Inhibition of microRNA-328 Increases Ocular Mucin Expression and Conjunctival Goblet Cells
Abstract
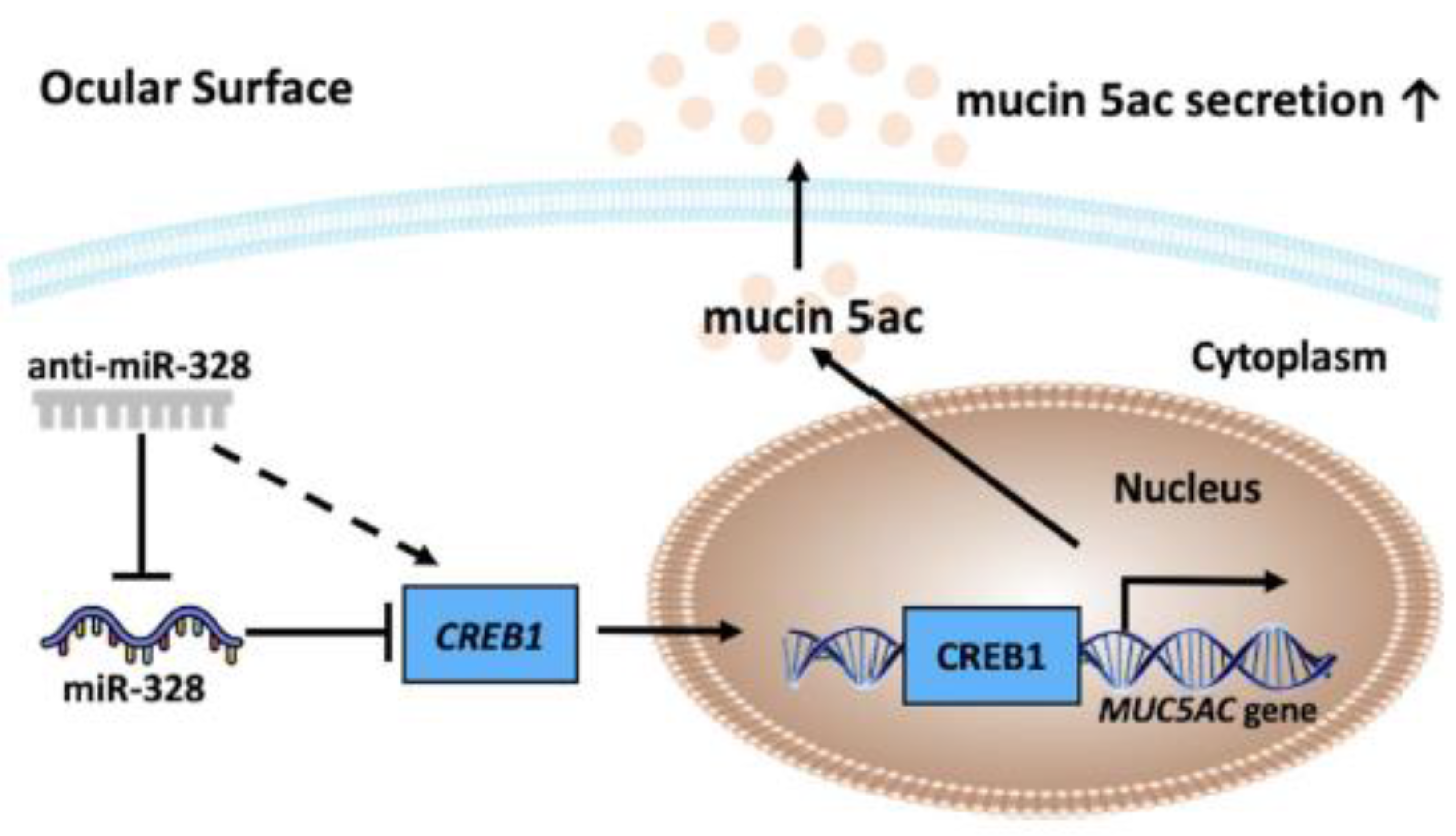
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Induction of Desiccation Stress
2.3. Anti-miR-328 Eye Drops
2.4. miRNA Targets and Pathway Prediction
2.5. MicroRNA Transfection Assay
2.6. Construction of Reporter Plasmids
2.7. Transient Transfection and Luciferase Reporter Assay
2.8. Reverse Transcription and Quantitative Polymerase Chain Reaction
2.9. Western Blotting
2.10. Immunofluorescence Staining
2.11. ELISA
2.12. Impression Cytology and Periodic Acid–Schiff (PAS) Staining
2.13. Statistical Analysis
3. Results
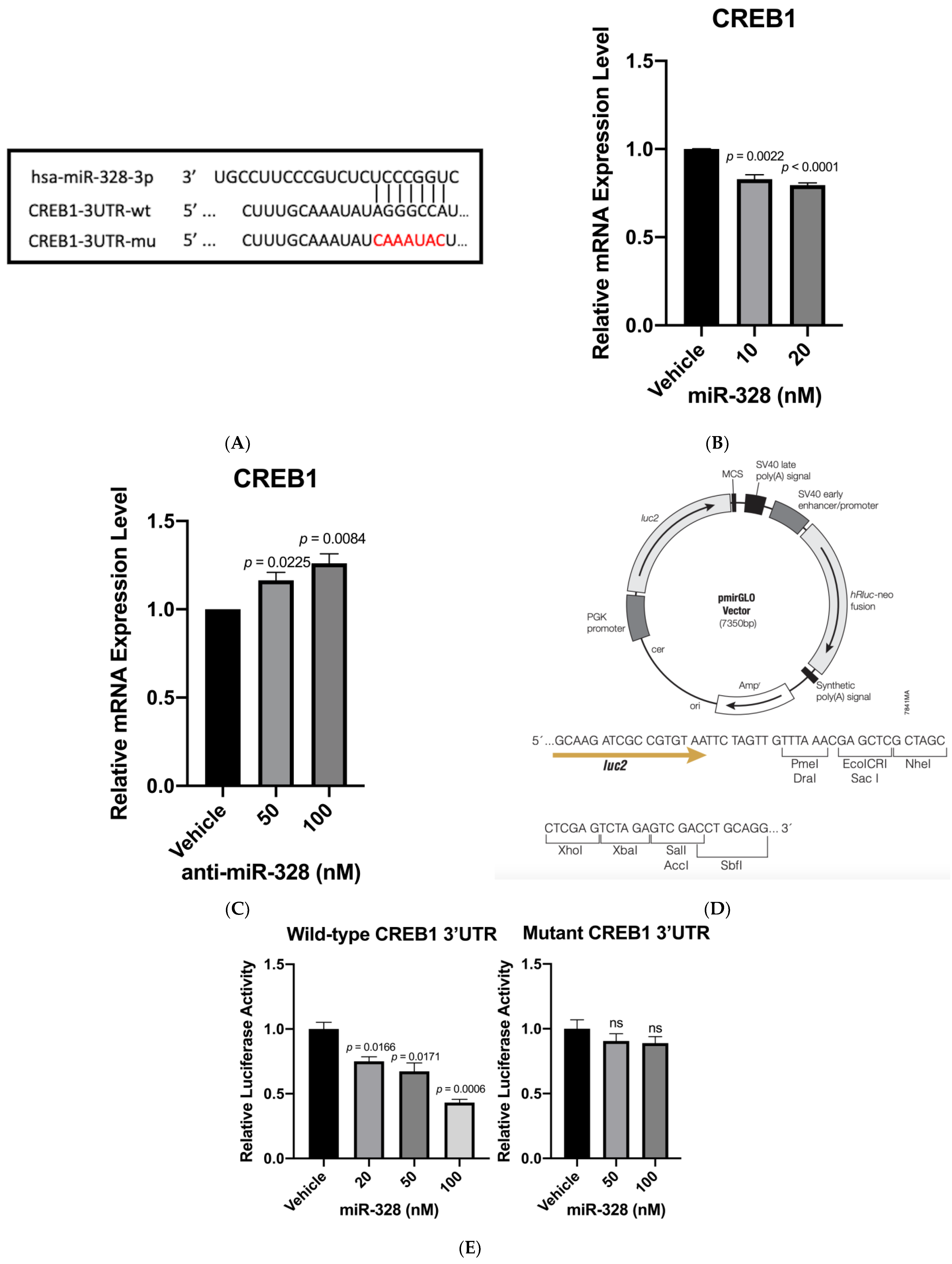
3.1. CREB1 Is a Direct Target of miR-328
3.2. Confirmation of CREB1 as a miR-328 Direct Target
