Analyzing the Differential Impact of Semen Preparation Methods on the Outcomes of Assisted Reproductive Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Clearance
2.2. Participants
2.3. Semen Collection and Analysis
2.3.1. Hypo-Osmotic Swelling Test (HOS)
2.3.2. Reactive Oxygen Species (ROS)
2.3.3. Sperm DNA Fragmentation (SDF)
2.3.4. Chromomycin A3 Staining
2.4. Experimental Design
2.4.1. Density-Gradient Centrifugation (DGC) Technique
2.4.2. Swim-Up (SU) Technique
2.4.3. DGC-SU Technique
2.4.4. DGC-MACS Technique
2.4.5. Ovarian Stimulation, IVF, ICSI and Embryo Development
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Sperm Standard and Quality Parameters
3.3. Post-Sperm Selection Technique Improvement in Standard and Quality Parameters of Sperm
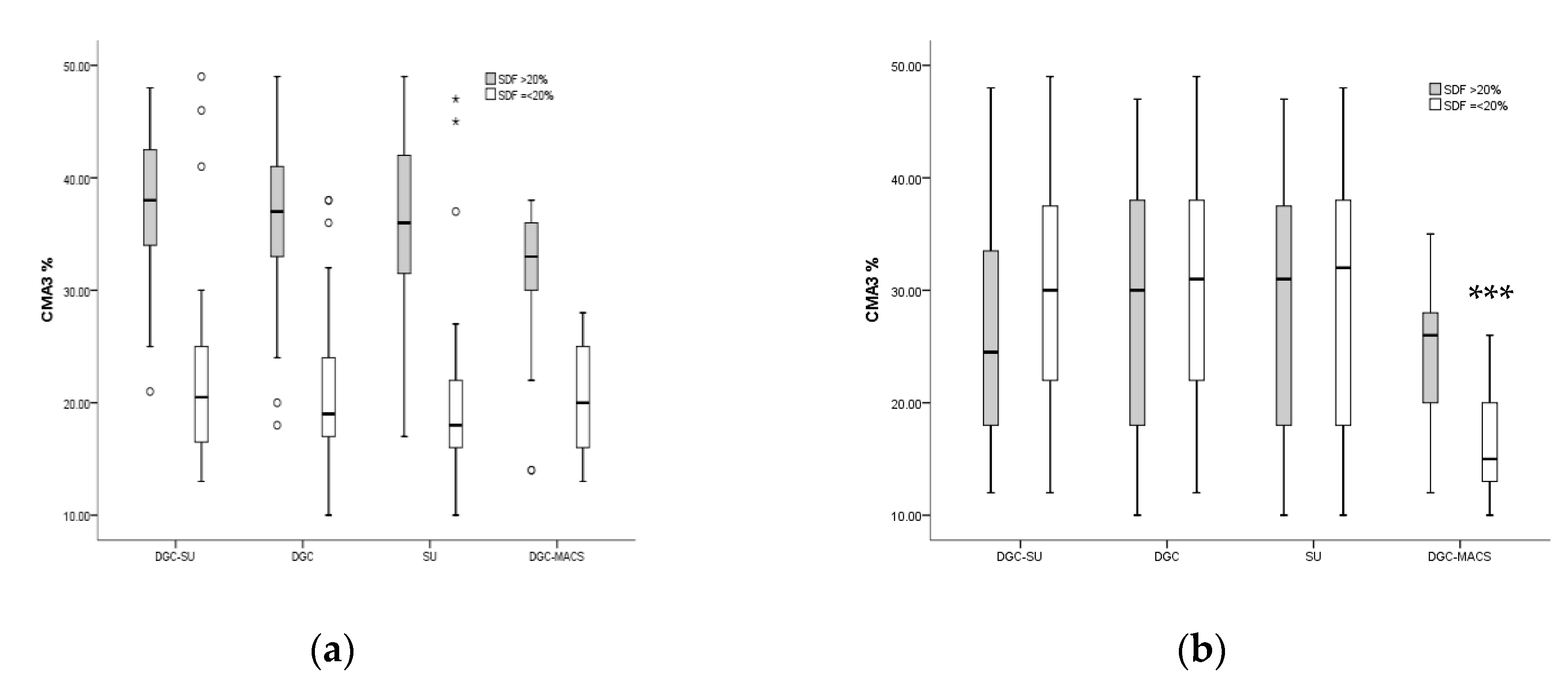
3.4. Effect of Sperm Preparation Techniques on Quality Sperm Selection in Men with Normal and Increased Sperm DNA Fragmentation
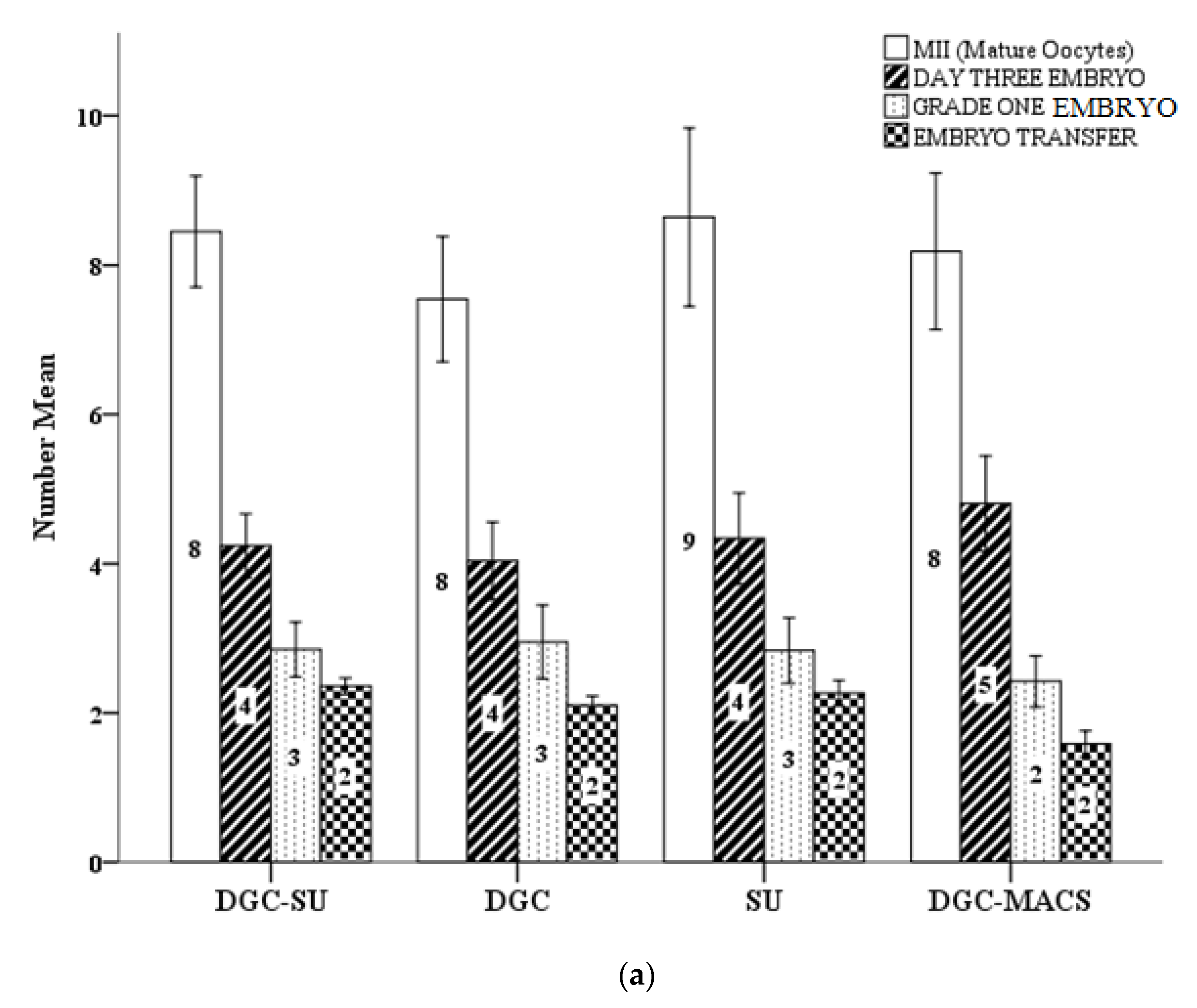
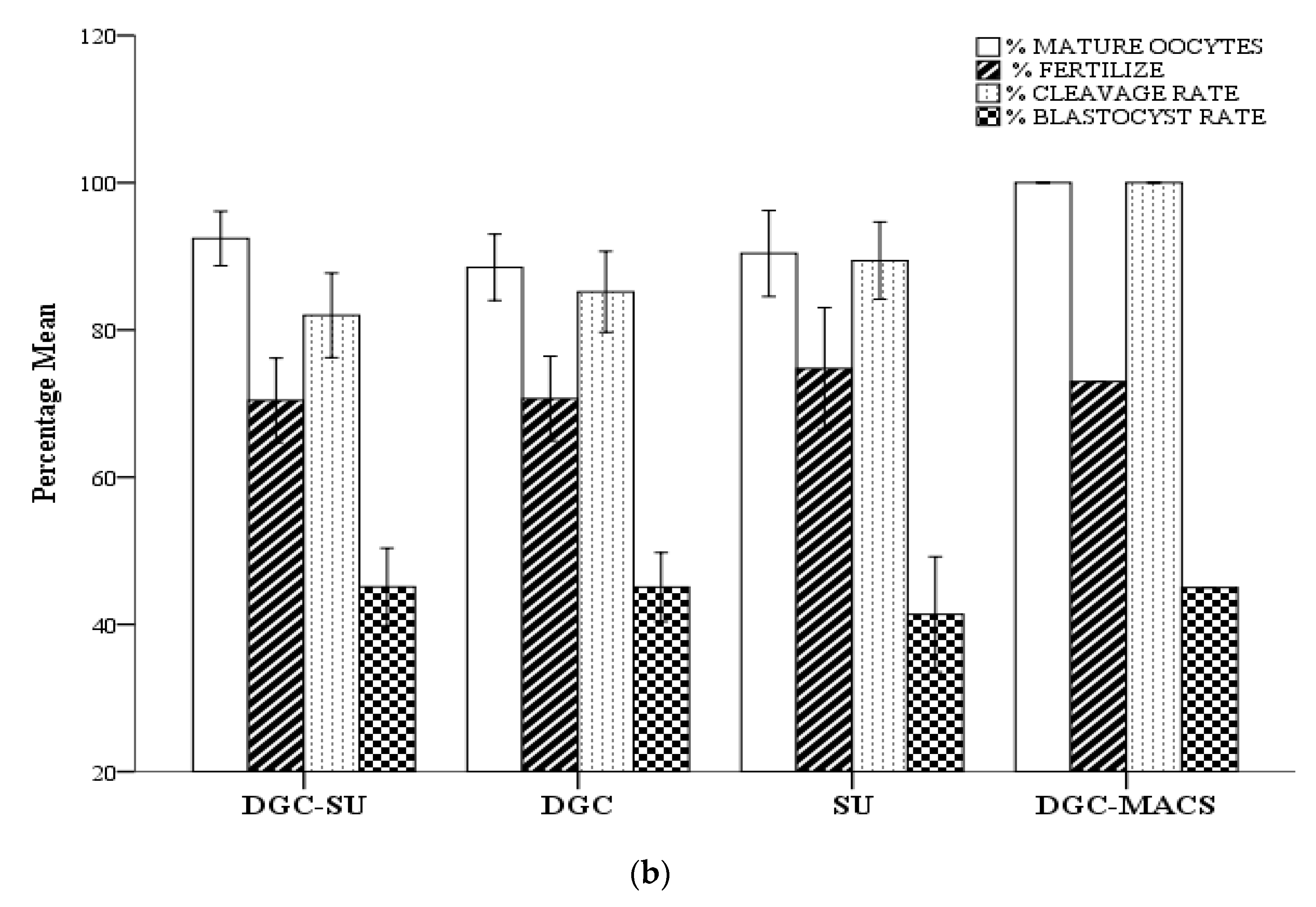
3.5. Effect of Sperm Selection Techniques on Assisted Reproductive Technology Cycle Parameters
3.6. Effect of Sperm Selection Techniques on Assisted Reproductive Technology Cycle Parameters in Men with Normal and Increased Sperm DNA Fragmentation
3.7. Effect of Sperm Selection Techniques on Assisted Reproductive Technology Cycle Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skakkebaek, N.E.; Jorgensen, N.; Main, K.M.; Rajpert-De Meyts, E.; Leffers, H.; Andersson, A.M.; Juul, A.; Carlsen, E.; Mortensen, G.K.; Jensen, T.K.; et al. Is human fecundity declining? Int. J. Androl. 2006, 29, 2–11. [Google Scholar] [CrossRef]
- Simopoulou, M.; Gkoles, L.; Bakas, P.; Giannelou, P.; Kalampokas, T.; Pantos, K.; Koutsilieris, M. Improving ICSI: A review from the spermatozoon perspective. Syst. Biol. Reprod. Med. 2016, 62, 359–371. [Google Scholar] [CrossRef]
- Okun, N.; Sierra, S.; Douglas Wilson, R.; Audibert, F.; Brock, J.-A.; Campagnolo, C.; Carroll, J.; Cartier, L.; Chitayat, D.; Gagnon, A.; et al. Pregnancy Outcomes After Assisted Human Reproduction. J. Obstet. Gynaecol. Can. 2014, 36, 64–83. [Google Scholar] [CrossRef]
- Engel, K.M.; Springsguth, C.H.; Grunewald, S. What happens to the unsuccessful spermatozoa? Andrology 2018, 6, 335–344. [Google Scholar] [CrossRef]
- Esteves, S.C.; Miyaoka, R.; Agarwal, A. An update on the clinical assessment of the infertile male. [corrected]. Clinics 2011, 66, 691–700. [Google Scholar] [CrossRef]
