The Role and Involvement of Stem Cells in Periodontology
Abstract
1. Introduction
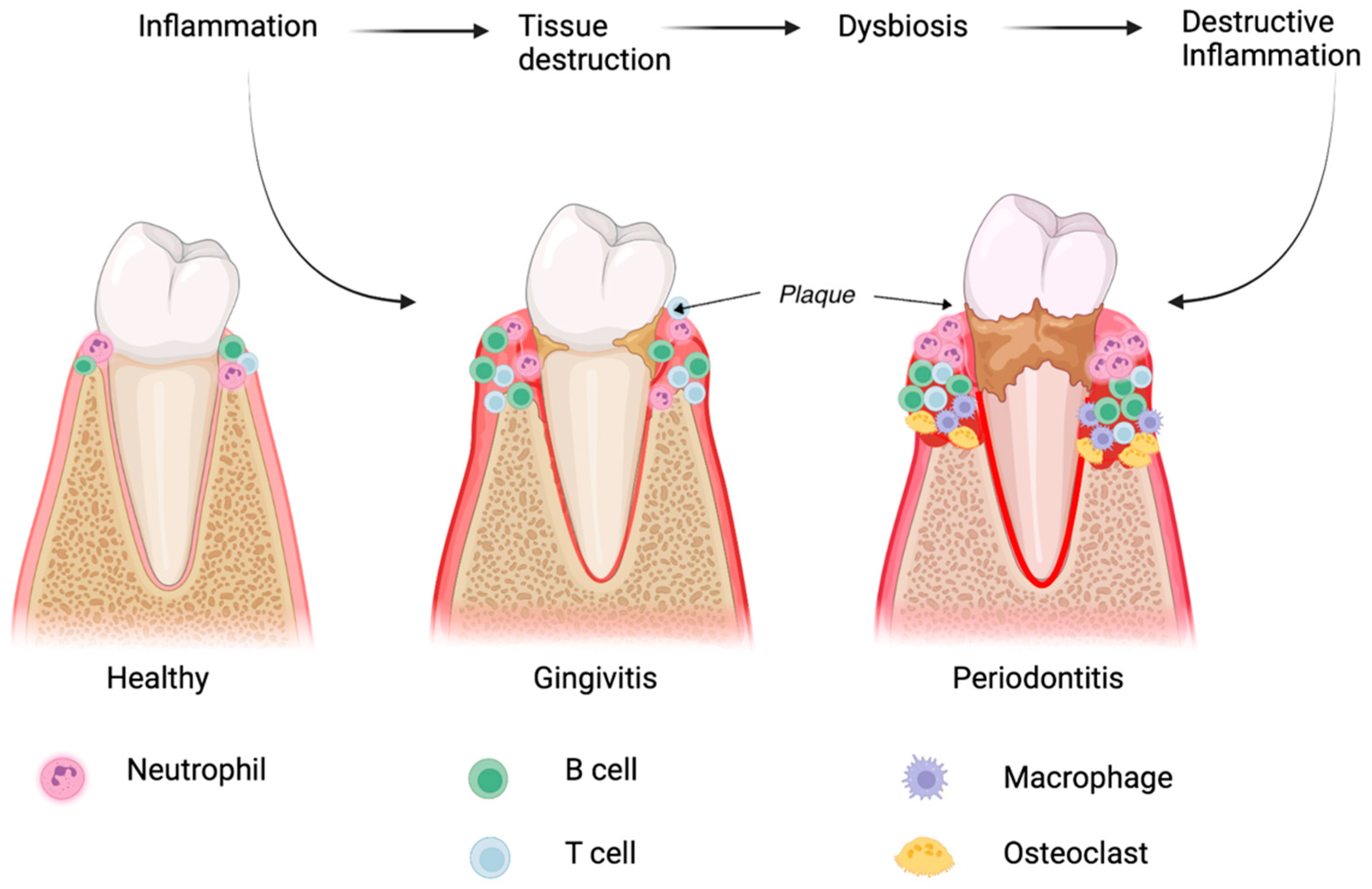
2. Pathological Processes Involved in Periodontitis
2.1. Microbial Dysbiosis and Its Role in Periodontitis
2.2. The Immune Response in Periodontal Diseases
3. Characteristics, Immunological Features, and the Evaluation of Periodontal Stem Cell Regeneration
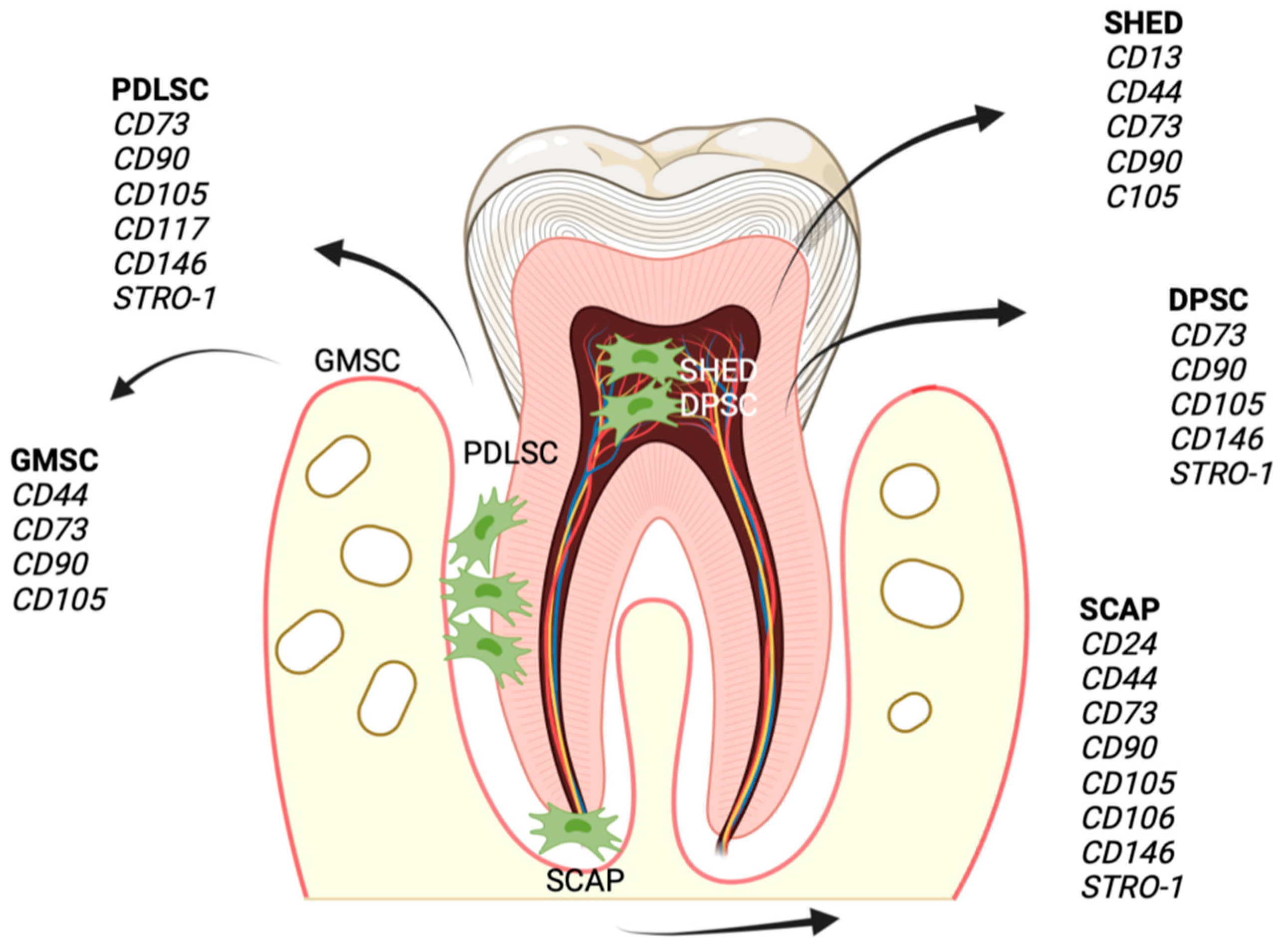
3.1. Dental Mesenchymal Stem Cells (DMSCs)
3.1.1. Dental Follicle Stem Cells (DFSCs)
3.1.2. Dental Pulp Stem Cells (DPSCs)
3.1.3. Stem Cells from the Apical Papilla (SCAPs)
3.1.4. Gingiva-Derived Mesenchymal Stem Cells (GMSCs)
3.1.5. Human Exfoliated Deciduous Teeth Stem Cells (SHEDs)
3.1.6. Periodontal Ligament Stem Cells (PDLSCs)
3.2. Non-Dental Stem Cells
3.2.1. Bone Marrow-Derived Mesenchymal Stem/Stromal Cells (BMSCs)
3.2.2. Adipose-Derived Stem Cells (ASCs)
3.3. Induced Pluripotent Stem Cells (iPSCs)
4. Interaction between Stem Cells and the Periodontal Inflammatory Environment
4.1. Stem Cells in the Inflammatory Environment
4.2. Infected Microenvironment and Its Influence on Stem Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| DMSCs | Dental mesenchymal stem cells |
| iPSCs | Induced pluripotent stem cells |
| iDMSCs | DMSCs derived from infected tissue |
| PDL | Periodontal ligament |
| TLRs | Toll-like receptors |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| IL | Interleukin |
| CCL | Chemokine ligand |
| CCR | Chemokine receptor |
| TNF-α | Tumor necrosis factor α |
| PDLSCs | Periodontal ligament stem cells |
| DFSCs | Dental follicle stem cells |
| DPSCs | Dental-pulp-derived stem cells |
| SCAPs | Stem cells from apical papilla |
| SHED | Stem cells from exfoliated deciduous teeth |
| GMSCs | Gingival mesenchymal stem cells |
| DSSCs | Dental-socket-derived stem cells |
| ALP | Alkaline phosphatase |
| PGE2 | Prostaglandin E2 |
| PBMCs | Peripheral blood mononuclear cells |
| IFN | Interferon |
| TGF-ββ | Transforming growth factor-β |
| IDO-1 | Indoleamine 2,3-dioxygenase-1 |
