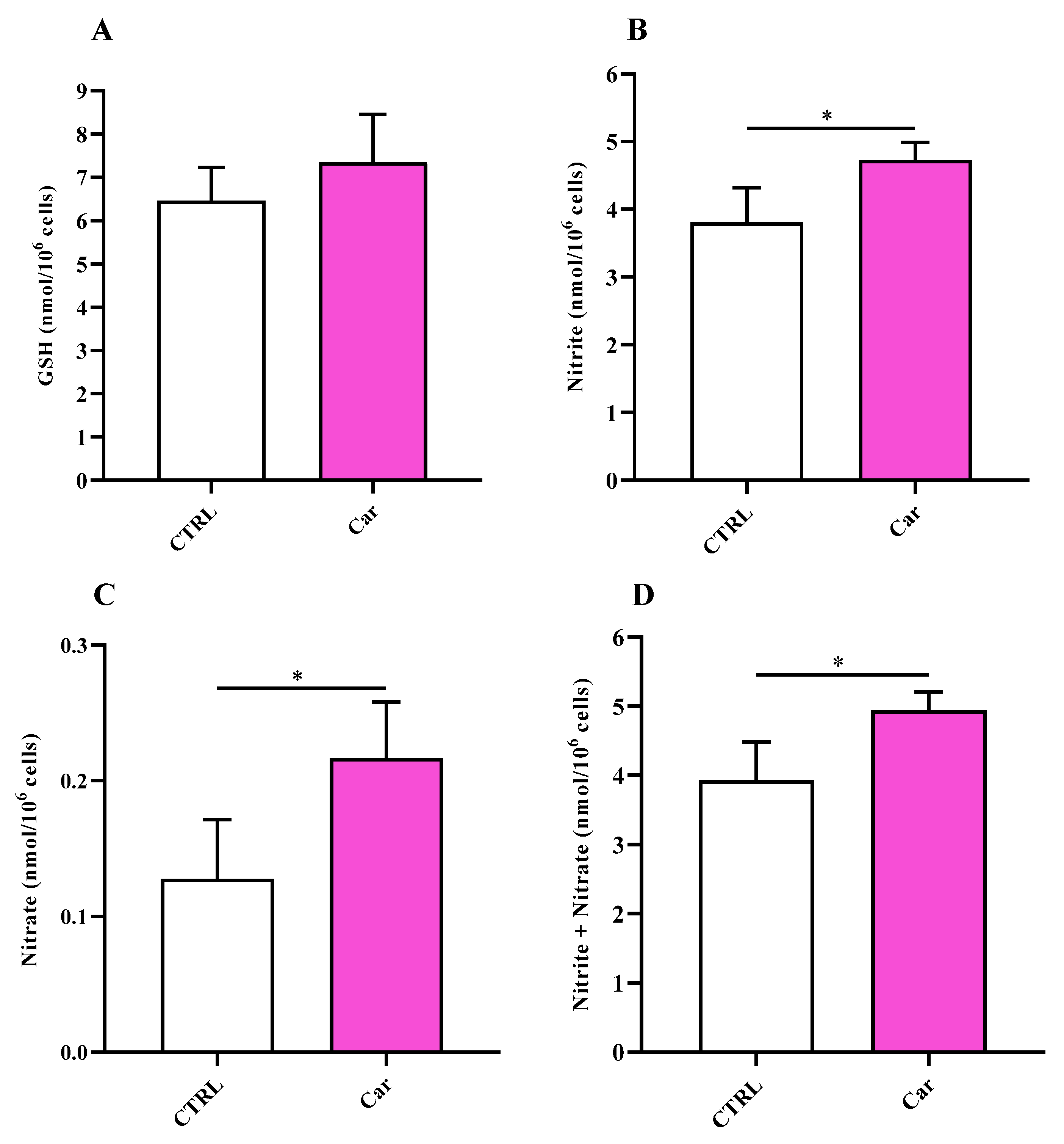
3.3. Carnosine Decreases NO Bioavailability
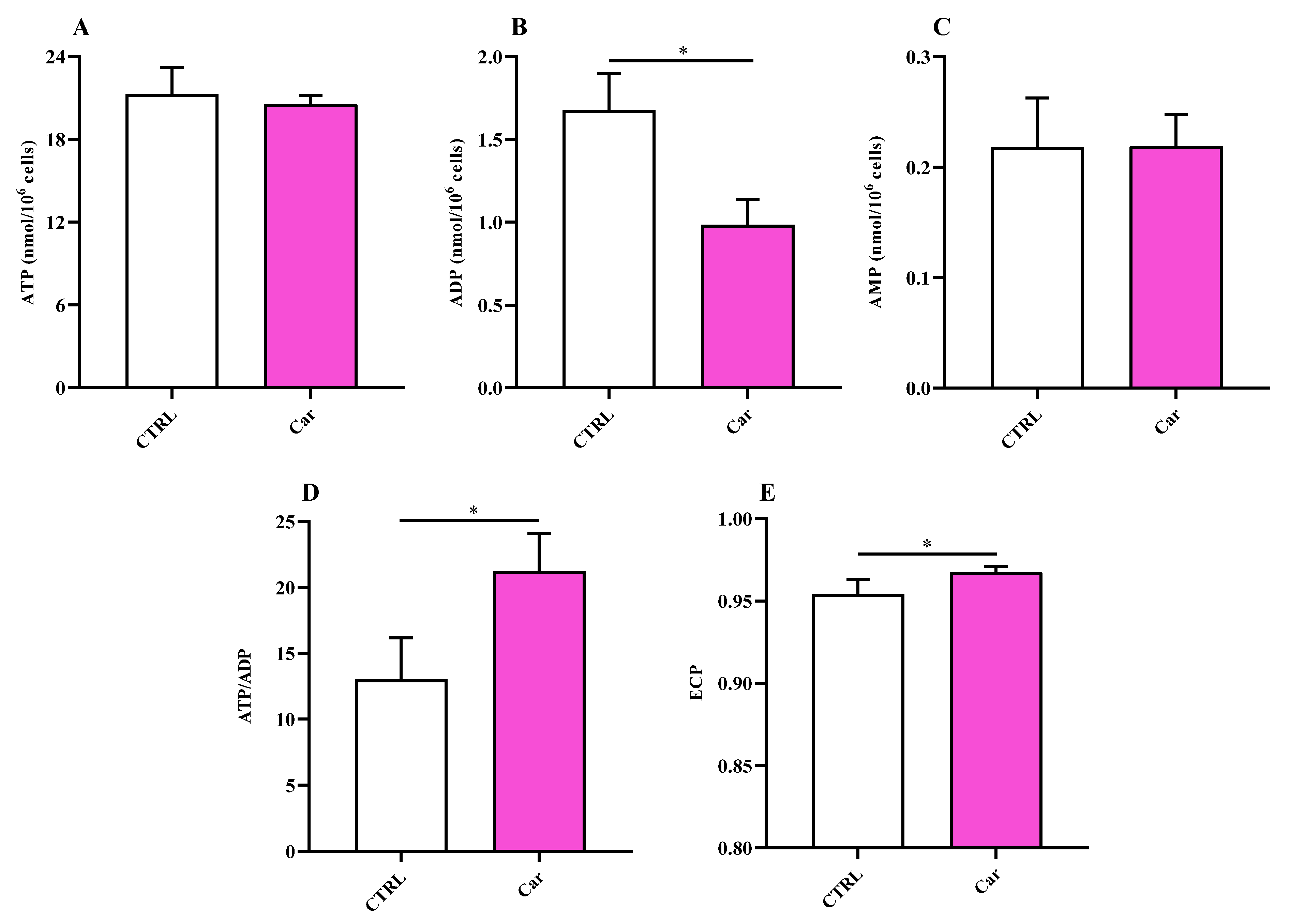
3.4. Carnosine Ameliorates Cellular Energy Metabolism Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Ursin, R.; Furset, K.; Aanderud, L. Eeg power spectra in rats during compression and during pentobarbital infusion at pressure. Undersea Biomed. Res. 1989, 16, 41–51. [Google Scholar] [PubMed]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, X.F.; Liu, C.S.; Wen, Z.L.; Du, J.L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 2012, 23, 1189–1202. [Google Scholar] [CrossRef]
- Delpech, J.C.; Madore, C.; Nadjar, A.; Joffre, C.; Wohleb, E.S.; Layé, S. Microglia in neuronal plasticity: Influence of stress. Neuropharmacology 2015, 96, 19–28. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Barron, K.D. The microglial cell. A historical review. J. Neurol. Sci. 1995, 134, 57–68. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in cns neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Liu, B.; Hong, J.-S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef]
- Dello Russo, C.; Lisi, L.; Tentori, L.; Navarra, P.; Graziani, G.; Combs, C.K. Exploiting microglial functions for the treatment of glioblastoma. Curr. Cancer Drug Targets 2017, 17, 267–281. [Google Scholar] [CrossRef]
- Mainz, E.R.; Gunasekara, D.B.; Caruso, G.; Jensen, D.T.; Hulvey, M.K.; Da Silva, J.A.F.; Metto, E.C.; Culbertson, A.H.; Culbertson, C.T.; Lunte, S.M. Monitoring intracellular nitric oxide production using microchip electrophoresis and laser-induced fluorescence detection. Anal. Methods 2012, 4, 414–420. [Google Scholar] [CrossRef]
- Gunasekara, D.B.; Siegel, J.M.; Caruso, G.; Hulvey, M.K.; Lunte, S.M. Microchip electrophoresis with amperometric detection method for profiling cellular nitrosative stress markers. Analyst 2014, 139, 3265–3273. [Google Scholar] [CrossRef]
- Caruso, G.; Spampinato, S.F.; Cardaci, V.; Caraci, F.; Sortino, M.A.; Merlo, S. Β-amyloid and oxidative stress: Perspectives in drug development. Curr. Pharm. Des. 2019, 25, 4771–4781. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (o&ns) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar]
- de Campos, R.P.; Siegel, J.M.; Fresta, C.G.; Caruso, G.; da Silva, J.A.; Lunte, S.M. Indirect detection of superoxide in raw 264.7 macrophage cells using microchip electrophoresis coupled to laser-induced fluorescence. Anal. Bioanal. Chem. 2015, 407, 7003–7012. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Preventing ca2+-mediated nitrosative stress in neurodegenerative diseases: Possible pharmacological strategies. Cell Calcium 2010, 47, 190–197. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Du, L.; Zhang, Y.; Chen, Y.; Zhu, J.; Yang, Y.; Zhang, H.L. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol. Neurobiol. 2017, 54, 7567–7584. [Google Scholar] [CrossRef]