- Lewis, S.E. Is sperm evaluation useful in predicting human fertility? Reproduction 2007, 134, 31–40. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Laboratory Manual for the Examination and Processing of human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Ammar, O.; Mehdi, M.; Muratori, M. Teratozoospermia: Its association with sperm DNA defects, apoptotic alterations, and oxidative stress. Andrology 2020, 8, 1095–1106. [Google Scholar] [CrossRef]
- Baskaran, S.; Cho, C.; Agarwal, A.; Rizk, B.; Sabanegh, E. Male Infertility in Reproductive Medicine: Diagnosis and Management; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Men’s Health 2020, 38, 412. [Google Scholar] [CrossRef] [PubMed]
- McDowell, S.; Kroon, B.; Ford, E.; Hook, Y.; Glujovsky, D.; Yazdani, A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst. Rev. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Candela, L.; Boeri, L.; Capogrosso, P.; Cazzaniga, W.; Pozzi, E.; Belladelli, F.; Baudo, A.; Ravizzoli, A.; Ventimiglia, E.; Vigano, P.; et al. Correlation among isolated teratozoospermia, sperm DNA fragmentation and markers of systemic inflammation in primary infertile men. PLoS ONE 2021, 16, e0251608. [Google Scholar] [CrossRef]
- Hernandez-Silva, G.; Lopez-Torres, A.S.; Maldonado-Rosas, I.; Mata-Martinez, E.; Larrea, F.; Torres-Flores, V.; Trevino, C.L.; Chirinos, M. Effects of Semen Processing on Sperm Function: Differences between Swim-Up and Density Gradient Centrifugation. World J. Men’s Health 2021, 39, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.D.; Zhang, Y.P.; Zhai, L.P.; Zhang, M.H.; Dong, Y.L.; Yang, H.J.; Qiu, Y. Sperm Parameters, ASAs and Apoptosis After Processing by the Double Tube and Swim up Methods. Am. J. Men’s Health 2021, 15, 15579883211001202. [Google Scholar] [CrossRef] [PubMed]
- Ayad, B.M.; Oyeyipo, I.P.; Van der Horst, G.; Du Plessis, S.S. Cementing the relationship between conventional and advanced semen parameters. Middle East Fertil. Soc. J. 2021, 26, 39. [Google Scholar] [CrossRef]
- Sharma, R.; Kattoor, A.J.; Ghulmiyyah, J.; Agarwal, A. Effect of sperm storage and selection techniques on sperm parameters. Syst. Biol. Reprod. Med. 2015, 61, 1–12. [Google Scholar] [CrossRef]
- Tavalaee, M.; Kiani-Esfahani, A.; Nasr-Esfahani, M.H. Relationship between phospholipase C-zeta, semen parameters, and chromatin status. Syst. Biol. Reprod. Med. 2017, 63, 259–268. [Google Scholar] [CrossRef]