| HGF | Hepatocyte growth factor |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death protein ligand 1 |
| HLA-ABC | Human leukocyte antigen |
| NK cells | Natural killer cells |
| MAPK | Mitogen-activated protein kinase |
| Tregs | Regulatory T cells |
| DCs | Dendritic cells |
| BMSCs | Bone marrow stromal stem cells |
| ASCs | Adipose-tissue-derived stem cells |
| iPDLSCs | Inflammatory periodontal ligament stem cells |
| ROS | Reactive oxygen species |
| MMPs | Matrix metalloproteinases |
| Th | T helper cells |
| TIMPs | Tissue inhibitors of metalloproteinases |
| CD | Surface cluster of differentiation |
| HLA-ABC | Human leukocyte antigen |
| NOTCH 1 | Neurogenic locus notch homolog protein 1 |
| LPS | Lipopolysaccharides |
| sEVs | cell-derived small extracellular vesicles |
| OPG | Osteoprotegerin |
| RANK | Receptor activator of NF-κB |
| HA-sECM | Hydroxyapatite -synthetic extracellular matrix |
| TDM | Treated dentin matrix |
| PRP | Platelet-rich plasma |
| EDM gel | Enamel matrix-derived gel |
References
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem Cells from Human Exfoliated Deciduous Teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.-Y.; Shi, S. Investigation of Multipotent Postnatal Stem Cells from Human Periodontal Ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal Ligament Stem Cell-Mediated Treatment for Periodontitis in Miniature Swine. Stem Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem Cell-Based Tooth and Periodontal Regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef]
- Wang, L.; Shen, H.; Zheng, W.; Tang, L.; Yang, Z.; Gao, Y.; Yang, Q.; Wang, C.; Duan, Y.; Jin, Y. Characterization of Stem Cells from Alveolar Periodontal Ligament. Tissue Eng. Part A 2011, 17, 1015–1026. [Google Scholar] [CrossRef]
- Ford, P.J.; Gamonal, J.; Seymour, G.J. Immunological Differences and Similarities between Chronic Periodontitis and Aggressive Periodontitis. Periodontol. 2000 2010, 53, 111–123. [Google Scholar] [CrossRef]
