Role of Hyaluronic Acid in Selected Malignant Neoplasms in Women
Abstract
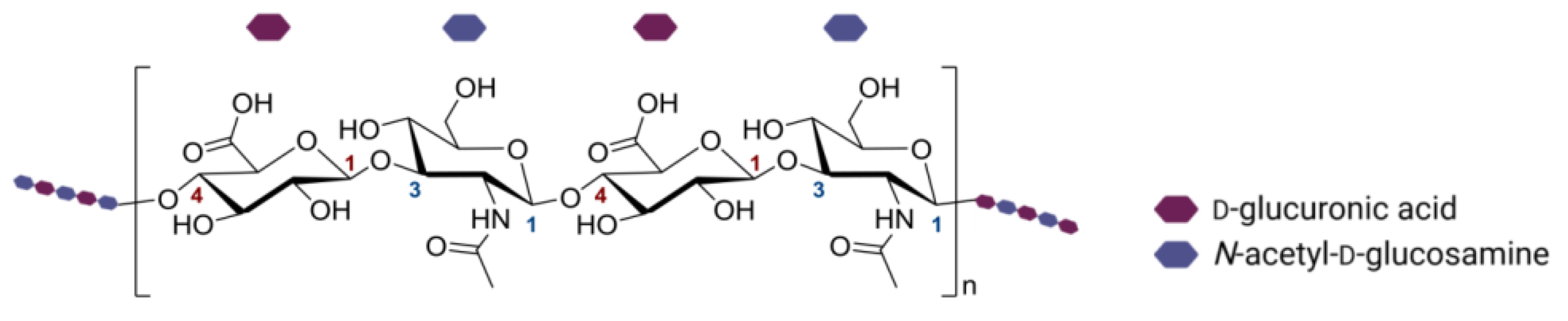
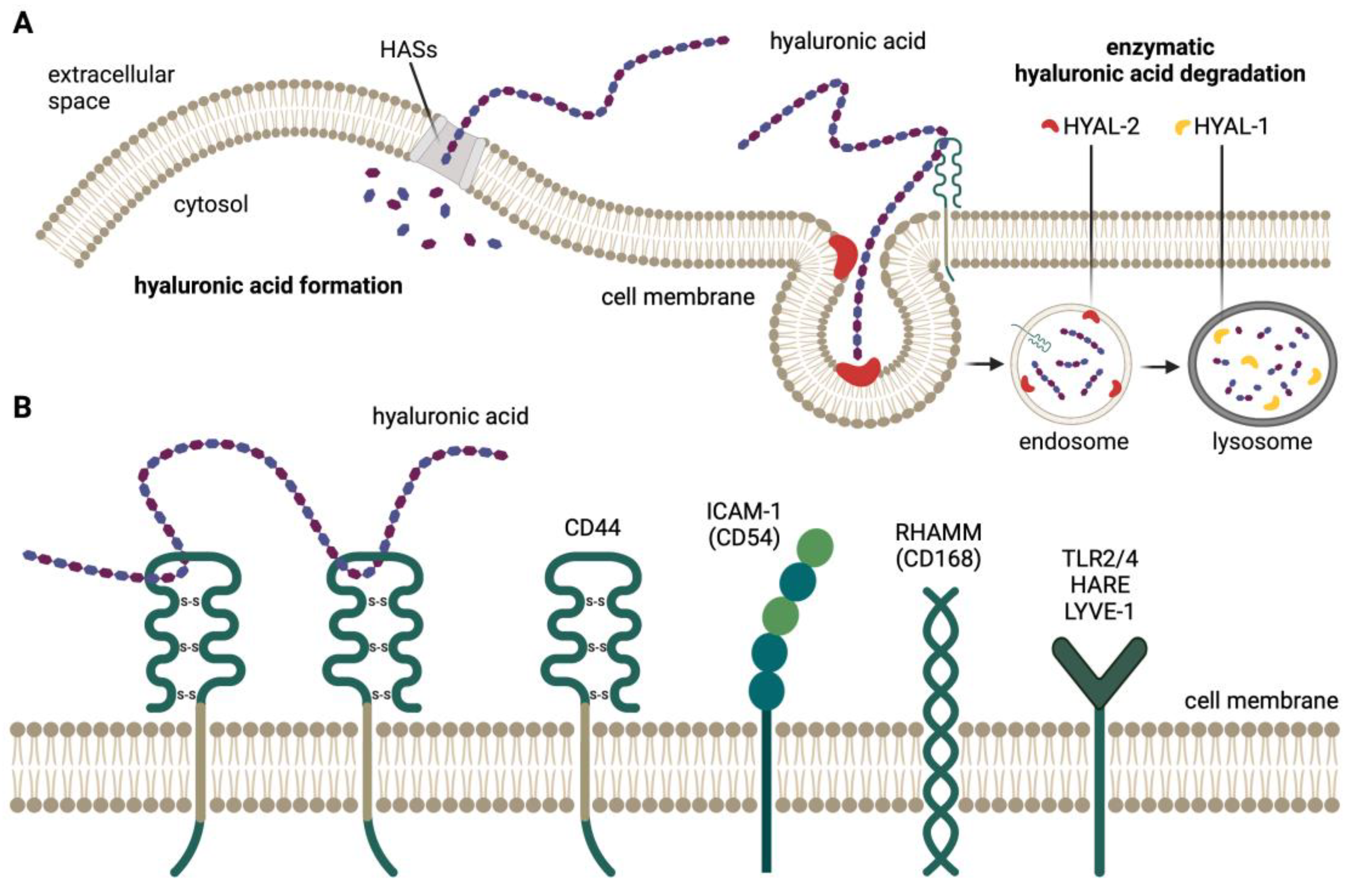
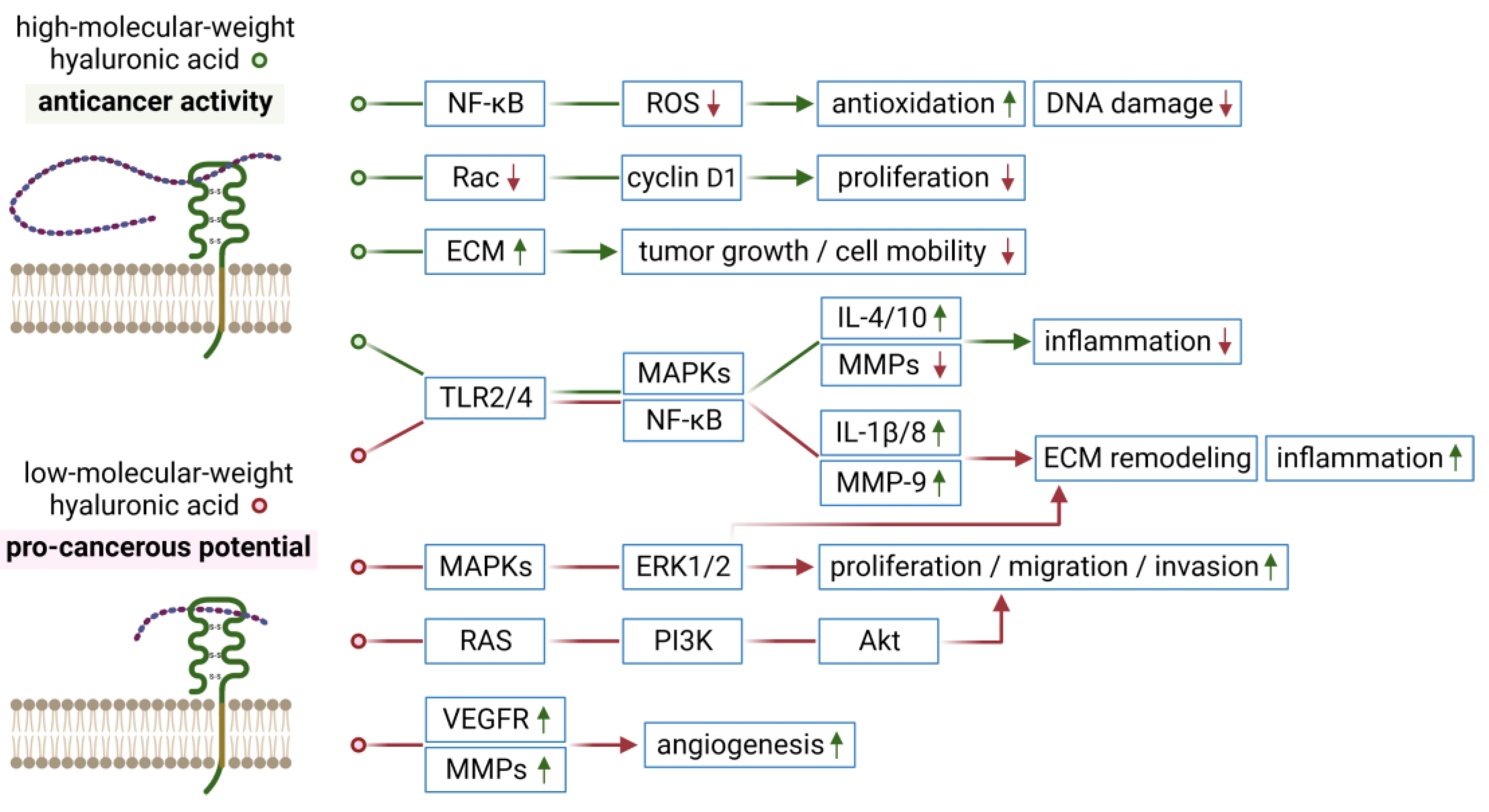
1. Introduction
2. Breast Cancer
3. Cervical Cancer
4. Endometrial Cancer
5. Ovarian Cancer
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Qiao, M.; Ji, Y.; Lin, L.; Zhang, X.; Linhardt, R.J. Chemical, enzymatic and biological synthesis of hyaluronic acids. Int. J. Biol. Macromol. 2020, 152, 199–206. [Google Scholar] [CrossRef]
- Queisser, K.A.; Mellema, R.A.; Petrey, A.C. Hyaluronan and its receptors as regulatory molecules of the endothelial interface. J. Histochem. Cytochem. 2021, 69, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Maloney, F.P.; Kuklewicz, J.; Corey, R.A.; Bi, Y.; Ho, R.; Mateusiak, L.; Pardon, E.; Steyaert, J.; Stansfeld, P.J.; Zimmer, J. Structure, substrate recognition and initiation of hyaluronan synthase. Nature 2022, 604, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Garantziotis, S.; Savani, R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Sang, C.; Cao, G.; Wang, S. Diagnostic and prognostic value of HABP2 as a novel biomarker for endometrial cancer. Ann. Transl. Med. 2020, 8, 1164. [Google Scholar] [CrossRef]
- Suto, S.; Kakizaki, I.; Tatara, Y.; Endo, M. Essential hyaluronan structure for binding with hyaluronan-binding protein (HABP) determined by glycotechnological approach. Carbohydr. Polym. 2021, 251, 116989. [Google Scholar] [CrossRef]
- Heldin, P.; Kolliopoulos, C.; Lin, C.Y.; Heldin, C.H. Involvement of hyaluronan and CD44 in cancer and viral infections. Cell. Signal. 2020, 65, 109427. [Google Scholar] [CrossRef]
- Sacks, J.; Barbolina, M. Expression and function of CD44 in epithelial ovarian carcinoma. Biomolecules 2015, 5, 3051–3066. [Google Scholar] [CrossRef]
- Elbasateeny, S.S.; Salem, A.A.; Abdelsalam, W.A.; Salem, R.A. Immunohistochemical expression of cancer stem cell related markers CD44 and CD133 in endometrial cancer. Pathol. Res. Pract. 2016, 212, 10–16. [Google Scholar] [CrossRef]
- Tan, S.; Yamashita, A.; Gao, S.J.; Kurisawa, M. Hyaluronic acid hydrogels with defined crosslink density for the efficient enrichment of breast cancer stem cells. Acta Biomater. 2019, 94, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Jariyal, H.; Gupta, C.; Srivastava, A. Hyaluronic acid induction on breast cancer stem cells unfolds subtype specific variations in stemness and epithelial-to-mesenchymal transition. Int. J. Biol. Macromol. 2020, 160, 1078–1089. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Sleeman, J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv. Cancer Res. 2014, 123, 231–254. [Google Scholar] [CrossRef]
- Spadea, A.; Rios de la Rosa, J.M.; Tirella, A.; Ashford, M.B.; Williams, K.J.; Stratford, I.J.; Tirelli, N.; Mehibel, M. Evaluating the efficiency of hyaluronic acid for tumor targeting via CD44. Mol. Pharm. 2019, 16, 2481–2493. [Google Scholar] [CrossRef] [PubMed]
- Karan Križanac, D.; Krasić Arapović, A.; Skočibušić, S.; Pintarić, I.; Trgo, G.; Tomić, S. CD44 immunoexpression is unfavorable predictor in ovarian serous cancer. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Nikitovic, D.; Tzardi, M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N. Cancer microenvironment and inflammation: Role of hyaluronan. Front. Immunol. 2015, 6, 169. [Google Scholar] [CrossRef]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y.W. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- McCourt, P.; Ek, B.; Forsberg, N.; Gustafson, S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. J. Biol. Chem. 1994, 269, 30081–30084. [Google Scholar] [CrossRef]
- Caon, I.; Bartolini, B.; Parnigoni, A.; Caravà, E.; Moretto, P.; Viola, M.; Karousou, E.; Vigetti, D.; Passi, A. Revisiting the hallmarks of cancer: The role of hyaluronan. Semin. Cancer Biol. 2020, 62, 9–19. [Google Scholar] [CrossRef]