- Hichri, R.; Amor, H.; Khammari, M.; Harzallah, M.; El Fekih, S.; Saad, A.; Ajina, M.; Ben Ali, H. Apoptotic sperm biomarkers and the correlation between conventional sperm parameters and clinical characteristics. Andrologia 2018, 50, e12813. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm Oxidative Stress during In Vitro Manipulation and Its Effects on Sperm Function and Embryo Development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef]
- Oseguera-López, I.; Ruiz-Díaz, S.; Ramos-Ibeas, P.; Pérez-Cerezales, S. Novel Techniques of Sperm Selection for Improving IVF and ICSI Outcomes. Front. Cell Dev. Biol. 2019, 7, 298. [Google Scholar] [CrossRef]
- Chen, Z.; Hauser, R.; Trbovich, A.M.; Shifren, J.L.; Dorer, D.J.; Godfrey-Bailey, L.; Singh, N.P. The relationship between human semen characteristics and sperm apoptosis: A pilot study. J. Androl. 2006, 27, 112–120. [Google Scholar] [CrossRef]
- Mahfouz, R.Z.; Sharma, R.K.; Said, T.M.; Erenpreiss, J.; Agarwal, A. Association of sperm apoptosis and DNA ploidy with sperm chromatin quality in human spermatozoa. Fertil. Steril. 2009, 91, 1110–1118. [Google Scholar] [CrossRef]
- Romany, L.; Garrido, N.; Motato, Y.; Aparicio, B.; Remohi, J.; Meseguer, M. Removal of annexin V-positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: A controlled and randomized trial in unselected males. Fertil. Steril. 2014, 102, 1567–1575.e1561. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.A.; Paasch, U.; Villegas, J.V. Mitochondrial membrane potential disruption pattern in human sperm. Hum. Reprod. 2009, 24, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Tavalaee, M.; Deemeh, M.R.; Arbabian, M.; Nasr-Esfahani, M.H. Density gradient centrifugation before or after magnetic-activated cell sorting: Which technique is more useful for clinical sperm selection? J. Assist. Reprod. Genet. 2012, 29, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.R.; Ghosh, S.S.; East, J.M.; Franson, R.C. Role of Human Sperm Phospholipase A2 in Fertilization: Effects of a Novel Inhibitor of Phospholipase A2 Activity on Membrane Perturbations and Oocyte Penetration1. Biol. Reprod. 1992, 47, 751–759. [Google Scholar] [CrossRef]