- Barth, K.; Remick, D.G.; Genco, C.A. Disruption of Immune Regulation by Microbial Pathogens and Resulting Chronic Inflammation. J. Cell. Physiol. 2013, 228, 1413–1422. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immuno-Microbial Pathogenesis of Periodontitis: Keystones, Pathobionts, and the Host Response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Mira, A.; Simon-Soro, A.; Curtis, M.A. Role of Microbial Communities in the Pathogenesis of Periodontal Diseases and Caries. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S23–S38. [Google Scholar] [CrossRef]
- Roberts, F.A.; Darveau, R.P. Microbial Protection and Virulence in Periodontal Tissue as a Function of Polymicrobial Communities: Symbiosis and Dysbiosis. Periodontol. 2000 2015, 69, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Baraniya, D.; Naginyte, M.; Chen, T.; Albandar, J.M.; Chialastri, S.M.; Devine, D.A.; Marsh, P.D.; Al-Hebshi, N.N. Modeling Normal and Dysbiotic Subgingival Microbiomes: Effect of Nutrients. J. Dent. Res. 2020, 99, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Gaudilliere, D.K.; Culos, A.; Djebali, K.; Tsai, A.S.; Ganio, E.A.; Choi, W.M.; Han, X.; Maghaireh, A.; Choisy, B.; Baca, Q.; et al. Systemic Immunologic Consequences of Chronic Periodontitis. J. Dent. Res. 2019, 98, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S. Contemporary Approaches for Identifying Individual Risk for Periodontitis. Periodontol. 2000 2018, 78, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.L.; Crielaard, W.; Loos, B.G. Genetic Susceptibility to Periodontitis. Periodontol. 2000 2012, 58, 37–68. [Google Scholar] [CrossRef]
- Stabholz, A.; Soskolne, W.A.; Shapira, L. Genetic and Environmental Risk Factors for Chronic Periodontitis and Aggressive Periodontitis. Periodontol. 2000 2010, 53, 138–153. [Google Scholar] [CrossRef]
- Modifiable Risk Factors in Periodontitis: At the Intersection of Aging and Disease - Reynolds - 2014 - Periodontology 2000 - Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/prd.12047 (accessed on 18 November 2022).