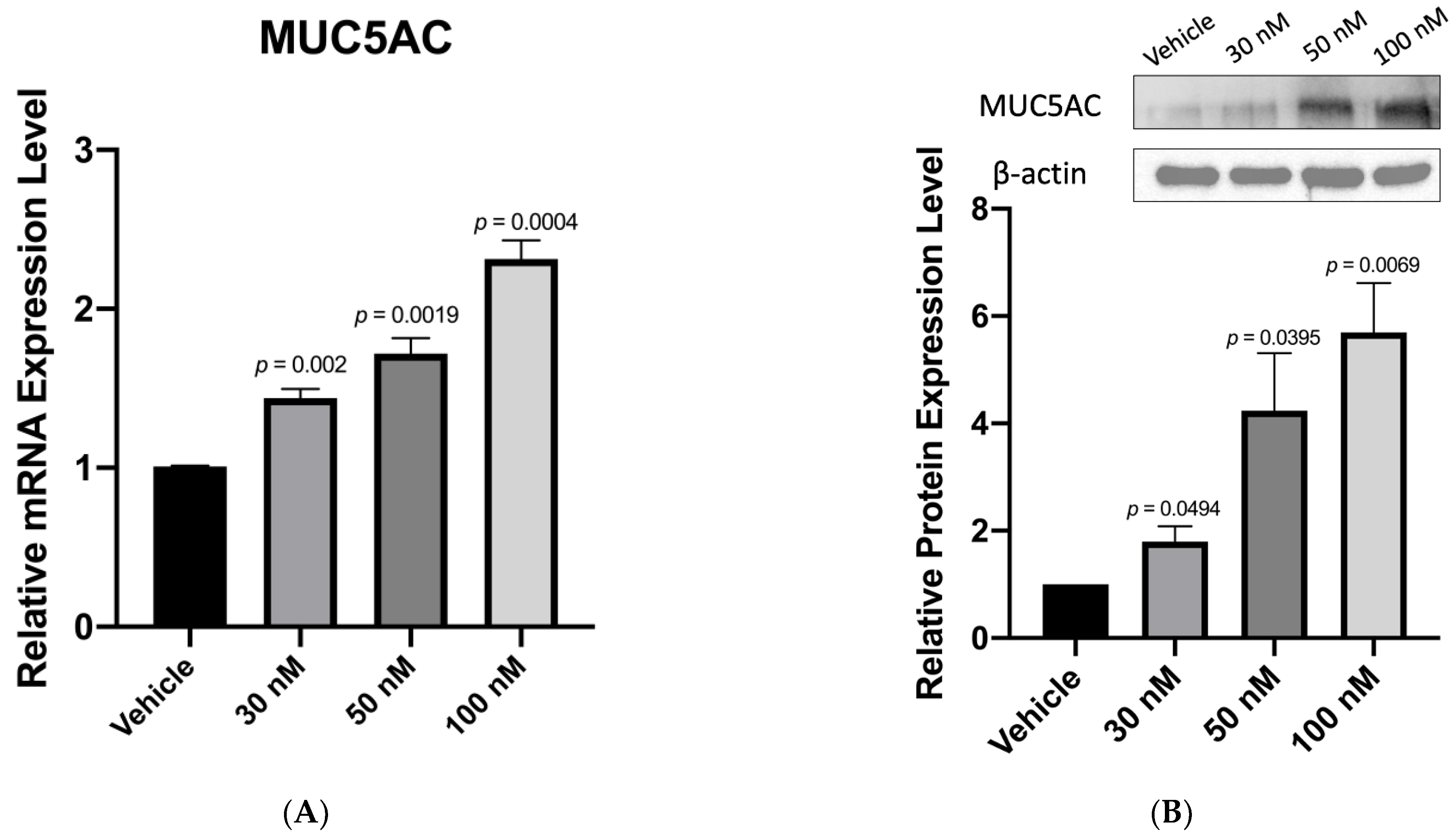
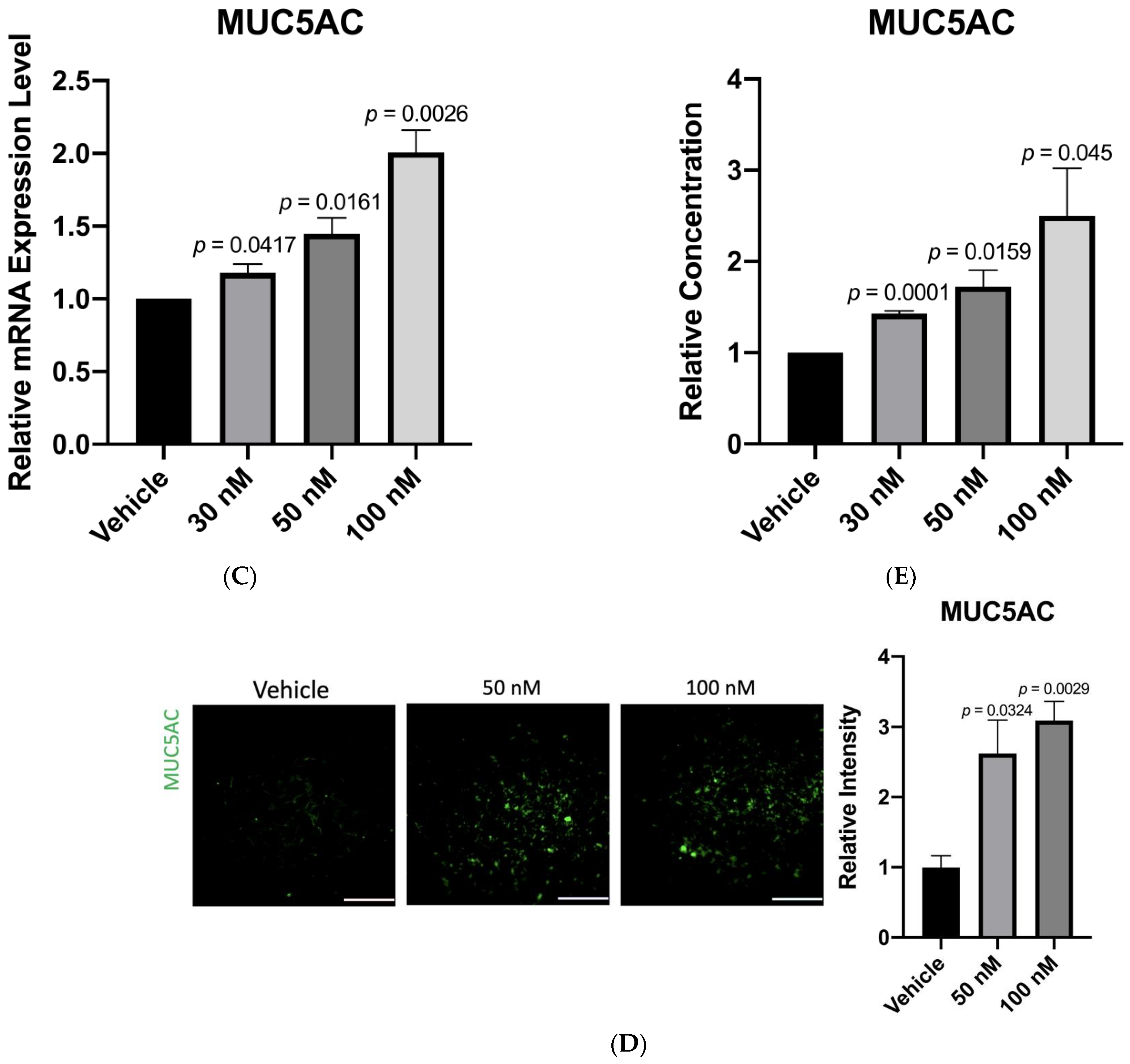
3.3. Anti-miR-328 Increases MUC5AC Expression in Goblet Cells
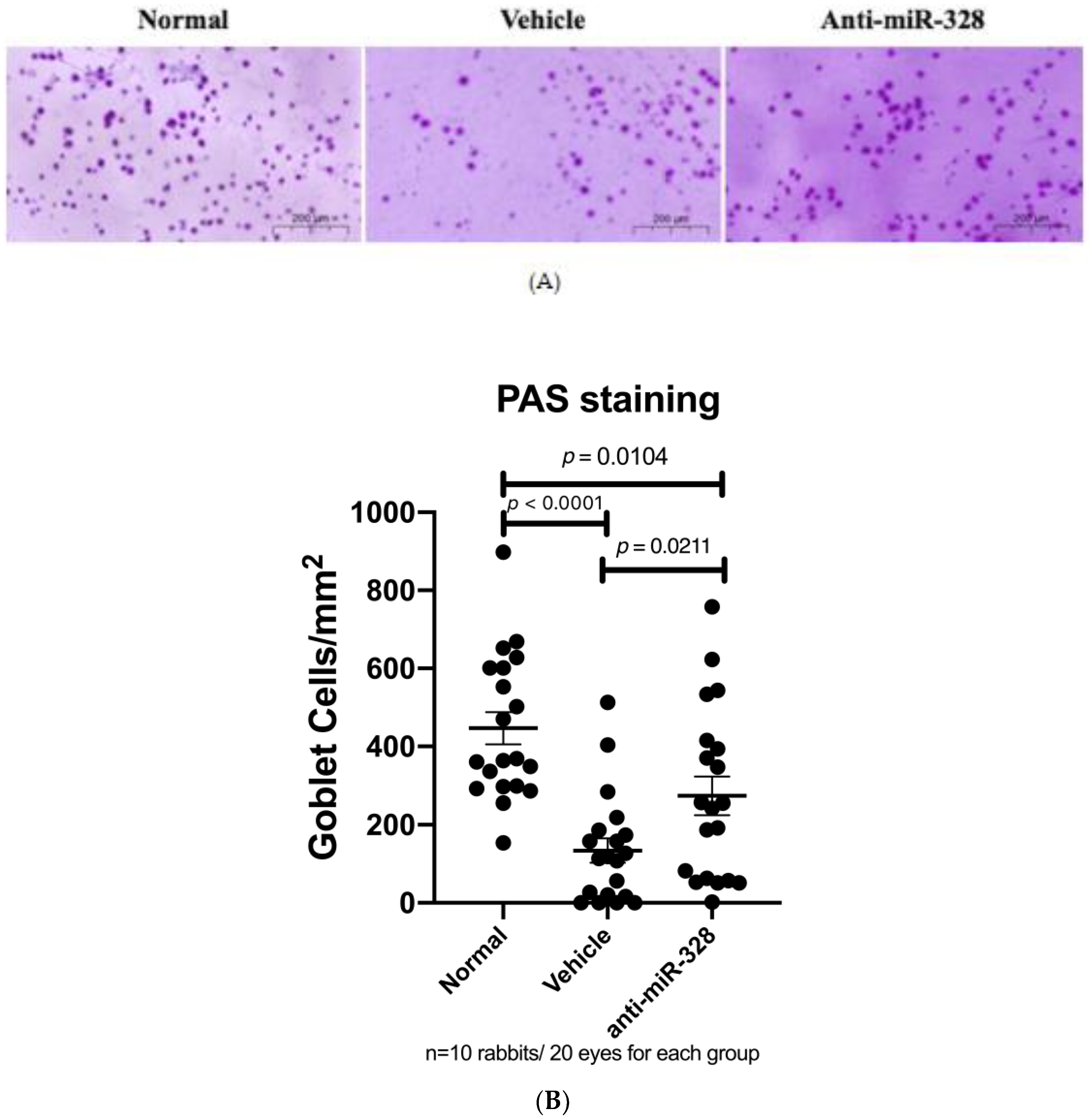
3.4. Anti-miR-328 Treatment Increases Goblet Cells in Rabbit Eyes
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yazdani, M.; Elgstøen, K.B.P.; Rootwelt, H.; Shahdadfar, A.; Utheim, Ø.A.; Utheim, T.P. Tear Metabolomics in Dry Eye Disease: A Review. Int. J. Mol. Sci. 2019, 20, 3755. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Davidson, H.J.; Kuonen, V.J. The tear film and ocular mucins. Vet. Ophthalmol. 2004, 7, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.J.; Liu, Y.; Zou, H.D.; Jiang, Y.J.; Liang, X.Q.; Sheng, M.J.; Li, B.; Xu, X. Epidemiologic study of dry eye in