- Karousou, E.; D’Angelo, M.L.; Kouvidi, K.; Vigetti, D.; Viola, M.; Nikitovic, D.; de Luca, G.; Passi, A. Collagen VI and hyaluronan: The common role in breast cancer. Biomed. Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Rilla, K.; Siiskonen, H.; Tammi, M.; Tammi, R. Hyaluronan-coated extracellular vesicles—A novel link between hyaluronan and cancer. Adv. Cancer Res. 2014, 123, 121–148. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Karamanos, N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019, 75–76, 220–259. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.G.; Kovar, J.L.; Loughman, E.; Elowsky, C.; Oakley, G.G.; Simpson, M.A. Spontaneous metastasis of prostate cancer is promoted by excess hyaluronan synthesis and processing. Am. J. Pathol. 2009, 174, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.L.; Muthukumar, L.; Herzberg, O. Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry 2007, 46, 6911–6920. [Google Scholar] [CrossRef]
- Gurski, L.A.; Xu, X.; Labrada, L.N.; Nguyen, N.T.; Xiao, L.; van Golen, K.L.; Jia, X.; Farach-Carson, M.C. Hyaluronan (HA) interacting proteins RHAMM and hyaluronidase impact prostate cancer cell behavior and invadopodia formation in 3D HA-based hydrogels. PLoS ONE 2012, 7, e50075. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Brown, T.J.; Heldin, P. Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells. Int. J. Cancer 2007, 120, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Cerwinka, W.H.; Lokeshwar, B.L. HYAL1 hyaluronidase: A molecular determinant of bladder tumor growth and invasion. Cancer Res. 2005, 65, 2243–2250. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Estrella, V.; Lopez, L.; Kramer, M.; Gomez, P.; Soloway, M.S.; Lokeshwar, B.L. HYAL1-v1, an alternatively spliced variant of HYAL1 hyaluronidase: A negative regulator of bladder cancer. Cancer Res. 2006, 66, 11219–11227. [Google Scholar] [CrossRef]
- Hanna, S.; Mari, P.; Kristiina, T.K.; Reijo, S.; Sanna, P.S. Inverse expression of hyaluronidase 2 and hyaluronan synthases 1–3 is associated with reduced hyaluronan content in malignant cutaneous melanoma. BMC Cancer 2013, 13, 181. [Google Scholar] [CrossRef]
- McAtee, C.O.; Berkebile, A.R.; Elowsky, C.G.; Fangman, T.; Barycki, J.J.; Wahl, J.K., 3rd; Khalimonchuk, O.; Naslavsky, N.; Caplan, S.; Simpson, M.A. Hyaluronidase Hyal1 increases tumor cell proliferation and motility through accelerated vesicle trafficking. J. Biol. Chem. 2015, 290, 13144–13156. [Google Scholar] [CrossRef]
- Menaa, F.; Menaa, A.; Menaa, B. Hyaluronic acid and derivatives for tissue engineering. J. Biotechnol. Biomater. 2013, S3, 1. [Google Scholar] [CrossRef]
- Ekici, S.; Cerwinka, W.H.; Duncan, R.; Gomez, P.; Civantos, F.; Soloway, M.S.; Lokeshwar, V.B. Comparison of the prognostic potential of hyaluronic acid, hyaluronidase (HYAL-1), CD44v6 and microvessel density for prostate cancer. Int. J. Cancer 2004, 112, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.S.; Gomez, P.; Knapp, J.; Jorda, M.; Soloway, M.S.; Lokeshwar, V.B. Hyaluronic acid and HYAL-1 in prostate biopsy specimens: Predictors of biochemical recurrence. J. Urol. 2009, 182, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.W.; Escudero, D.O.; Lokeshwar, S.D.; Golshani, R.; Ekwenna, O.O.; Acosta, K.; Merseburger, A.S.; Soloway, M.; Lokeshwar, V.B. Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis. Cancer 2011, 117, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Poola, I.; Abraham, J.; Marshalleck, J.J.; Yue, Q.; Lokeshwar, V.B.; Bonney, G.; Dewitty, R.L. Molecular risk assessment for breast cancer development in patients with ductal hyperplasias. Clin. Cancer Res. 2008, 14, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Rilla, K.; Tumelius, R.; Tammi, M.; Sironen, R.; Soini, Y.; Kosma, V.M.; Mannermaa, A.; Viikari, J.; Tammi, R. Hyaluronan synthases (HAS1–3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res. Treat. 2014, 143, 277–286. [Google Scholar] [CrossRef]
- Bouga, H.; Tsouros, I.; Bounias, D.; Kyriakopoulou, D.; Stavropoulos, M.S.; Papageorgakopoulou, N.; Theocharis, D.A.; Vynios, D.H. Involvement of hyaluronidases in colorectal cancer. BMC Cancer 2010, 10, 499. [Google Scholar] [CrossRef]
- Chi, A.; Shirodkar, S.P.; Escudero, D.O.; Ekwenna, O.O.; Yates, T.J.; Ayyathurai, R.; Garcia-Roig, M.; Gahan, J.C.; Manoharan, M.; Bird, V.G.; et al. Molecular characterization of kidney cancer. Cancer 2012, 118, 2394–2402. [Google Scholar] [CrossRef]