- Dirican, E.K.; Ozgun, O.D.; Akarsu, S.; Akin, K.O.; Ercan, O.; Ugurlu, M.; Camsari, C.; Kanyilmaz, O.; Kaya, A.; Unsal, A. Clinical outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. J. Assist. Reprod. Genet. 2008, 25, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Noureen, A.; Uzair, M.; Jabeen, F.; Abdel Daim, M.; Cappello, T. Perspectives of Nanoparticles in Male Infertility: Evidence for Induced Abnormalities in Sperm Production. Int. J. Environ. Res. Public Health 2021, 18, 1758. [Google Scholar] [CrossRef]
- Pacheco, A.; Blanco, A.; Bronet, F.; Cruz, M.; García-Fernández, J.; García-Velasco, J.A. Magnetic-Activated Cell Sorting (MACS): A Useful Sperm-Selection Technique in Cases of High Levels of Sperm DNA Fragmentation. J. Clin. Med. 2020, 9, 3976. [Google Scholar] [CrossRef] [PubMed]
- Baldini, D.; Ferri, D.; Baldini, G.M.; Lot, D.; Catino, A.; Vizziello, D.; Vizziello, G. Sperm Selection for ICSI: Do We Have a Winner? Cells 2021, 10, 3566. [Google Scholar] [CrossRef] [PubMed]
- Cakar, Z.; Cetinkaya, B.; Aras, D.; Koca, B.; Ozkavukcu, S.; Kaplanoglu, I.; Can, A.; Cinar, O. Does combining magnetic-activated cell sorting with density gradient or swim-up improve sperm selection? J. Assist. Reprod. Genet. 2016, 33, 1059–1065. [Google Scholar] [CrossRef]
- Gil, M.; Sar-Shalom, V.; Melendez Sivira, Y.; Carreras, R.; Checa, M.A. Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 479–485. [Google Scholar] [CrossRef]
- Said, T.M.; Land, J.A. Effects of advanced selection methods on sperm quality and ART outcome: A systematic review. Hum. Reprod. Update 2011, 17, 719–733. [Google Scholar] [CrossRef]
- Romany, L.; Garrido, N.; Cobo, A.; Aparicio-Ruiz, B.; Serra, V.; Meseguer, M. Obstetric and perinatal outcome of babies born from sperm selected by MACS from a randomized controlled trial. J. Assist. Reprod. Genet. 2017, 34, 201–207. [Google Scholar] [CrossRef]
- Sergerie, M.; Laforest, G.; Bujan, L.; Bissonnette, F.; Bleau, G. Sperm DNA fragmentation: Threshold value in male fertility. Hum. Reprod. 2005, 20, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Chandrashekara, S. Sample size estimation and power analysis for clinical research studies. J. Hum. Reprod. Sci. 2012, 5, 7–13. [Google Scholar] [CrossRef]
- Bibi, R.; Jahan, S.; Afsar, T.; Almajwal, A.; Hammadeh, M.E.; Alruwaili, N.W.; Razak, S.; Amor, H. The influence of paternal overweight on sperm chromatin integrity, fertilization rate and pregnancy outcome among males attending fertility clinic for IVF/ICSI treatment. BMC Pregnancy Childbirth 2022, 22, 620. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Uchida, A.; Kitao, M. Hypoosmotic Swelling Test of Sperm. Arch. Androl. 1990, 25, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, I.; Morishita, Y.; Imai, K.; Nakamura, M.; Nakachi, K.; Hayashi, T. High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 631, 55–61. [Google Scholar] [CrossRef]