populations equal or over 20 years old in Jiangning District of Shanghai. Zhonghua Yan Ke Za Zhi 2009, 45, 486–491. [Google Scholar]
- Viso, E.; Rodriguez-Ares, M.T.; Gude, F. Prevalence of and associated factors for dry eye in a Spanish adult population (the Salnes Eye Study). Ophthalmic Epidemiol. 2009, 16, 15–21. [Google Scholar] [CrossRef]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Ärzteblatt Int. 2015, 112, 71–81. [Google Scholar] [CrossRef]
- Abidi, A.; Shukla, P.; Ahmad, A. Lifitegrast: A novel drug for treatment of dry eye disease. J. Pharmacol. Pharmacother. 2016, 7, 194–198. [Google Scholar] [CrossRef]
- Dunn, J.D.; Karpecki, P.M.; Meske, M.E.; Reissman, D. Evolving knowledge of the unmet needs in dry eye disease. Am. J. Manag. Care 2021, 27 (Suppl. 2), S23–S32. [Google Scholar]
- Sullivan, D.A.; Hammitt, K.M.; Schaumberg, D.A.; Sullivan, B.D.; Begley, C.G.; Gjorstrup, P.; Garrigue, J.S.; Nakamura, M.; Quentric, Y.; Barabino, S.; et al. Report of the TFOS/ARVO Symposium on global treatments for dry eye disease: An unmet need. Ocul. Surf. 2012, 10, 108–116. [Google Scholar] [CrossRef]
- Mantelli, F.; Argüeso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef]
- Baudouin, C.; Rolando, M.; Benitez Del Castillo, J.M.; Messmer, E.M.; Figueiredo, F.C.; Irkec, M.; Van Setten, G.; Labetoulle, M. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog. Retin. Eye Res. 2019, 71, 68–87. [Google Scholar] [CrossRef]
- Gipson, I.K.; Argüeso, P. Role of mucins in the function of the corneal and conjunctival epithelia. Int. Rev. Cytol 2003, 231, 1–49. [Google Scholar]