- Nussbaum, G.; Shapira, L. How Has Neutrophil Research Improved Our Understanding of Periodontal Pathogenesis? J. Clin. Periodontol. 2011, 38 (Suppl. 11), 49–59. [Google Scholar] [CrossRef]
- Alfakry, H.; Malle, E.; Koyani, C.N.; Pussinen, P.J.; Sorsa, T. Neutrophil Proteolytic Activation Cascades: A Possible Mechanistic Link between Chronic Periodontitis and Coronary Heart Disease. Innate Immun. 2016, 22, 85–99. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Chakravarti, A.; Raquil, M.-A.; Tessier, P.; Poubelle, P.E. Surface RANKL of Toll-like Receptor 4-Stimulated Human Neutrophils Activates Osteoclastic Bone Resorption. Blood 2009, 114, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, M.; Giro, G.; Taubman, M.A.; Han, X.; Mayer, M.P.A.; Kawai, T. Role of Periodontal Pathogenic Bacteria in RANKL-Mediated Bone Destruction in Periodontal Disease. J. Oral Microbiol. 2010, 2, 10–3402. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a Cross-Talk between Human Neutrophils and Th17 Cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 Cells in Chronic Inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef]
- Kurgan, S.; Kantarci, A. Molecular Basis for Immunohistochemical and Inflammatory Changes during Progression of Gingivitis to Periodontitis. Periodontol. 2000 2018, 76, 51–67. [Google Scholar] [CrossRef]
- Berglundh, T.; Donati, M.; Zitzmann, N. B Cells in Periodontitis - Friends or Enemies? Periodontol. 2000 2007, 45, 51–66. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular Aspects of the Pathogenesis of Periodontitis. Periodontol. 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jeon, S.H.; Choung, P.-H. Efficacy of Periodontal Stem Cell Transplantation in the Treatment of Advanced Periodontitis. Cell Transplant. 2011, 20, 271–285. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Konoki, R.; Baba, S. Promising Advances in Clinical Trials of Dental Tissue-Derived Cell-Based Regenerative Medicine. Stem Cell Res. Ther. 2020, 11, 175. [Google Scholar] [CrossRef]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental Follicle Cells: Roles in Development and Beyond. Stem Cells Int. 2019, 2019, 9159605. [Google Scholar] [CrossRef]
- Guo, S.; Guo, W.; Ding, Y.; Gong, J.; Zou, Q.; Xie, D.; Chen, Y.; Wu, Y.; Tian, W. Comparative Study of Human Dental Follicle Cell Sheets and Periodontal Ligament Cell Sheets for Periodontal Tissue Regeneration. Cell Transplant. 2013, 22, 1061–1073. [Google Scholar] [CrossRef]
- Khorsand, A.; Eslaminejad, M.B.; Arabsolghar, M.; Paknejad, M.; Ghaedi, B.; Rokn, A.R.; Moslemi, N.; Nazarian, H.; Jahangir, S. Autologous Dental Pulp Stem Cells in Regeneration of Defect Created in Canine Periodontal Tissue. J. Oral Implantol. 2013, 39, 433–443. [Google Scholar] [CrossRef]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of Immunomodulatory Properties of Exosomes Derived from Bone Marrow Mesenchymal Stem Cells and Dental Pulp Stem Cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wang, J.; Wu, C.-T.; Wang, S. Periodontal Regeneration in Swine after Cell Injection and Cell Sheet Transplantation of Human Dental Pulp Stem Cells Following Good Manufacturing Practice. Stem Cell Res Ther 2016, 7, 130. [Google Scholar] [CrossRef]
- Li, G.; Han, N.; Zhang, X.; Yang, H.; Cao, Y.; Wang, S.; Fan, Z. Local Injection of Allogeneic Stem Cells from Apical Papilla Enhanced Periodontal Tissue Regeneration in Minipig Model of Periodontitis. Biomed. Res. Int. 2018, 2018, 3960798. [Google Scholar] [CrossRef]
- Ding, G.; Liu, Y.; Wang, W.; Wei, F.; Liu, D.; Fan, Z.; An, Y.; Zhang, C.; Wang, S. Allogeneic Periodontal Ligament Stem Cell Therapy for Periodontitis in Swine. Stem Cells 2010, 28, 1829–1838. [Google Scholar] [CrossRef]
- Subba, T.A.; Varma, S.; Thomas, B.; Rao, S.; Kumar, M.; Talwar, A.; Shashidhar, K. Comparison of Cellular and Differentiation Characteristics of Mesenchymal Stem Cells Derived from Human Gingiva and Periodontal Ligament. J. Int. Soc. Prev. Community Dent. 2022, 12, 235–244. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Nguyen, A.L.; Yu, W.H.; Le, A.D. Human Oral Mucosa and Gingiva: A Unique Reservoir for Mesenchymal Stem Cells. J. Dent. Res. 2012, 91, 1011–1018. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Mekhemar, M.K.; Beck-Broichsitter, B.E.; Bähr, T.; Hegab, M.; Receveur, J.; Heneweer, C.; Becker, S.T.; Wiltfang, J.; Dörfer, C.E. Periodontal Regeneration Employing Gingival Margin-Derived Stem/Progenitor Cells in Conjunction with IL-1ra-Hydrogel Synthetic Extracellular Matrix. J. Clin. Periodontol. 2015, 42, 448–457. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Guo, W.; Yang, B.; Tian, W. Stem Cells from Human Exfoliated Deciduous Teeth as an Alternative Cell Source in Bio-Root Regeneration. Theranostics 2019, 9, 2694–2711. [Google Scholar] [CrossRef]