- Rangarajan, P.; Karthikeyan, A.; Dheen, S.T. Role of dietary phenols in mitigating microglia-mediated neuroinflammation. Neuromol. Med. 2016, 18, 453–464. [Google Scholar] [CrossRef]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; Grosso, G.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022, 232, 108013. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic acids and prevention of cognitive decline: Polyphenols with a neuroprotective role in cognitive disorders and alzheimer’s disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Kalyankar, G.D.; Meister, A. Enzymatic synthesis of carnosine and related beta-alanyl and gamma-aminobutyryl peptides. J. Biol. Chem. 1959, 234, 3210–3218. [Google Scholar] [CrossRef]
- Winnick, R.E.; Winnick, T. Carnosineanserine synthetase of muscle. I. Preparation and properties of soluble enzyme from chick muscle. Biochim. Biophys.Acta 1959, 31, 47–55. [Google Scholar] [CrossRef]
- Gariballa, S.E.; Sinclair, A.J. Carnosine: Physiological properties and therapeutic potential. Age Ageing 2000, 29, 207–210. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Preston, J.E.; Himsworth, D.T.; Worthington, V.C.; Keown, M.; Michaelis, J.; Lawrence, J.; Mateen, A.; Allende, L.; Eagles, P.A.; et al. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann. N. Y. Acad. Sci. 1998, 854, 37–53. [Google Scholar] [CrossRef]
- Prokopieva, V.D.; Yarygina, E.G.; Bokhan, N.A.; Ivanova, S.A. Use of carnosine for oxidative stress reduction in different pathologies. Oxid. Med. Cell. Longev. 2016, 2016, 2939087. [Google Scholar] [CrossRef]
- Kubota, M.; Kobayashi, N.; Sugizaki, T.; Shimoda, M.; Kawahara, M.; Tanaka, K.I. Carnosine suppresses neuronal cell death and inflammation induced by 6-hydroxydopamine in an in vitro model of parkinson’s disease. PLoS ONE 2020, 15, e0240448. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine decreases pma-induced oxidative stress and inflammation in murine macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.F.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine prevents aβ-induced oxidative stress and inflammation in microglial cells: A key role of tgf-β1. Cells 2019, 8, 64. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; de Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine raw 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef]
- Dello Russo, C.; Cappoli, N.; Coletta, I.; Mezzogori, D.; Paciello, F.; Pozzoli, G.; Navarra, P.; Battaglia, A. The human microglial hmc3 cell line: Where do we stand? A systematic literature review. J. Neuroinflamm. 2018, 15, 259. [Google Scholar] [CrossRef]
- Caruso, G.; Distefano, D.A.; Parlascino, P.; Fresta, C.G.; Lazzarino, G.; Lunte, S.M.; Nicoletti, V.G. Receptor-mediated toxicity of human amylin fragment aggregated by short- and long-term incubations with copper ions. Mol. Cell. Biochem. 2017, 425, 85–93. [Google Scholar] [CrossRef]
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.M.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water- and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants 2019, 8, 96. [Google Scholar] [CrossRef]