- Franzmann, E.J.; Schroeder, G.L.; Goodwin, W.J.; Weed, D.T.; Fisher, P.; Lokeshwar, V.B. Expression of tumor markers hyaluronic acid and hyaluronidase (HYAL1) in head and neck tumors. Int. J. Cancer 2003, 106, 438–445. [Google Scholar] [CrossRef]
- Golshani, R.; Hautmann, S.H.; Estrella, V.; Cohen, B.L.; Kyle, C.C.; Manoharan, M.; Jorda, M.; Soloway, M.S.; Lokeshwar, V.B. HAS1 expression in bladder cancer and its relation to urinary HA test. Int. J. Cancer 2007, 120, 1712–1720. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Schroeder, G.L.; Selzer, M.G.; Hautmann, S.H.; Posey, J.T.; Duncan, R.C.; Watson, R.; Rose, L.; Markowitz, S.; Soloway, M.S. Bladder tumor markers for monitoring recurrence and screening comparison of hyaluronic acid-hyaluronidase and BTA-Stat tests. Cancer 2002, 95, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Paiva, P.; van Damme, M.P.; Tellbach, M.; Jones, R.L.; Jobling, T.; Salamonsen, L.A. Expression patterns of hyaluronan, hyaluronan synthases and hyaluronidases indicate a role for hyaluronan in the progression of endometrial cancer. Gynecol. Oncol. 2005, 98, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Matsuda, Y.; Naito, Z.; Ishiwata, T. CD44 in human glioma correlates with histopathological grade and cell migration. Pathol. Int. 2012, 62, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, B.; Liu, J. CD44 standard form expression is correlated with high-grade and advanced-stage ovarian carcinoma but not prognosis. Hum. Pathol. 2013, 44, 1882–1889. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Choi, K.Y.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef]
- Auzenne, E.; Ghosh, S.C.; Khodadadian, M.; Rivera, B.; Farquhar, D.; Price, R.E.; Ravoori, M.; Kundra, V.; Freedman, R.S.; Klostergaard, J. Hyaluronic acid-paclitaxel: Antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia 2007, 9, 479–486. [Google Scholar] [CrossRef]
- de Stefano, I.; Battaglia, A.; Zannoni, G.F.; Prisco, M.G.; Fattorossi, A.; Travaglia, D.; Baroni, S.; Renier, D.; Scambia, G.; Ferlini, C.; et al. Hyaluronic acid-paclitaxel: Effects of intraperitoneal administration against CD44(+) human ovarian cancer xenografts. Cancer Chemother. Pharmacol. 2011, 68, 107–116. [Google Scholar] [CrossRef]
- Delpech, B.; Chevallier, B.; Reinhardt, N.; Julien, J.P.; Duval, C.; Maingonnat, C.; Bastit, P.; Asselain, B. Serum hyaluronan (hyaluronic acid) in breast cancer patients. Int. J. Cancer 1990, 46, 388–390. [Google Scholar] [CrossRef]
- Peng, C.; Wallwiener, M.; Rudolph, A.; Ćuk, K.; Eilber, U.; Celik, M.; Modugno, C.; Trumpp, A.; Heil, J.; Marmé, F.; et al. Plasma hyaluronic acid level as a prognostic and monitoring marker of metastatic breast cancer. Int. J. Cancer 2016, 138, 2499–2509. [Google Scholar] [CrossRef]
- Yahya, R.; El-Bindary, A.; El-Mezayen, H.; Abdelmasseh, H.; Eissa, M. Biochemical evaluation of hyaluronic acid in breast cancer. Clin. Lab. 2014, 60, 1115–1121. [Google Scholar] [CrossRef]
- Chen, Y.B.; Jiang, C.T.; Zhang, G.Q.; Wang, J.S.; Pang, D. Increased expression of hyaluronic acid binding protein 1 is correlated with poor prognosis in patients with breast cancer. J. Surg. Oncol. 2009, 100, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Cao, M.; Liu, Y.; He, Y.; Zhang, G.; Du, Y.; Gao, F.; Yang, C. Hyaluronan synthase 2 (HAS2) regulates cell phenotype and invadopodia formation in luminal-like breast cancer cells. Mol. Cell. Biochem. 2021, 476, 3383–3391. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-X.; Wang, X.-Y.; Li, H.-Y.; Su, X.-L.; Wang, L.; Ran, L.; Zheng, K.; Ren, G.-S. HYAL1 Overexpression Is Correlated with the Malignant Behavior of Human Breast Cancer. Int J Cancer 2011, 128, 1303–1315. [Google Scholar] [CrossRef]
- Roy, R.; Mandal, S.; Chakrabarti, J.; Saha, P.; Panda, C.K. Downregulation of hyaluronic acid-CD44 signaling pathway in cervical cancer cell by natural polyphenols plumbagin, pongapin and karanjin. Mol. Cell. Biochem. 2021, 476, 3701–3709. [Google Scholar] [CrossRef]