- Queiroz, A.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Pelissari, C.; Trierveiler, M.; Holzhausen, M. Therapeutic Potential of Periodontal Ligament Stem Cells. World J. Stem Cells 2021, 13, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Kim, M.; Han, J.-A.; Choi, B.; Hwang, D.; Do, Y.; Yun, J.-H. Human Periodontal Ligament Stem Cells Suppress T-Cell Proliferation via down-Regulation of Non-Classical Major Histocompatibility Complex-like Glycoprotein CD1b on Dendritic Cells. J. Periodontal Res. 2017, 52, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura, S.; Ito, K.; Umemura, E.; Hara, K.; Nagasaka, T.; Abe, A.; Baba, S.; Furuichi, Y.; Izumi, Y.; et al. Injectable Bone Tissue Engineering Using Expanded Mesenchymal Stem Cells. Stem Cells 2013, 31, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wei, Y.; Liang, Y.; Hu, Q.; Xie, H. Stem Cell Homing in Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1017613. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, Y.; Zhu, L.; Yang, S.; Huang, R.; Shi, W.; Peng, B.; Xiao, Y. SPHK1-S1PR1-RANKL Axis Regulates the Interactions Between Macrophages and BMSCs in Inflammatory Bone Loss. J. Bone Miner. Res. 2018, 33, 1090–1104. [Google Scholar] [CrossRef]
- Du, J.; Shan, Z.; Ma, P.; Wang, S.; Fan, Z. Allogeneic Bone Marrow Mesenchymal Stem Cell Transplantation for Periodontal Regeneration. J. Dent. Res. 2014, 93, 183–188. [Google Scholar] [CrossRef]
- Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Animal Models for Periodontal Regeneration and Peri-Implant Responses. Periodontol. 2000 2015, 68, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Venkataiah, V.S.; Handa, K.; Njuguna, M.M.; Hasegawa, T.; Maruyama, K.; Nemoto, E.; Yamada, S.; Sugawara, S.; Lu, L.; Takedachi, M.; et al. Periodontal Regeneration by Allogeneic Transplantation of Adipose Tissue Derived Multi-Lineage Progenitor Stem Cells in Vivo. Sci. Rep. 2019, 9, 921. [Google Scholar] [CrossRef]
- Tobita, M.; Uysal, C.A.; Guo, X.; Hyakusoku, H.; Mizuno, H. Periodontal Tissue Regeneration by Combined Implantation of Adipose Tissue-Derived Stem Cells and Platelet-Rich Plasma in a Canine Model. Cytotherapy 2013, 15, 1517–1526. [Google Scholar] [CrossRef]
- Du, M.; Duan, X.; Yang, P. Induced Pluripotent Stem Cells and Periodontal Regeneration. Curr. Oral Health Rep. 2015, 2, 257–265. [Google Scholar] [CrossRef]
- Duan, X.; Tu, Q.; Zhang, J.; Ye, J.; Sommer, C.; Mostoslavsky, G.; Kaplan, D.; Yang, P.; Chen, J. Application of Induced Pluripotent Stem (IPS) Cells in Periodontal Tissue Regeneration. J. Cell. Physiol. 2011, 226, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.L.; Furuhashi, A.; Zhang, Q.Z.; Jiang, C.M.; Chang, T.-H.; Le, A.D. Induction of Salivary Gland-Like Cells from Dental Follicle Epithelial Cells. J. Dent. Res. 2017, 96, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, L.; Liu, W.; Li, Q.; Jin, Z.; Jin, Y. Dental Follicle Cells Rescue the Regenerative Capacity of Periodontal Ligament Stem Cells in an Inflammatory Microenvironment. PLoS ONE 2014, 9, e108752. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, B.; Tian, J.; Hong, H.; Du, Y.; Li, K.; Li, X.; Wang, N.; Yu, X.; Wei, X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-Β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell. Physiol. Biochem. 2018, 51, 2290–2308. [Google Scholar] [CrossRef]
- Guo, W.; Chen, L.; Gong, K.; Ding, B.; Duan, Y.; Jin, Y. Heterogeneous Dental Follicle Cells and the Regeneration of Complex Periodontal Tissues. Tissue Eng. Part A 2012, 18, 459–470. [Google Scholar] [CrossRef]
- Ma, L.; Rao, N.; Jiang, H.; Dai, Y.; Yang, S.; Yang, H.; Hu, J. Small Extracellular Vesicles from Dental Follicle Stem Cells Provide Biochemical Cues for Periodontal Tissue Regeneration. Stem Cell Res. Ther. 2022, 13, 92. [Google Scholar] [CrossRef]