- Lazzarino, G.; Listorti, I.; Muzii, L.; Amorini, A.M.; Longo, S.; Di Stasio, E.; Caruso, G.; D’Urso, S.; Puglia, I.; Pisani, G.; et al. Low-molecular weight compounds in human seminal plasma as potential biomarkers of male infertility. Hum. Reprod. 2018, 33, 1817–1828. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Fazzina, G.; Vagnozzi, R.; Signoretti, S.; Donzelli, S.; Di Stasio, E.; Giardina, B.; Tavazzi, B. Single-sample preparation for simultaneous cellular redox and energy state determination. Anal. Biochem. 2003, 322, 51–59. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Lazzarino, G.; Musso, N.; Grasso, M.; Merlo, S.; Amorini, A.M.; Bucolo, C.; Tavazzi, B.; Lazzarino, G.; et al. Modulation of pro-oxidant and pro-inflammatory activities of m1 macrophages by the natural dipeptide carnosine. Int. J. Mol. Sci. 2020, 21, 776. [Google Scholar] [CrossRef]
- Pelicci, S.; Diaspro, A.; Lanzanò, L. Chromatin nanoscale compaction in live cells visualized by acceptor-to-donor ratio corrected förster resonance energy transfer between DNA dyes. J. Biophotonics 2019, 12, e201900164. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Siegel, J.M.; Wijesinghe, M.B.; Lunte, S.M. Microchip electrophoresis with laser-induced fluorescence detection for the determination of the ratio of nitric oxide to superoxide production in macrophages during inflammation. Anal. Bioanal. Chem. 2017, 409, 4529–4538. [Google Scholar] [CrossRef]
- Fidilio, A.; Grasso, M.; Turnaturi, R.; Caruso, G.; Spitale, F.M.; Vicario, N.; Parenti, R.; Spoto, S.; Musso, N.; Marrazzo, A.; et al. The multimodal mopr/dopr agonist lp2 reduces allodynia in chronic constriction injured rats by rescue of tgf-β1 signalling. Front. Pharmacol. 2021, 12, 749365. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Choudhury, M.E.; Kigami, Y.; Utsunomiya, R.; Matsumoto, S.; Watanabe, H.; Kumon, Y.; Kunieda, T.; Yano, H.; Tanaka, J. Sustained anti-inflammatory effects of tgf-β1 on microglia/macrophages. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tyml, K.; Wilson, J.X. Inos expression requires nadph oxidase-dependent redox signaling in microvascular endothelial cells. J. Cell. Physiol. 2008, 217, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Vomhof-Dekrey, E.E.; Picklo, M.J., Sr. The nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Mehlal, S.; Jeljeli, M.; Saidu, N.E.B.; Nicco, C.; Cerles, O.; Chouzenoux, S.; Cauvet, A.; Camus, C.; Ait-Djoudi, M.; et al. Corrigendum: The nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front. Immunol. 2021, 12, 737303. [Google Scholar] [CrossRef]
- Akella, N.M.; Ciraku, L.; Reginato, M.J. Fueling the fire: Emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019, 17, 52. [Google Scholar] [CrossRef]
- Giallongo, C.; Tibullo, D.; Puglisi, F.; Barbato, A.; Vicario, N.; Cambria, D.; Parrinello, N.L.; Romano, A.; Conticello, C.; Forte, S.; et al. Inhibition of tlr4 signaling affects mitochondrial fitness and overcomes bortezomib resistance in myeloma plasma cells. Cancers 2020, 12, 1999. [Google Scholar] [CrossRef]