- Fernandez, J.; Muriel, L.; Goyanes, V.; Segrelles, E.; Gosalvez, J.; Enciso, M.; Lafromboise, M.; Dejonge, C. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil. Steril. 2005, 84, 833–842. [Google Scholar] [CrossRef]
- Lolis, D.; Georgiou, I.; Syrrou, M.; Zikopoulos, K.; Konstantelli, M.; Messinis, I. Chromomycin A3-staining as an indicator of protamine deficiency and fertilization. Int. J. Androl. 1996, 19, 23–27. [Google Scholar] [CrossRef]
- Agarwal, A.; Henkel, R.; Majzoub, A. Manual of Sperm Function Testing in Human Assisted Reproduction; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- World Medical Association Declaration of Helsinki. JAMA 2013, 310, 2191. [CrossRef]
- Lee, T.H.; Liu, C.H.; Shih, Y.T.; Tsao, H.M.; Huang, C.C.; Chen, H.H.; Lee, M.S. Magnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum. Reprod. 2010, 25, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, S.; Reinhardt, M.; Blumenauer, V.; Said, T.M.; Agarwal, A.; Abu Hmeidan, F.; Glander, H.J.; Paasch, U. Increased sperm chromatin decondensation in selected nonapoptotic spermatozoa of patients with male infertility. Fertil. Steril. 2009, 92, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Lin, D.P.-C.; Tsao, H.-M.; Cheng, T.-C.; Liu, C.-H.; Lee, M.-S. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil. Steril. 2005, 84, 130–140. [Google Scholar] [CrossRef]
- Said, T.M.; Agarwal, A.; Zborowski, M.; Grunewald, S.; Glander, H.J.; Paasch, U. Utility of magnetic cell separation as a molecular sperm preparation technique. J. Androl. 2008, 29, 134–142. [Google Scholar] [CrossRef]
- Hikim, A.P.S.; Swerdloff, R.S. Hormonal and genetic control of germ cell apoptosis in the testis. Rev. Reprod. 1999, 4, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Glander, H.J.; Schaller, J. Binding of annexin V to plasma membranes of human spermatozoa: A rapid assay for detection of membrane changes after cryostorage. Mol. Hum. Reprod. 1999, 5, 109–115. [Google Scholar] [CrossRef]
- Nadalini, M.; Tarozzi, N.; Di Santo, M.; Borini, A. Annexin V magnetic-activated cell sorting versus swim-up for the selection of human sperm in ART: Is the new approach better then the traditional one? J. Assist. Reprod. Genet. 2014, 31, 1045–1051. [Google Scholar] [CrossRef]