- Zhang, M.; Li, N.; Liang, Y.; Liu, J.; Zhou, Y.; Liu, C. Hyaluronic acid binding protein 1 overexpression is an indicator for disease-free survival in cervical cancer. Int. J. Clin. Oncol. 2017, 22, 347–352. [Google Scholar] [CrossRef]
- Yabushita, H.; Kishida, T.; Fusano, K.; Kanyama, K.; Zhuo, L.; Itano, N.; Kimata, K.; Noguchi, M. Role of hyaluronan and hyaluronan synthase in endometrial cancer. Oncol. Rep. 2005, 13, 1101–1105. [Google Scholar] [CrossRef]
- Nykopp, T.K.; Rilla, K.; Tammi, M.I.; Tammi, R.H.; Sironen, R.; Hämäläinen, K.; Kosma, V.M.; Heinonen, S.; Anttila, M. Hyaluronan synthases (HAS1-3) and hyaluronidases (HYAL1-2) in the accumulation of hyaluronan in endometrioid endometrial carcinoma. BMC Cancer 2010, 10, 512. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Yu, G.; Wang, J. Overexpression of HABP1 correlated with clinicopathological characteristics and unfavorable prognosis in endometrial cancer. Tumor Biol. 2015, 36, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Nykopp, T.K.; Pasonen-Seppänen, S.; Tammi, M.I.; Tammi, R.H.; Kosma, V.M.; Anttila, M.; Sironen, R. Decreased hyaluronidase 1 expression is associated with early disease recurrence in human endometrial cancer. Gynecol. Oncol. 2015, 137, 152–159. [Google Scholar] [CrossRef]
- Klarić, M.; Haller, H.; Brnčić Fischer, A.; Babarović, E.; Prijić, A.; Eminović, S. The role of CD44 and RHAMM in endometrial (endometrioid type) cancer: An immunohistochemical study. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Schatz-Siemers, N.; Chen, Y.T.; Chen, Z.; Wang, D.; Ellenson, L.H.; Du, Y.C.N. Expression of the receptor for hyaluronic acid-mediated motility (RHAMM) in endometrial cancer is associated with adverse histologic parameters and tumor progression. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.T.; Roehrig, K.; Schöndorf, T.; Lazar, A.; Fleisch, M.; Niederacher, D.; Bender, H.G.; Dall, P. Expression of the hyaluronan receptor RHAMM in endometrial carcinomas suggests a role in tumour progression and metastasis. J. Cancer Res. Clin. Oncol. 2003, 129, 161–164. [Google Scholar] [CrossRef]
- Anttila, M.A.; Tammi, R.H.; Tammi, M.I.; Syrjänen, K.J.; Saarikoski, S.V.; Kosma, V.M. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res. 2000, 60, 150–155. [Google Scholar] [PubMed]
- Hiltunen, E.L.J.; Anttila, M.; Kultti, A.; Ropponen, K.; Penttinen, J.; Yliskoski, M.; Kuronen, A.T.; Juhola, M.; Tammi, R.; Tammi, M.; et al. Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Res. 2002, 62, 6410–6413. [Google Scholar]
- Ricciardelli, C.; Ween, M.P.; Lokman, N.A.; Tan, I.A.; Pyragius, C.E.; Oehler, M.K. Chemotherapy-induced hyaluronan production: A novel chemoresistance mechanism in ovarian cancer. BMC Cancer 2013, 13, 476. [Google Scholar] [CrossRef]
- Sillanpää, S.; Anttila, M.A.; Voutilainen, K.; Tammi, R.H.; Tammi, M.I.; Saarikoski, S.V.; Kosma, V.M. CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clin. Cancer Res. 2003, 9, 5318–5324. [Google Scholar]
- Yu, H.; Liu, Q.; Xin, T.; Xing, L.; Dong, G.; Jiang, Q.; Lv, Y.; Song, X.; Teng, C.; Huang, D.; et al. Elevated expression of hyaluronic acid binding protein 1 (HABP1)/P32/C1QBP is a novel indicator for lymph node and peritoneal metastasis of epithelial ovarian cancer patients. Tumor Biol. 2013, 34, 3981–3987. [Google Scholar] [CrossRef]
- Nykopp, T.K.; Rilla, K.; Sironen, R.; Tammi, M.I.; Tammi, R.H.; Hämäläinen, K.; Heikkinen, A.M.; Komulainen, M.; Kosma, V.M.; Anttila, M. Expression of hyaluronan synthases (HAS1–3) and hyaluronidases (HYAL1–2) in serous ovarian carcinomas: Inverse correlation between HYAL1 and hyaluronan content. BMC Cancer 2009, 9, 143. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.M.; Gu, F.; Ambati, C.R.; Rasaily, U.; Ramirez-Pena, E.; Joseph, R.; Manikkam, M.; San Martin, R.; Charles, C.; Pan, Y.; et al. UDP-glucose 6-dehydrogenase regulates hyaluronic acid production and promotes breast cancer progression. Oncogene 2020, 39, 3089–3101. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Wang, Y.; Jin, J.; Xie, X.; Zhang, J. Hyaluronic acid predicts poor prognosis in breast cancer patients. Medicine 2020, 99, e20438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, H.; Liu, J.; Chen, Y.; Xie, J.; Zhao, Y.; Pang, D. Increased breast cancer risk with HABP1/P32/GC1qR genetic polymorphism Rs2285747 and its upregulation in Northern Chinese women. Oncotarget 2017, 8, 13932–13941. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Ryoo, I.; Sim, K.H.; Ahn, H.; Lee, Y.J.; Kwak, M.K. High levels of hyaluronic acid synthase-2 mediate NRF2-driven chemoresistance in breast cancer cells. Biomol. Ther. 2022, 30, 368–379. [Google Scholar] [CrossRef]