- Gholami, L.; Nooshabadi, V.T.; Shahabi, S.; Jazayeri, M.; Tarzemany, R.; Afsartala, Z.; Khorsandi, K. Extracellular Vesicles in Bone and Periodontal Regeneration: Current and Potential Therapeutic Applications. Cell Biosci. 2021, 11, 16. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.; Chen, Y.; Liu, B.; Chen, G. Dental Stem Cell-Derived Extracellular Vesicles as Promising Therapeutic Agents in the Treatment of Diseases. Int. J. Oral Sci. 2022, 14, 1–9. [Google Scholar] [CrossRef]
- Huynh, N.; VonMoss, L.; Smith, D.; Rahman, I.; Felemban, M.F.; Zuo, J.; Rody, W.J.; McHugh, K.P.; Holliday, L.S. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. J. Dent. Res. 2016, 95, 673–679. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Cortez de SantAnna, J.P.; Frisene, I.; Gazarini, J.P.; Gomes Pinheiro, C.C.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Franco Bueno, D. Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration. Tissue Eng. Part B Rev. 2020, 26, 1–12. [Google Scholar] [CrossRef]
- Maioli, M.; Basoli, V.; Santaniello, S.; Cruciani, S.; Delitala, A.P.; Pinna, R.; Milia, E.; Grillari-Voglauer, R.; Fontani, V.; Rinaldi, S.; et al. Osteogenesis from Dental Pulp Derived Stem Cells: A Novel Conditioned Medium Including Melatonin within a Mixture of Hyaluronic, Butyric, and Retinoic Acids. Stem Cells Int. 2016, 2016, 2056416. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhang, Q.Z.; Karabucak, B.; Le, A.D. DPSCs from Inflamed Pulp Modulate Macrophage Function via the TNF-α/IDO Axis. J. Dent. Res. 2016, 95, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Amghar-Maach, S.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Regeneration of Periodontal Bone Defects with Dental Pulp Stem Cells Grafting: Systematic Review. J. Clin. Exp. Dent. 2019, 11, e373–e381. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Tan, J.; Wang, T.; Wang, Q. The Immunomodulatory Properties of Periodontal Ligament Stem Cells Isolated from Inflamed Periodontal Granulation. Cells Tissues Organs 2014, 199, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Dörfer, C.E. Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-R.; Zhang, Q.-Z.; Shi, S.-H.; Nguyen, A.L.; Le, A.D. Human Gingiva-Derived Mesenchymal Stromal Cells Attenuate Contact Hypersensitivity via Prostaglandin E2-Dependent Mechanisms. Stem Cells 2011, 29, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Zibandeh, N.; Genc, D.; Ozcan, E.M.; Goker, K.; Akkoc, T. The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int. 2016, 2016, 4682875. [Google Scholar] [CrossRef]
- Silva, F.d.S.; Ramos, R.N.; de Almeida, D.C.; Bassi, E.J.; Gonzales, R.P.; Miyagi, S.P.H.; Maranduba, C.P.; Sant’Anna, O.A.B.E.; Marques, M.M.; Barbuto, J.A.M.; et al. Mesenchymal Stem Cells Derived from Human Exfoliated Deciduous Teeth (SHEDs) Induce Immune Modulatory Profile in Monocyte-Derived Dendritic Cells. PLoS ONE 2014, 9, e98050. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Z.; Guan, M.; Huang, Q.; Chen, L.; Qin, W.; Ge, X.; Chen, H.; Xiao, Y.; Lin, Z. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng. Part A 2018, 24, 1341–1353. [Google Scholar] [CrossRef]
- Hynes, K.; Menicanin, D.; Han, J.; Marino, V.; Mrozik, K.; Gronthos, S.; Bartold, P.M. Mesenchymal Stem Cells from IPS Cells Facilitate Periodontal Regeneration. J. Dent. Res. 2013, 92, 833–839. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, X.; Zhou, H.; Zhang, C.; Wang, Y.; Huang, J.; Liu, M.; Yang, P.; Song, A. Enhancement of Periodontal Tissue Regeneration by Conditioned Media from Gingiva-Derived or Periodontal Ligament-Derived Mesenchymal Stem Cells: A Comparative Study in Rats. Stem Cell Res. Ther. 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Hong, J.S.-A.; Andrukhova, O.; Blufstein, A.; Moritz, A.; Rausch-Fan, X. Response of Human Periodontal Ligament Stem Cells to IFN-γ and TLR-Agonists. Sci. Rep. 2017, 7, 12856. [Google Scholar] [CrossRef] [PubMed]
- Paknejad, M.; Eslaminejad, M.B.; Ghaedi, B.; Rokn, A.-R.; Khorsand, A.; Etemad-Moghadam, S.; Alaeddini, M.; Dehghan, M.M.; Moslemi, N.; Nowzari, H. Isolation and Assessment of Mesenchymal Stem Cells Derived From Bone Marrow: Histologic and Histomorphometric Study in a Canine Periodontal Defect. J. Oral Implantol. 2015, 41, 284–291. [Google Scholar] [CrossRef]