- Degheidy, T.; Abdelfattah, H.; Seif, A.; Albuz, F.K.; Gazi, S.; Abbas, S. Magnetic activated cell sorting: An effective method for reduction of sperm DNA fragmentation in varicocele men prior to assisted reproductive techniques. Andrologia 2015, 47, 892–896. [Google Scholar] [CrossRef]
- Ziarati, N.; Tavalaee, M.; Bahadorani, M.; Nasr Esfahani, M.H. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Hum. Fertil. 2019, 22, 118–125. [Google Scholar] [CrossRef]
- Bucar, S.; Goncalves, A.; Rocha, E.; Barros, A.; Sousa, M.; Sa, R. DNA fragmentation in human sperm after magnetic-activated cell sorting. J. Assist. Reprod. Genet. 2015, 32, 147–154. [Google Scholar] [CrossRef]
- Gil Juliá, M.; Hervás, I.; Navarro-Gómez Lechón, A.; Quintana, F.; Amorós, D.; Pacheco, A.; González-Ravina, C.; Rivera-Egea, R.; Garrido, N. Sperm Selection by Magnetic-Activated Cell Sorting before Microinjection of Autologous Oocytes Increases Cumulative Live Birth Rates with Limited Clinical Impact: A Retrospective Study in Unselected Males. Biology 2021, 10, 430. [Google Scholar] [CrossRef]
- Aydos, K.; Aydos, O.S. Sperm Selection Procedures for Optimizing the Outcome of ICSI in Patients with NOA. J. Clin. Med. 2021, 10, 2687. [Google Scholar] [CrossRef] [PubMed]
- Akerlöf, E.; Fredricson, B.; Gustafsson, O.; Lundin, A.; Lunell, N.O.; Nylund, L.; Rosenborg, L.; Pousette, A. Comparison between a swim-up and a Percoll gradient technique for the separation of human spermatozoa. Int. J. Androl. 1987, 10, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296. [Google Scholar] [CrossRef] [PubMed]
- Esbert, M.; Godo, A.; Soares, S.R.; Florensa, M.; Amoros, D.; Ballesteros, A.; Vidal, F. Spermatozoa with numerical chromosomal abnormalities are more prone to be retained by Annexin V-MACS columns. Andrology 2017, 5, 807–813. [Google Scholar] [CrossRef]
- Martinez, M.G.; Sanchez-Martin, P.; Dorado-Silva, M.; Fernandez, J.L.; Girones, E.; Johnston, S.D.; Gosalvez, J. Magnetic-activated cell sorting is not completely effective at reducing sperm DNA fragmentation. J. Assist. Reprod. Genet. 2018, 35, 2215–2221. [Google Scholar] [CrossRef]
- Grützkau, A.; Radbruch, A. Small but mighty: How the MACS®-technology based on nanosized superparamagnetic particles has helped to analyze the immune system within the last 20 years. Cytometry Part A 2010, 77, 643–647. [Google Scholar] [CrossRef]