- Gao, R.; Liu, Y.; Li, D.; Xun, J.; Zhou, W.; Wang, P.; Liu, C.; Li, X.; Shen, W.; Su, W.; et al. PFKFB4 promotes breast cancer metastasis via induction of hyaluronan production in a P38-dependent manner. Cell. Physiol. Biochem. 2018, 50, 2108–2123. [Google Scholar] [CrossRef]
- Kirova, Y.M.; Fromantin, I.; de Rycke, Y.; Fourquet, A.; Morvan, E.; Padiglione, S.; Falcou, M.-C.; Campana, F.; Bollet, M.A. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy? Results of phase III randomised trial. Radiother. Oncol. 2011, 100, 205–209. [Google Scholar] [CrossRef]
- Pinnix, C.; Perkins, G.H.; Strom, E.A.; Tereffe, W.; Woodward, W.; Oh, J.L.; Arriaga, L.; Munsell, M.F.; Kelly, P.; Hoffman, K.E.; et al. Topical hyaluronic acid vs. standard of care for the prevention of radiation dermatitis after adjuvant radiotherapy for breast cancer: Single-blind randomized phase III clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1089–1094. [Google Scholar] [CrossRef]
- Lee, C.J.; Fang, H.F.; Wang, C.Y.; Chou, K.R.; Huang, T.W. Effect of hyaluronic acid on radiodermatitis in patients with breast cancer: A meta-analysis of randomized controlled trials. Support. Care Cancer 2022, 30, 3965–3975. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Ono, A.; Koshiyama, M.; Nakagawa, M.; Watanabe, Y.; Ikuta, E.; Seki, K.; Oowaki, M. The preventive effect of dietary antioxidants on cervical cancer development. Medicina 2020, 56, 604. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, M.; Hertapanndika, I.; Kusuma, F. The prognostic value of cancer stem cell markers in cervical cancer: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2021, 22, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiao, M.; Zhao, M.; Xu, T.; Guo, M.; Wang, C.; Li, Y.; Zhu, B.; Liu, H. Doxorubicin-loaded functionalized selenium nanoparticles for enhanced antitumor efficacy in cervical carcinoma therapy. Mater. Sci. Eng. C 2020, 106, 110100. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Ge, Z.; Yang, W.; Liu, C.; Yuan, Y. Inhibition of tumor metastasis in vivo by combination of paclitaxel and hyaluronic acid. Cancer Lett. 2006, 243, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Delia, P.; Sansotta, G.; Pontoriero, A.; Iati, G.; De Salvo, S.; Pisana, M.; Potami, A.; Lopes, S.; Messina, G.; Pergolizzi, S. Clinical evaluation of low-molecular-weight hyaluronic acid-based treatment on onset of acute side effects in women receiving adjuvant radiotherapy after cervical surgery: A randomized clinical trial. Oncol. Res. Treat. 2019, 42, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Riemma, G.; Schettino, M.T.; Munno, G.M.; Fasulo, D.D.; Sandullo, L.; Amabile, E.; la Verde, M.; Torella, M. Echinacea angustifolia and Echinacea purpurea supplementation combined with vaginal hyaluronic acid to boost the remission of cervical low-grade squamous intraepithelial lesions (L-SILs): A randomized controlled trial. Medicina 2022, 58, 646. [Google Scholar] [CrossRef]
- Patino, E.G.; Garrido, N.S.; Caneda, L.C.; Gomez, E.C.; Pazos, A.V.; Ferreira, A.V.; Conde, C.R.; Vazquez, C.P. Protective effect on the urinary bladder mucosa of intravesical hyaluronic acid in cervix cancer patients treated with pelvic radiotherapy, weekly chemotherapy and high-dose-rate brachytherapy. Brachytherapy 2008, 7, 152–153. [Google Scholar] [CrossRef]
- Giannone, G.; Attademo, L.; Scotto, G.; Genta, S.; Ghisoni, E.; Tuninetti, V.; Aglietta, M.; Pignata, S.; Valabrega, G. Endometrial cancer stem cells: Role, characterization and therapeutic implications. Cancers 2019, 11, 1820. [Google Scholar] [CrossRef]