- Perry, V.H.; Teeling, J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia morphophysiological diversity and its implications for the cns. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Kádasi, A.; Maruniaková, N.; Štochmaľová, A.; Bauer, M.; Grossmann, R.; Harrath, A.H.; Kolesárová, A.; Sirotkin, A.V. Direct effect of curcumin on porcine ovarian cell functions. Anim. Reprod. Sci. 2017, 182, 77–83. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, J.J.; Cai, Z.N.; Xu, C.J. The effect of curcumin on the differentiation, apoptosis and cell cycle of neural stem cells is mediated through inhibiting autophagy by the modulation of atg7 and p62. Int. J. Mol. Med. 2018, 42, 2481–2488. [Google Scholar] [CrossRef]
- Fu, Y.C.; Jin, X.P.; Wei, S.M. The effects on cell growth of tea polyphenols acting as a strong anti-peroxidatant and an inhibitor of apoptosis in primary cultured rat skin cells. Biomed. Environ. Sci. 2000, 13, 170–179. [Google Scholar]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Characterization of resveratrol, oxyresveratrol, piceatannol and roflumilast as modulators of phosphodiesterase activity. Study of yeast lifespan. Pharmaceuticals 2020, 13, 225. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting camp phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef]
- Bissacotti, B.F.; Copetti, P.M.; Bottari, N.B.; Gündel, S.D.S.; Machado, A.K.; Sagrillo, M.R.; Ourique, A.F.; Morsch, V.M.M.; da Silva, A.S. Impact of free curcumin and curcumin nanocapsules on viability and oxidative status of neural cell lines. Drug Chem. Toxicol. 2021, 46, 155–165. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, W.; Liu, T.; Mosenthin, R.; Bao, Y.; Chen, P.; Hao, W.; Zhao, L.; Zhang, J.; Ji, C. Improved satellite cell proliferation induced by l-carnosine benefits muscle growth of pigs in part through activation of the akt/mtor/s6k signaling pathway. Agriculture 2022, 12, 988. [Google Scholar] [CrossRef]
- Rybakova, Y.S.; Boldyrev, A.A. Effect of carnosine and related compounds on proliferation of cultured rat pheochromocytoma pc-12 cells. Bull. Exp. Biol. Med. 2012, 154, 136–140. [Google Scholar] [CrossRef]
- Ikeda, D.; Wada, S.; Yoneda, C.; Abe, H.; Watabe, S. Carnosine stimulates vimentin expression in cultured rat fibroblasts. Cell Struct. Funct. 1999, 24, 79–87. [Google Scholar] [CrossRef]
- Vishniakova Kh, S.; Babizhaev, M.A.; Aliper, A.M.; Buzdin, A.A.; Kudriavtseva, A.V.; Egorov, E.E. Stimulation of proliferation by carnosine: Cellular and transcriptome approaches. Mol. Biol. 2014, 48, 824–833. [Google Scholar] [CrossRef]
- Maier, A.B.; Cohen, R.; Blom, J.; van Heemst, D.; Westendorp, R.G. Marked heterogeneity in growth characteristics of myoblast clonal cultures and myoblast mixed cultures obtained from the same individual. Gerontology 2012, 58, 150–155. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Castellano, S.; Micek, A.; Murabito, P.; Galvano, F.; Ferri, R.; Grosso, G.; Caraci, F. The therapeutic potential of carnosine/anserine supplementation against cognitive decline: A systematic review with meta-analysis. Biomedicines 2021, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Cripps, M.J.; Hanna, K.; Lavilla, C., Jr.; Sayers, S.R.; Caton, P.W.; Sims, C.; De Girolamo, L.; Sale, C.; Turner, M.D. Carnosine scavenging of glucolipotoxic free radicals enhances insulin secretion and glucose uptake. Sci. Rep. 2017, 7, 13313. [Google Scholar] [CrossRef] [PubMed]