- Hasegawa, N.; Kawaguchi, H.; Hirachi, A.; Takeda, K.; Mizuno, N.; Nishimura, M.; Koike, C.; Tsuji, K.; Iba, H.; Kato, Y.; et al. Behavior of Transplanted Bone Marrow-Derived Mesenchymal Stem Cells in Periodontal Defects. J. Periodontol. 2006, 77, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue Source Determines the Differentiation Potentials of Mesenchymal Stem Cells: A Comparative Study of Human Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yang, H.; Aprecio, R.M.; Zhou, X.; Wang, Q.; Zhang, W.; Ding, Y.; Li, Y. Therapeutic Effect of TSG-6 Engineered IPSC-Derived MSCs on Experimental Periodontitis in Rats: A Pilot Study. PLoS ONE 2014, 9, e100285. [Google Scholar] [CrossRef]
- Iwasaki, K.; Peng, Y.; Kanda, R.; Umeda, M.; Ishikawa, I. Stem Cell Transplantation and Cell-Free Treatment for Periodontal Regeneration. Int. J. Mol. Sci. 2022, 23, 1011. [Google Scholar] [CrossRef]
- Shimauchi, H.; Nemoto, E.; Ishihata, H.; Shimomura, M. Possible Functional Scaffolds for Periodontal Regeneration. Jpn. Dent. Sci. Rev. 2013, 49, 118–130. [Google Scholar] [CrossRef]
- Fawzy-El-Sayed, K.; Mekhemar, M.; Adam-Klages, S.; Kabelitz, D.; Dörfer, C. TlR Expression Profile of Human Gingival Margin-Derived Stem Progenitor Cells. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e30–e38. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem Cell-Based Bone and Dental Regeneration: A View of Microenvironmental Modulation. Int. J. Oral Sci. 2019, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental Stem Cell and Dental Tissue Regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal Stem Cell-Based Tissue Regeneration Is Governed by Recipient T Lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, J.; Liu, O.; Fan, Z.; Liu, Y.; Wang, F.; Ding, G.; Wei, F.; Zhang, C.; Wang, S. Mesenchymal Stem Cells Derived from Inflamed Periodontal Ligaments Exhibit Impaired Immunomodulation. J. Clin. Periodontol. 2012, 39, 1174–1182. [Google Scholar] [CrossRef]
- Li, Y.; Nan, X.; Zhong, T.-Y.; Li, T.; Li, A. Treatment of Periodontal Bone Defects with Stem Cells from Inflammatory Dental Pulp Tissues in Miniature Swine. Tissue Eng. Regen. Med. 2019, 16, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.J.; Yamaza, T.; Song, Y.; Fouad, A.F.; Romberg, E.E.; Shi, S.; Tuan, R.S.; Huang, G.T.-J. Stem/Progenitor Cells from Inflamed Human Dental Pulp Retain Tissue Regeneration Potential. Regen. Med. 2010, 5, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-D.; Kim, K.-H.; Ryoo, H.-M.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Recent Advances of Useful Cell Sources in the Periodontal Regeneration. Curr. Stem Cell Res. Ther. 2019, 14, 3–8. [Google Scholar] [CrossRef]
- Chien, K.-H.; Chang, Y.-L.; Wang, M.-L.; Chuang, J.-H.; Yang, Y.-C.; Tai, M.-C.; Wang, C.-Y.; Liu, Y.-Y.; Li, H.-Y.; Chen, J.-T.; et al. Promoting Induced Pluripotent Stem Cell-Driven Biomineralization and Periodontal Regeneration in Rats with Maxillary-Molar Defects Using Injectable BMP-6 Hydrogel. Sci. Rep. 2018, 8, 114. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory Properties of Dental Tissue-Derived Mesenchymal Stem Cells: Implication in Disease and Tissue Regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef]
- Onizuka, S.; Iwata, T. Application of Periodontal Ligament-Derived Multipotent Mesenchymal Stromal Cell Sheets for Periodontal Regeneration. Int. J. Mol. Sci. 2019, 20, 2796. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, L.; Zhou, X.; Li, J.; Xu, X. Dental Mesenchymal Stem Cells in Inflamed Microenvironment: Potentials and Challenges for Regeneration. Curr. Stem Cell Res. Ther. 2015, 10, 412–421. [Google Scholar] [CrossRef]
- Whiting, D.; Chung, W.O.; Johnson, J.D.; Paranjpe, A. Characterization of the Cellular Responses of Dental Mesenchymal Stem Cells to the Immune System. J. Endod. 2018, 44, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Lau, A.S.-Y.; Li, J.C.-B.; Law, H.K.-W.; Lau, Y.L.; Chan, G.C.-F. MHC Expression Kinetics and Immunogenicity of Mesenchymal Stromal Cells after Short-Term IFN-γ Challenge. Exp. Hematol. 2008, 36, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. Porphyromonas Gingivalis LPS Inhibits Osteoblastic Differentiation and Promotes Pro-Inflammatory Cytokine Production in Human Periodontal Ligament Stem Cells. Arch. Oral Biol. 2014, 59, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kukolj, T.; Trivanović, D.; Djordjević, I.O.; Mojsilović, S.; Krstić, J.; Obradović, H.; Janković, S.; Santibanez, J.F.; Jauković, A.; Bugarski, D. Lipopolysaccharide Can Modify Differentiation and Immunomodulatory Potential of Periodontal Ligament Stem Cells via ERK1,2 Signaling. J. Cell. Physiol. 2018, 233, 447–462. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, W.; Ren, H.; Zhao, X.; Wang, S.; Ma, H.; Shi, X. Mesenchymal Stem Cells Increase Expression of Heme Oxygenase-1 Leading to Anti-Inflammatory Activity in Treatment of Acute Liver Failure. Stem Cell Res. Ther. 2017, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of Stem Cells on Periodontal Regeneration: Systematic Review of Pre-Clinical Studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef]