| DGC-SU (N = 100) | DGC (N = 99) | SU (N = 92) | DGC-MACS (N = 94) | p-Value | |
|---|---|---|---|---|---|
| Male patient age (Years) | 34.89 ± 3.9 | 34.90 ± 4.50 | 34.9 ± 646 | 39.07 ± 8.59 | 0.93 |
| Male patient BMI (Kg/m2) | 25.34 ± 3.46 | 25.25 ± 3.1 | 24.5 ± 3.02 | 25.39 ± 2.98 | 0.09 |
| Infertility duration (Years) | 9.32 ± 5.38 | 9.54 ± 5.57 | 9.24 ± 6.42 | 10.34 ± 6.16 | 0.19 |
| Female patient age (Years) | 30.7 ± 5.31 | 30.86 ± 5.61 | 31.8 ± 6.08 | 33.01 ± 5.42 | 0.28 |
| Female patient BMI (Kg/m2) | 27.10 ± 4.52 | 28.95 ± 2.58 | 28.78 ± 3.66 | 28.14 ± 3.81 | 0.49 |
| AMH (ng/mL) | 3.98 ± 2.94 | 4 ± 3.70 | 3.30 ± 3.30 | 3.6 ± 3.5 | 0.56 |
| Total gonadotropin dose (IU) | 2529 ± 1536 | 2539 ± 1878 | 2925 ± 1755 | 2625 ± 1834 | 0.92 |
| Total gonadotropin dose/oocyte | 647.6 ± 891 | 605 ± 1079 | 747 ± 1011 | 651 ± 987 | 0.72 |
| Stimulation days | 15 ± 3 | 15 ± 2 | 14.5 ± 2.8 | 15 ± 2 | 0.89 |
| Estradiol level on the day of HCG (pg/mL) | 1974 ± 1316 | 1791 ± 1076 | 2040 ± 1408 | 1804 ± 1601 | 0.38 |
| Estradiol level/oocyte | 237 ± 385 | 220 ± 302 | 202 ± 304 | 200 ± 392 | 0.586 |
| DGC-SU (N = 100) | DGC (N = 99) | SU (N = 92) | DGC-MACS (N = 94) | p-Value | |
|---|---|---|---|---|---|
| Sperm concentration M/mL | 22.39 ± 2.76 | 27.33 ± 9.18 | 40.00 ± 23.9 | 30.17 ± 9.44 | 0.26 |
| Volume (mL) | 3.65 ± 1.59 | 3.95 ± 1.97 | 3.93 ± 1.52 | 3.63 ± 1.65 | 0.33 |
| WBC/HPF | 2.89 ± 1.96 | 2.91 ± 1.79 | 3.16 ± 1.78 | 4.49 ± 7.45 | 0.78 |
| TNMS % | 32.25 ± 20.7 | 43.9 ± 22.5 | 44.7 ± 22.65 | 37.40 ± 22.13 | 0.07 |
| Normal % | 2.8 ± 0.68 | 2.58 ± 0.65 | 2.4 ± 0.81 | 2.70 ± 0.93 | 0.36 |
| HOS % | 39.25 ± 27.13 | 49.09 ± 25.31 | 51.13 ± 25.9 | 54.65 ± 19.90 | 0.79 |
| SDF % | 25.15 ± 11.8 | 20.9 ± 13.2 | 23.1 ± 12.6 | 25.3 ± 11.7 | 0.68 |
| CMA3+ % | 27.5 ± 10.3 | 28.7 ± 10.7 | 29.3 ± 103 | 29.6 ± 12.4 | 0.45 |
| DGC-SU (N = 100) | DGC (N = 99) | SU (N = 92) | DGC-MACS (N = 94) | p-Value | |
|---|---|---|---|---|---|
| HOS % | 65.8 ± 25.8 | 66.0 ± 23.3 | 65.1 ± 24.7 | 73.8 ± 17.5 | 0.253 |
| SDF % | 14.2 ± 3.5 | 14.7 ± 4.7 | 14.5 ± 3.9 | 12.3 ± 4.7 bc | 0.01 |
| CMA3+ % | 27.5 ± 10.1 | 29.7 ± 10.48 | 28.1 ± 11.1 | 21.9 ± 7.4 abc | 0.001 |
| NF (Control) | Teratozoospermia (TZs) | |||||
|---|---|---|---|---|---|---|
| DGC-SU | DGC | SU | DGC-MACS | p-Value | ||