- Jukić, I.; Kolobarić, N.; Stupin, A.; Matić, A.; Kozina, N.; Mihaljević, Z.; Mihalj, M.; Šušnjara, P.; Stupin, M.; Ćurić Ž, B.; et al. Carnosine, small but mighty-prospect of use as functional ingredient for functional food formulation. Antioxidants 2021, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Książek, K. L-carnosine prevents the pro-cancerogenic activity of senescent peritoneal mesothelium towards ovarian cancer cells. Anticancer Res. 2016, 36, 665–671. [Google Scholar] [PubMed]
- Alpsoy, L.; Akcayoglu, G.; Sahin, H. Anti-oxidative and anti-genotoxic effects of carnosine on human lymphocyte culture. Hum. Exp. Toxicol. 2011, 30, 1979–1985. [Google Scholar] [CrossRef]
- Fresta, C.G.; Hogard, M.L.; Caruso, G.; Melo Costa, E.E.; Lazzarino, G.; Lunte, S.M. Monitoring carnosine uptake by raw 264.7 macrophage cells using microchip electrophoresis with fluorescence detection. Anal. Methods 2017, 9, 402–408. [Google Scholar] [CrossRef]
- Zhang, A.; Suzuki, T.; Adachi, S.; Naganuma, E.; Suzuki, N.; Hosoya, T.; Itoh, K.; Sporn, M.B.; Yamamoto, M. Distinct regulations of ho-1 gene expression for stress response and substrate induction. Mol. Cell. Biol. 2021, 41, e0023621. [Google Scholar] [CrossRef]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; De Galarreta, C.M.; Cuadrado, A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/akt pathway and the nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef]
- Lee, P.J.; Jiang, B.H.; Chin, B.Y.; Iyer, N.V.; Alam, J.; Semenza, G.L.; Choi, A.M. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef]
- Li, R.; Wall, S.; Li, Q.; Dunigan, K.; Tipple, T. Nrf2 independent heme oxygenase-1 induction by auranofin is in lung macrophages. Free Radic. Biol. Med. 2018, 128, S30. [Google Scholar] [CrossRef]
- Caruso, G.; Benatti, C.; Musso, N.; Fresta, C.G.; Fidilio, A.; Spampinato, G.; Brunello, N.; Bucolo, C.; Drago, F.; Lunte, S.M.; et al. Carnosine protects macrophages against the toxicity of aβ1-42 oligomers by decreasing oxidative stress. Biomedicines 2021, 9, 477. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakashima, Y.; Toda, K. Carnosine facilitates nitric oxide production in endothelial f-2 cells. Biol. Pharm. Bull. 2009, 32, 1836–1839. [Google Scholar] [CrossRef]
- Fedorova, T.; Belyaev, M.; Trunova, O.; Gnezditsky, V.; Maximova, M.Y.; Boldyrev, A. Neuropeptide carnosine increases stability of lipoproteins and red blood cells as well as efficiency of immune competent system in patients with chronic discirculatory encephalopathy. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2009, 3, 62–65. [Google Scholar] [CrossRef]
- Ahshin-Majd, S.; Zamani, S.; Kiamari, T.; Kiasalari, Z.; Baluchnejadmojarad, T.; Roghani, M. Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: Possible involved mechanisms. Peptides 2016, 86, 102–111. [Google Scholar] [CrossRef]