- Afify, A.M.; Craig, S.; Paulino, A.F.G.; Stern, R. Expression of hyaluronic acid and its receptors, CD44s and CD44v6, in normal, hyperplastic, and neoplastic endometrium. Ann. Diagn. Pathol. 2005, 9, 312–318. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Tellez Santos, M.; Binkley, P.A.; Tekmal, R.R.; Schenken, R.S.; Knudtson, J.F. Endometrial cell invasion is decreased with inhibition of hyaluronic acid synthesis. Fertil. Steril. 2018, 110, e391. [Google Scholar] [CrossRef]
- Laliscia, C.; Delishaj, D.; Fabrini, M.G.; Gonnelli, A.; Morganti, R.; Perrone, F.; Tana, R.; Paiar, F.; Gadducci, A. Acute and late vaginal toxicity after adjuvant high-dose-rate vaginal brachytherapy in patients with intermediate risk endometrial cancer: Is local therapy with hyaluronic acid of clinical benefit? J. Contemp. Brachytherapy 2016, 6, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Nakamura, S.; Kashihara, T.; Kato, T.; Shibata, Y.; Takahashi, K.; Inaba, K.; Okuma, K.; Igaki, H.; Nakayama, Y.; et al. Hyaluronic acid gel injection in rectovaginal septum reduced incidence of rectal bleeding in brachytherapy for gynecological malignancies. Brachytherapy 2020, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Goldfarb, S.; Baser, R.E.; Goldfrank, D.J.; Seidel, B.; Milli, L.; Saban, S.; Stabile, C.; Canty, J.; Gardner, G.J.; et al. A single-arm clinical trial investigating the effectiveness of a non-hormonal, hyaluronic acid-based vaginal moisturizer in endometrial cancer survivors. Gynecol. Oncol. 2020, 158, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Baser, R.E.; Goldfrank, D.J.; Seidel, B.; Milli, L.; Stabile, C.; Canty, J.; Saban, S.; Goldfarb, S.; Dickler, M.N.; et al. A single-arm, prospective trial investigating the effectiveness of a non-hormonal vaginal moisturizer containing hyaluronic acid in postmenopausal cancer survivors. Support. Care Cancer 2021, 29, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.Y.; Li, S.; Lin, H.; Wu, J.B.; Wang, Y.Q. Relationship between expression of hyaluronan and pathologic features of ovarian adenocarcinoma. Ai Zheng 2004, 23, 177–180. [Google Scholar]
- Riecks, J.; Parnigoni, A.; Győrffy, B.; Kiesel, L.; Passi, A.; Vigetti, D.; Götte, M. The hyaluronan-related genes HAS2, HYAL1-4, PH20 and HYALP1 are associated with prognosis, cell viability and spheroid formation capacity in ovarian cancer. J. Cancer Res. Clin. Oncol. 2022, 148, 3399–3419. [Google Scholar] [CrossRef]
- Wang, S.; Yin, C.; Zhang, Y.; Zhang, L.; Tao, L.; Liang, W.; Pang, L.; Fu, R.; Ding, Y.; Li, F.; et al. Overexpression of ICAM-1 predicts poor survival in high-grade serous ovarian carcinoma: A study based on TCGA and GEO databases and tissue microarray. Biomed Res. Int. 2019, 2019, 2867372. [Google Scholar] [CrossRef]
- Balduit, A.; Agostinis, C.; Mangogna, A.; Maggi, V.; Zito, G.; Romano, F.; Romano, A.; Ceccherini, R.; Grassi, G.; Bonin, S.; et al. The extracellular matrix influences ovarian carcinoma cells’ sensitivity to cisplatinum: A first step towards personalized medicine. Cancers 2020, 12, 1175. [Google Scholar] [CrossRef]
- Karousou, E.; Misra, S.; Ghatak, S.; Dobra, K.; Götte, M.; Vigetti, D.; Passi, A.; Karamanos, N.K.; Skandalis, S.S. Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer. Matrix Biol. 2017, 59, 3–22. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W.; Peyrollier, K.; Xia, W.; Gilad, E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J. Biol. Chem. 2008, 283, 17635–17651. [Google Scholar] [CrossRef]
- Lee, S.J.; Ghosh, S.C.; Han, H.D.; Stone, R.L.; Bottsford-Miller, J.; Shen, D.Y.; Auzenne, E.J.; Lopez-Araujo, A.; Lu, C.; Nishimura, M.; et al. Metronomic activity of CD44-targeted hyaluronic acid-paclitaxel in ovarian carcinoma. Clin. Cancer Res. 2012, 18, 4114–4121. [Google Scholar] [CrossRef]
- Pang, J.; Jiang, P.; Wang, Y.; Jiang, L.; Qian, H.; Tao, Y.; Shi, R.; Gao, J.; Chen, Y.; Wu, Y. Cross-linked hyaluronan gel inhibits the growth and metastasis of ovarian carcinoma. J. Ovarian Res. 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Talih, T.; Oz, B.; Halaclar, B.; Caglayan, K.; Sipahi, M. Comparison of lovastatin and hyaluronic acid/carboxymethyl cellulose on experimental created peritoneal adhesion model in rats. Int. J. Surg. 2014, 12, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Wolny, P.M.; Banerji, S.; Gounou, C.; Brisson, A.R.; Day, A.J.; Jackson, D.G.; Richter, R.P. Analysis of CD44-hyaluronan interactions in an artificial membrane system. J. Biol. Chem. 2010, 285, 30170–30180. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Humeniuk, E.; Adamczuk, G.; Korga-Plewko, A. Hyaluronic acid as a modern approach in anticancer therapy—Review. Int. J. Mol. Sci. 2022, 24, 103. [Google Scholar] [CrossRef]