- Bindal, P.; Ramasamy, T.S.; Kasim, N.H.A.; Gnanasegaran, N.; Chai, W.L. Immune Responses of Human Dental Pulp Stem Cells in Lipopolysaccharide-Induced Microenvironment. Cell Biol. Int. 2018, 42, 832–840. [Google Scholar] [CrossRef]
| Stem Cells | Multipotent Differentiation | Mechanism of Action | Therapeutic Intervention | Animal Experiment | Tissue Types | Reference |
|---|---|---|---|---|---|---|
| Dental Mesenchymal Stem Cells | ||||||
| DFSCs | Cementoblasts Osteoblasts Chondrocytes | Migration, proliferation and osteogenic differentiation of PDLSCs | Transplantation of cell sheets with Treated Dentin Matrix complexes (TDM) | Experimental nude mice | Periodontal ligament (PDL) Cementum | [1,3,6,30,31,32] |
| DPSCs | Odontoblasts Osteoblasts Chondrocytes Adipocytes Neurogenic cells | Mediate both innate and the specific immune responses | Transplantation of cell sheets without additional materials | Experimental periodontitis in miniature pigs | Dentin pulp Blood vessel Neuronal | [6,29,33,34,35] |
| SCAPs | Osteoblasts Odontoblasts Adipocytes | Inhibit T lymphocyte proliferation | Local Injection | Minipig Model of Periodontitis | Dentin pulp Blood vessel | [2,29,36,37] |
| GMSCs | Osteoblasts Adipocytes Neurogenic cells | Inhibit the activity of M1 macrophages, reduce DC activation and maturation | Transplantation of a combination of GMSCs and hydroxyapatite synthetic extracellular matrix (HA-sECM) | Experimental periodontal defects in miniature pigs | Cementum Alveolar bone | [7,29,38,39,40] |
| SHEDs | Osteoblasts Adipocytes Myogenics Neurogenic cells | Mediate activation, maturation, and differentiation of T lymphocytes | Transplantation of cell sheets with TDM | Study on Sprague Dawley rats | Dentin pulp Blood vessel Neuronal | [1,3,6,36,41] |
| PDLSCs | Osteoblasts Cementoblasts Adipocytes Chondrocytes Neurogenic cells | Reduce the proliferation of PBMCs and secretion of glycoprotein 1b and PGE2 originating from dendritic cells | Transplantation of a combination of cells and materials | Experimental periodontitis in miniature pigs and canines | PDL Cementum | [4,5,40,42,43] |
| Non-dental stem cells | ||||||
| BMSCs | Osteoblasts Odontoblasts Chondrocytes Adipocytes Neurogenic cells | Stimulate expression of odontogenic genes Anti-inflammatory and immunosuppressive functions | Injection of cell solution without additional materials | Experimental periodontitis in rats | Cartilage Bone Neuronal Muscle | [5,44,45,46,47] |
| ASCs | Osteoblasts Chondrocytes Adipocytes Neurogenic cells | Secrete growth factors (insulin-like growth factor binding protein-6 | Cells Transplantation combined with platelet-rich plasma (PRP) | Periodontitis canine model | Cartilage Bone Blood vessel Adipose | [5,6,48,49,50] |
| Induced pluripotent stem cells | ||||||
| iPSCs | Somatic cells | Transplantation of combined cells with scaffolds such as enamel matrix-derived (EMD) gel | Mouse experimental periodontitis | Bone Cartilage Myocardium Blood vessel Adipose Neuronal | [1,5,6,51,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goriuc, A.; Foia, L.; Cojocaru, K.; Diaconu-Popa, D.; Sandu, D.; Luchian, I. The Role and Involvement of Stem Cells in Periodontology. Biomedicines 2023, 11, 387. https://doi.org/10.3390/biomedicines11020387
Goriuc A, Foia L, Cojocaru K, Diaconu-Popa D, Sandu D, Luchian I. The Role and Involvement of Stem Cells in Periodontology. Biomedicines. 2023; 11(2):387. https://doi.org/10.3390/biomedicines11020387
Chicago/Turabian StyleGoriuc, Ancuta, Liliana Foia, Karina Cojocaru, Diana Diaconu-Popa, Darius Sandu, and Ionut Luchian. 2023. "The Role and Involvement of Stem Cells in Periodontology" Biomedicines 11, no. 2: 387. https://doi.org/10.3390/biomedicines11020387
APA StyleGoriuc, A., Foia, L., Cojocaru, K., Diaconu-Popa, D., Sandu, D., & Luchian, I. (2023). The Role and Involvement of Stem Cells in Periodontology. Biomedicines, 11(2), 387. https://doi.org/10.3390/biomedicines11020387