- Tibullo, D.; Barbagallo, I.; Giallongo, C.; Vanella, L.; Conticello, C.; Romano, A.; Saccone, S.; Godos, J.; Di Raimondo, F.; Li Volti, G. Heme oxygenase-1 nuclear translocation regulates bortezomibinduced cytotoxicity and mediates genomic instability in myeloma cells. Oncotarget 2016, 7, 28868–28880. [Google Scholar] [CrossRef]
- DeVan, A.E.; Johnson, L.C.; Brooks, F.A.; Evans, T.D.; Justice, J.N.; Cruickshank-Quinn, C.; Reisdorph, N.; Bryan, N.S.; McQueen, M.B.; Santos-Parker, J.R.; et al. Effects of sodium nitrite supplementation on vascular function and related small metabolite signatures in middle-aged and older adults. J. Appl. Physiol. (1985) 2016, 120, 416–425. [Google Scholar] [CrossRef]
- Vanella, L.; Barbagallo, I.; Tibullo, D.; Forte, S.; Zappalà, A.; Li Volti, G. The non-canonical functions of the heme oxygenases. Oncotarget 2016, 7, 69075–69086. [Google Scholar] [CrossRef]
- Bryan, N.S.; Fernandez, B.O.; Bauer, S.M.; Garcia-Saura, M.F.; Milsom, A.B.; Rassaf, T.; Maloney, R.E.; Bharti, A.; Rodriguez, J.; Feelisch, M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat. Chem. Biol. 2005, 1, 290–297. [Google Scholar] [CrossRef]
- Gladwin, M.T. Nitrite as an intrinsic signaling molecule. Nat. Chem. Biol. 2005, 1, 245–246. [Google Scholar] [CrossRef]
- Pellegrino, D.; Parisella, M.L. Nitrite as a physiological source of nitric oxide and a signalling molecule in the regulation of the cardiovascular system in both mammalian and non-mammalian vertebrates. Recent Pat. Cardiovasc. Drug Discov. 2010, 5, 91–96. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological chemistry of superoxide radicals. ChemTexts 2020, 6, 7. [Google Scholar] [CrossRef]
- Buetler, T.M.; Krauskopf, A.; Ruegg, U.T. Role of superoxide as a signaling molecule. News Physiol. Sci. 2004, 19, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ros) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040–1052. [Google Scholar] [CrossRef]
- Bielanin, J.P.; Sun, D. Significance of microglial energy metabolism in maintaining brain homeostasis. Transl. Stroke Res. 2022. [Google Scholar] [CrossRef]
- Bernier, L.P.; York, E.M.; MacVicar, B.A. Immunometabolism in the brain: How metabolism shapes microglial function. Trends Neurosci. 2020, 43, 854–869. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, R.; Xu, Z.; Ke, Y.; Sun, R.; Yang, H.; Zhang, X.; Zhen, X.; Zheng, L.T. Early glycolytic reprogramming controls microglial inflammatory activation. J. Neuroinflamm. 2021, 18, 129. [Google Scholar] [CrossRef]
- Ghosh, S.; Castillo, E.; Frias, E.S.; Swanson, R.A. Bioenergetic regulation of microglia. Glia 2018, 66, 1200–1212. [Google Scholar] [CrossRef]
- Longhitano, L.; Distefano, A.; Murabito, P.; Astuto, M.; Nicolosi, A.; Buscema, G.; Sanfilippo, F.; Lazzarino, G.; Amorini, A.M.; Bruni, A.; et al. Propofol and α2-agonists attenuate microglia activation and restore mitochondrial function in an in vitro model of microglia hypoxia/reoxygenation. Antioxidants 2022, 11, 1682. [Google Scholar] [CrossRef]