| Cancer Type | HA Family Member | Methods | Main Results | Ref. |
|---|---|---|---|---|
| Breast cancer | HA level | 83 women with BC, including 57 with metastatic cancer | High serum levels of HA were found in patients with metastatic disease; lower levels of HA correlated with positive response to classical chemotherapy | [49] |
| HA level | 48 primary and 212 metastatic BC patients, and 60 healthy women | The median plasma level of HA in patients with metastatic BC was ~two-fold higher than that in primary BC patients and healthy women; plasma HA levels displayed prognostic and treatment monitoring values for women with metastatic variants of disease | [50] | |
| HA level HASs | 50 BC patients and 40 healthy women | Elevated serum levels of HA, together with increased HASs activity, were found in patients prior to chemotherapy compared to the control group; after the first cycle of chemotherapy, HA levels were decreased | [51] | |
| HABP1 | 63 BC and non-cancerous tissues | Elevated expression of HABP1 mRNA was found in 41 of 63 primary tumor samples; 5-year survival rates were 29% and 54% in cancer patients with high or low HABP1 mRNA levels, respectively | [52] | |
| HAS2 | Cell lines: MCF-7, MCF-7/DR (drug-resistant cancer cell line) | High expression of HAS2 was responsible for Nrf2 activation; pharmacological inhibition of HAS2 improved the sensitivity of MCF-7/DR cells to the action of doxorubicin; overexpression of HAS2 mediated activation of Nrf2 in drug-resistant cancer cells | [48] | |
| HAS2 | Cell lines: BT-474, BT-549, MCF-7, MDA-MB-231, MDA-MB-453, MDA-MB-468; NOD/SCID mice | HAS2 expression corresponded to the malignant phenotype of BC, and its exogenous expression regulated cell malignant phenotype and invadopodia formation in luminal BC; HAS2-HA signaling was required for the formation of invadopodia in cancer cells; HAS2 promoted both growth and metastatic potential of orthotopically injected luminal BC in the animal model | [53] | |
| HYAL-1 | Cell lines: MCF-7, MDA-MB-231; BALB/C nude mice | HYAL-1 was expressed in BC; knockdown of HYAL-1 expression reduced enzyme activity; inhibited the proliferation, adhesion, invasion as well as potential to angiogenesis in vitro; and led to the inhibition of tumorigenesis of BC in the animal model | [54] | |
| Cervical cancer | CD44s CD44v3 | Cell lines: HeLa | CD44s/CD44v3 expressions were inhibited by natural polyphenols; low-molecular-weight HA showed growth-promoting activity in HeLa cells, in contrast to high-molecular-weight HA | [55] |
| HABP1 | 30 CIN, 118 CC specimens, and 10 normal specimens | HABP1 expression was shown to be higher in CC than in high-grade CIN; overexpression of this protein correlated with advanced FIGO stage, poor histologic grade, large tumor size, LVSI, deep stromal infiltration, and lymph node metastasis, and seemed to be an independent factor for disease-free survival | [56] | |
| Endometrial cancer | HA level HAS1–3 | Sera obtained from 59 EC patients and 22 postmenopausal healthy women | Serum levels of HA were higher in the EC group than in the corresponding control group; the expression of HAS1 was related to the depth of myometrial invasion, histological grade, and LVSI | [57] |
| HA level HAS1–3 HYAL-1–3 | 39 EC biopsies with different histologic grade (grades I–III) | HA, HASs, and degradative enzymes of HA were identified in EC of all histologic grades; HA was predominantly localized to tumor-associated stroma, particularly to the basal surface of cells | [42] | |
| HA level HAS1–3 HYAL-1–2 | Endometrial tissue specimens collected from 35 patients | The immunoreactivity of HASs was increased in the cancer epithelium; HYAL-2 mRNA was reduced in EC and correlated with HYAL-1; an inverse correlation between HYAL-1 mRNA and the epithelial and stromal HA levels was found | [58] | |