| Neat sample | 1.8 ± 0.2 (N = 10) | 23.86 ± 1.4 (N = 100) | 29.7 ± 1.91 (N = 99) | 29.78 ± 1.18 (N = 92) | 28.95 ± 1.10 (N = 94) | 0.9 |
| Post-preparation | 0.4 ± 0.1 (N = 10) | 1.4 ± 1.0 (N = 100) | 1.1 ± 1.02 (N = 99) | 1.1 ± 1.0 (N = 92) | 0.53 ± 0.5 abc (N = 94) | 0.001 |
| SDF ≤ 20% | SDF > 20% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DGC (36) | SU (42) | DGC-SU (32) | DGC-MASC (39) | p-Value | DGC (64) | SU (50) | DGC-SU (55) | DGC-MASC (55) | p-Value | |
| MII | 8.2 + 6.1 | 8.1 + 6.3 | 7.1 + 4.8 | 8 + 5 | 0.83 | 7.3 + 7.1 | 9 + 5.6 | 8.5 + 56 | 8.6 + 5.8 | 0.74 |
| 2PN (Fertilization rate %) | 5 + 4.4 (66) | 5.7 + 4.5 (73) | 5.1 + 4.3 (69) | 5.7 + 4 (70) | 0.49 | 5.1 + 4.8 (73) | 6.1 + 4.4 (74) | 5.3 + 3.9 (68) | 5.8 + 4.2 (58) | 0.66 |
| D3 EMB (Cleavage rate %) | 3.4 + 3.1 (79) | 4.4 + 3.6 (81) | 4.3 + 4.02 (85) | 5.1 + 3.4 (93) | 0.25 | 4.08 + 4.4 (85) | 4.0 + 3.1 (75) | 3.6 + 2.6 (77) | 4.5 + 2.7 (87) c | 0.03 |
| Number of Embryos transferred | 2.3 ± 0.7 | 2.4 ± 0.7 | 2.4 ± 0.88 | 1.4 ± 0.75 | 0.66 | 1.8 ± 0.7 | 2.3 ± 0.76 | 2.1 ± 0.86 | 1.6 ± 0.8 abc | 0.00 |
| implantation rate % | 21 ± 42.5 | 35.7 ± 49 | 19 ± 40 | 36 ± 45 | 0.19 | 35 ± 48 | 28 ± 46 | 34 ± 48 | 38 ± 42 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi, R.; Jahan, S.; Afsar, T.; Almajwal, A.; Hammadeh, M.E.; Amor, H.; Abusharha, A.; Razak, S. Analyzing the Differential Impact of Semen Preparation Methods on the Outcomes of Assisted Reproductive Techniques. Biomedicines 2023, 11, 467. https://doi.org/10.3390/biomedicines11020467
Bibi R, Jahan S, Afsar T, Almajwal A, Hammadeh ME, Amor H, Abusharha A, Razak S. Analyzing the Differential Impact of Semen Preparation Methods on the Outcomes of Assisted Reproductive Techniques. Biomedicines. 2023; 11(2):467. https://doi.org/10.3390/biomedicines11020467
Chicago/Turabian StyleBibi, Riffat, Sarwat Jahan, Tayyaba Afsar, Ali Almajwal, Mohamad Eid Hammadeh, Houda Amor, Ali Abusharha, and Suhail Razak. 2023. "Analyzing the Differential Impact of Semen Preparation Methods on the Outcomes of Assisted Reproductive Techniques" Biomedicines 11, no. 2: 467. https://doi.org/10.3390/biomedicines11020467
APA StyleBibi, R., Jahan, S., Afsar, T., Almajwal, A., Hammadeh, M. E., Amor, H., Abusharha, A., & Razak, S. (2023). Analyzing the Differential Impact of Semen Preparation Methods on the Outcomes of Assisted Reproductive Techniques. Biomedicines, 11(2), 467. https://doi.org/10.3390/biomedicines11020467