- Di Pierro, D.; Tavazzi, B.; Perno, C.F.; Bartolini, M.; Balestra, E.; Caliò, R.; Giardina, B.; Lazzarino, G. An ion-pairing high-performance liquid chromatographic method for the direct simultaneous determination of nucleotides, deoxynucleotides, nicotinic coenzymes, oxypurines, nucleosides, and bases in perchloric acid cell extracts. Anal. Biochem. 1995, 231, 407–412. [Google Scholar] [CrossRef]
- Burgener, A.; Coombs, K.; Butler, M. Intracellular atp and total adenylate concentrations are critical predictors of reovirus productivity from vero cells. Biotechnol. Bioeng. 2006, 94, 667–679. [Google Scholar] [CrossRef]
- Tibullo, D.; Giallongo, C.; Romano, A.; Vicario, N.; Barbato, A.; Puglisi, F.; Parenti, R.; Amorini, A.M.; Wissam Saab, M.; Tavazzi, B.; et al. Mitochondrial functions, energy metabolism and protein glycosylation are interconnected processes mediating resistance to bortezomib in multiple myeloma cells. Biomolecules 2020, 10, 696. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Rong, H.; Zhang, X.; Dong, M. Ferulic acid ameliorates mpp(+)/mptp-induced oxidative stress via erk1/2-dependent nrf2 activation: Translational implications for parkinson disease treatment. Mol. Neurobiol. 2020, 57, 2981–2995. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Barnes, N.M.; Bruce, L.; Mordente, A.; Lazzarino, G.; Pietro, V.D.; Tavazzi, B.; Belli, A.; Logan, A. Low molecular weight dextran sulfate (ilb(®)) administration restores brain energy metabolism following severe traumatic brain injury in the rat. Antioxidants 2020, 9, 850. [Google Scholar] [CrossRef]
- Chakrabarty, R.P.; Chandel, N.S. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell 2021, 28, 394–408. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Kahmini, F.R. A comprehensive review of sirtuins: With a major focus on redox homeostasis and metabolism. Life Sci. 2021, 282, 119803. [Google Scholar] [CrossRef]
- Fago, A. New insights into survival strategies to oxygen deprivation in anoxia-tolerant vertebrates. Acta Physiol. 2022, 235, e13841. [Google Scholar] [CrossRef]
- Thöni, V.; Mauracher, D.; Ramalingam, A.; Fiechtner, B.; Sandbichler, A.M.; Egg, M. Quantum based effects of therapeutic nuclear magnetic resonance persistently reduce glycolysis. iScience 2022, 25, 105536. [Google Scholar] [CrossRef]
- Kay, L.; Potenza, L.; Hininger-Favier, I.; Roth, H.; Attia, S.; Tellier, C.; Zuppinger, C.; Calcabrini, C.; Sestili, P.; Wallimann, T.; et al. Supplementing soy-based diet with creatine in rats: Implications for cardiac cell signaling and response to doxorubicin. Nutrients 2022, 14, 583. [Google Scholar] [CrossRef]
- Nanni, S.; Aiello, A.; Salis, C.; Re, A.; Cencioni, C.; Bacci, L.; Pierconti, F.; Pinto, F.; Ripoli, C.; Ostano, P.; et al. Metabolic reprogramming by malat1 depletion in prostate cancer. Cancers 2020, 13, 15. [Google Scholar] [CrossRef]
- Ciccarone, F.; Di Leo, L.; Lazzarino, G.; Maulucci, G.; Di Giacinto, F.; Tavazzi, B.; Ciriolo, M.R. Aconitase 2 inhibits the proliferation of mcf-7 cells promoting mitochondrial oxidative metabolism and ros/foxo1-mediated autophagic response. Br. J. Cancer 2020, 122, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]