| HABP1 | 188 EC, 43 benign endometrial lesions, and 41 normal endometrium specimens | HABP1 was overexpressed in EC and benign endometrial lesions, compared with normal endometrium; cancer patients with high HABP1 expression had a poorer overall and disease-free survival than individuals with low expression of this protein | [59] | |
| HYAL-1 | Endometrial tissue specimens collected from 343 patients | Reduced HYAL-1 expression was associated with the progression of EC towards higher grades and large tumor sizes, lymph node metastasis, and lymphovascular invasion | [60] | |
| CD44 RHAMM | 104 tissue samples of EC | Higher CD44/RHAMM expression correlated with higher depth of myometrial invasion, LVSI, and FIGO stage of disease | [61] | |
| RHAMM | 225 samples of EC and 8 samples of normal endometrium | Increased expression of RHAMM protein was found in EC compared with no or weak expression in normal endometrium; higher RHAMM expression was related to more malignant tumors | [62] | |
| RHAMM | 89 EC and 15 normal endometrium specimens | Increased RHAMM expression was detected in 58% of 89 tumor samples; the positivity rates for RHAMM were 100% and 51% in patients with positive or negative lymph nodes, respectively; RHAMM overexpression correlated with higher histological grade of the tumors and occurrence of lymph node metastases | [63] | |
| Ovarian cancer | HA level | Histological sections of 309 epithelial OC and 45 matched metastatic lesions | High stromal levels of HA corresponded with poor differentiation, serous histological type, advanced stage, and large primary residual tumor, but was not associated with CD44 overexpression on cancer cells; high levels of this polysaccharide were found more frequently in metastatic lesions than in primary tumors | [64] |
| HA level HYALs | Ovarian tissue specimens from 78 patients | HA levels increased in cancers, especially in grade III tumors and metastases; HYALs activity slightly decreased from semi-malignant through low-grade to high-grade tumors | [65] | |
| HA level CD44 | Cell lines: OV-90, OVCAR-3/5, SKOV-3; serum from OC patients | HA level was increased following carboplatin treatment and predicted OC outcome; HA treatment increased resistance of OC to chemotherapy; HA regulated expression of ABC transporters | [66] | |
| CD44 | Samples collected from 307 patients with epithelial OC | 51% of the tumors had a high proportion of CD44-positive cells; overexpression of CD44 predicted better 5-year overall survival and recurrence-free survival | [67] | |
| CD44 | 81 OC tumor sections | CD44 expression was found in 43% of OC samples; the expression of CD44, FIGO stage III and IV, and the presence of vascular invasion was related to a shorter overall survival | [15] | |
| HABP1 | Samples collected from 161 patients with epithelial OC | HABP1 was overexpressed in most metastatic lesions; high expression of HABP1 correlated with peritoneal (95% cases) and lymph node metastases (48% cases) among patients with primary tumors | [68] | |
| HAS1–3 HYAL-1–2 | 39 ovarian tissue specimens from 39 patients | HASs expression was not consistently elevated in serous epithelial OC; expression of HYAL-1 was reduced and correlated with the accumulation of HA | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Role of Hyaluronic Acid in Selected Malignant Neoplasms in Women. Biomedicines 2023, 11, 304. https://doi.org/10.3390/biomedicines11020304
Markowska A, Antoszczak M, Markowska J, Huczyński A. Role of Hyaluronic Acid in Selected Malignant Neoplasms in Women. Biomedicines. 2023; 11(2):304. https://doi.org/10.3390/biomedicines11020304
Chicago/Turabian StyleMarkowska, Anna, Michał Antoszczak, Janina Markowska, and Adam Huczyński. 2023. "Role of Hyaluronic Acid in Selected Malignant Neoplasms in Women" Biomedicines 11, no. 2: 304. https://doi.org/10.3390/biomedicines11020304
APA StyleMarkowska, A., Antoszczak, M., Markowska, J., & Huczyński, A. (2023). Role of Hyaluronic Acid in Selected Malignant Neoplasms in Women. Biomedicines, 11(2), 304. https://doi.org/10.3390/biomedicines11020304