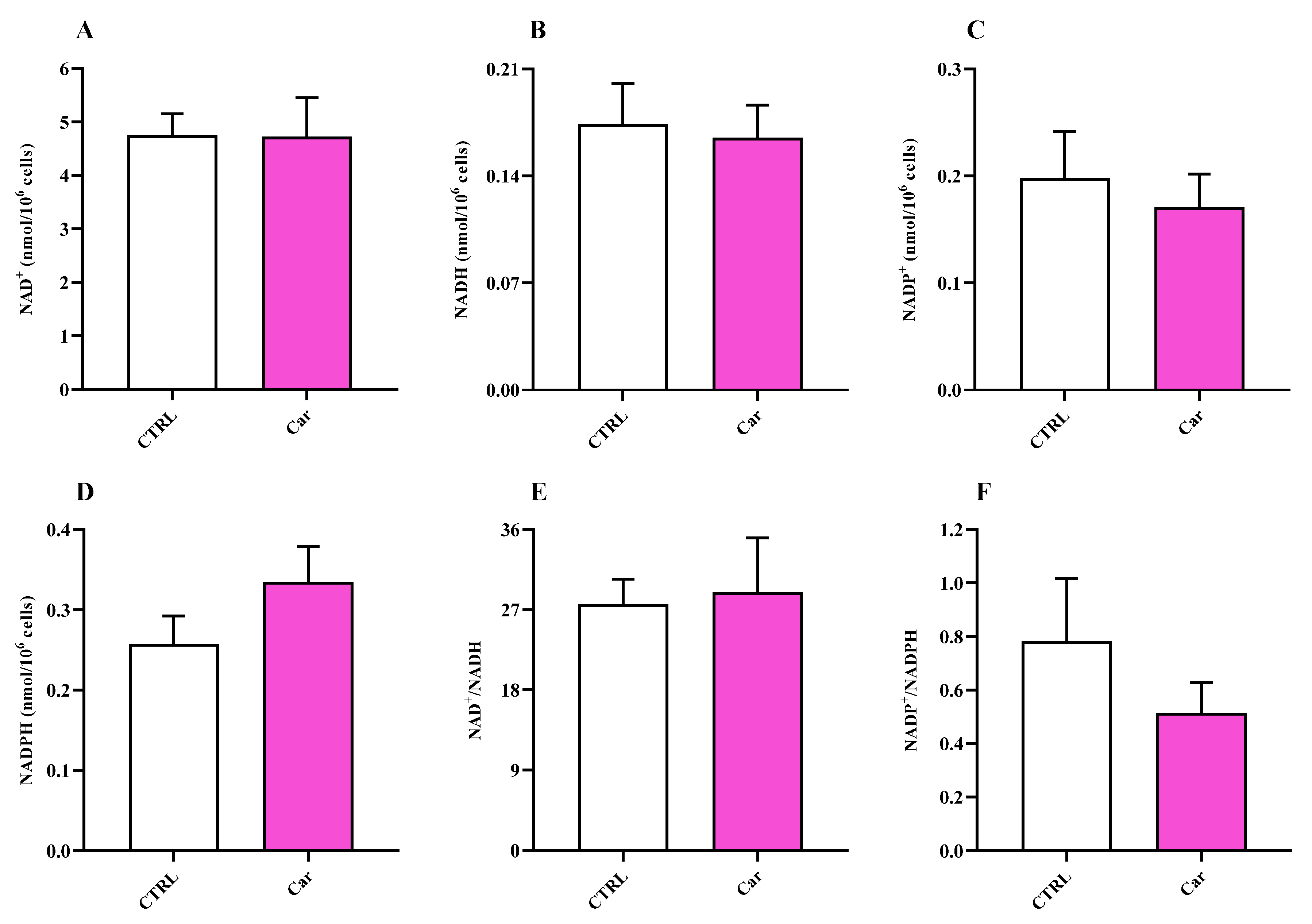

| Official Name # | Official Symbol | Alternative Titles/Symbols | Detected Transcript | Amplicon Length | Cat. No. § |
|---|---|---|---|---|---|
| interleukin 1, beta | IL1B | IL-1; IL1F2; IL1beta; IL1-BETA | NM_000576; XM_006712496 | 117 bp | QT00021385 |
| interleukin 6 | IL6 | CDF; HGF; HSF; BSF2; IL-6; BSF-2; IFNB2; IFN-beta-2 | NM_000600; XM_005249745 | 107 bp | QT00083720 |
| nitric oxide synthase 2 | NOS2 | NOS; INOS; NOS2A; HEP-NOS | NM_000625; NM_153292 | 92 bp | QT00068740 |
| cytochrome b-245 beta chain | CYBB | CGD; CGDX; NOX2; IMD34; AMCBX2; GP91-1; GP91PHOX; p91-PHOX; GP91-PHOX | NM_000397 | 124 bp | QT00029533 |
| heme oxygenase 1 | HMOX1 | HO-1; HSP32; HMOX1D; bK286B10 | NM_002133 | 99 bp | QT00092645 |
| kelch like ECH associated protein 1 | KEAP1 | INrf2; KLHL19 | XM_005260174; NM_012289; NM_203500; XM_005260173 | 109 bp | QT00080220 |
| NFE2 like bZIP transcription factor 2 | NFE2L2 | NRF2; HEBP1; Nrf-2; IMDDHH | NM_006164 | 153 bp | QT00027384 |
| transforming growth factor beta 1 | TGFB1 | CED; LAP; DPD1; TGFB; IBDIMDE; TGFbeta; TGF-beta1 | NM_000660 | 108 bp | QT00000728 |
| transforming growth factor beta receptor 2 | TGFB2 | AAT3; FAA3; LDS2; MFS2; RIIC; LDS1B; LDS2B; TAAD2; TBRII; TBR-ii; TGFR-2; tbetaR-II; TGFbeta-RII | NM_001024847; NM_003242; XM_006713316 | 108 bp | QT00014350 |
| glyceraldehyde-3-phosphate dehydrogenase | GAPDH | G3PD; GAPD; HEL-S-162eP | NM_001256799; NM_002046; NM_001289745; NM_001289746 | 95 bp | QT00079247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, G.; Privitera, A.; Saab, M.W.; Musso, N.; Maugeri, S.; Fidilio, A.; Privitera, A.P.; Pittalà, A.; Jolivet, R.B.; Lanzanò, L.; et al. Characterization of Carnosine Effect on Human Microglial Cells under Basal Conditions. Biomedicines 2023, 11, 474. https://doi.org/10.3390/biomedicines11020474
Caruso G, Privitera A, Saab MW, Musso N, Maugeri S, Fidilio A, Privitera AP, Pittalà A, Jolivet RB, Lanzanò L, et al. Characterization of Carnosine Effect on Human Microglial Cells under Basal Conditions. Biomedicines. 2023; 11(2):474. https://doi.org/10.3390/biomedicines11020474
Chicago/Turabian StyleCaruso, Giuseppe, Anna Privitera, Miriam Wissam Saab, Nicolò Musso, Salvatore Maugeri, Annamaria Fidilio, Anna Provvidenza Privitera, Alessandra Pittalà, Renaud Blaise Jolivet, Luca Lanzanò, and et al. 2023. "Characterization of Carnosine Effect on Human Microglial Cells under Basal Conditions" Biomedicines 11, no. 2: 474. https://doi.org/10.3390/biomedicines11020474
APA StyleCaruso, G., Privitera, A., Saab, M. W., Musso, N., Maugeri, S., Fidilio, A., Privitera, A. P., Pittalà, A., Jolivet, R. B., Lanzanò, L., Lazzarino, G., Caraci, F., & Amorini, A. M. (2023). Characterization of Carnosine Effect on Human Microglial Cells under Basal Conditions. Biomedicines, 11(2), 474. https://doi.org/10.3390/biomedicines11020474