- Xu, K.; Liu, X.N.; Zhang, H.B.; Zhu, X.P.; Zhang, X.J. Tear film instability is associated with weakened colocalization between occludin and MUC5AC in scopolamine-induced dry eye disease (DED) rats. Int. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Hori, Y. Secreted Mucins on the Ocular Surface. Invest. Ophthalmol. Vis. Sci. 2018, 59, DES151–DES156. [Google Scholar] [CrossRef]
- Portal, C.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. Ocular mucins in dry eye disease. Exp. Eye Res. 2019, 186, 107724. [Google Scholar] [CrossRef]
- Liao, C.H.; Tseng, C.L.; Lin, S.L.; Liang, C.L.; Juo, S.H. MicroRNA Therapy for Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2022, 38, 125–132. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Chung, W.C.; Ryu, S.H.; Sun, H.; Zeldin, D.C.; Koo, J.S. CREB mediates prostaglandin F2alpha-induced MUC5AC overexpression. J. Immunol. 2009, 182, 2349–2356. [Google Scholar] [CrossRef]
- Chen, Y.; Garvin, L.M.; Nickola, T.J.; Watson, A.M.; Colberg-Poley, A.M.; Rose, M.C. IL-1β induction of MUC5AC gene expression is mediated by CREB and NF-κB and repressed by dexamethasone. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L797–L807. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed]
- Shatos, M.A.; Rios, J.D.; Tepavcevic, V.; Kano, H.; Hodges, R.; Dartt, D.A. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1455–1464. [Google Scholar] [PubMed]
- Saha, P.; Kim, K.J.; Lee, V.H. A primary culture model of rabbit conjunctival epithelial cells exhibiting tight barrier properties. Curr. Eye Res. 1996, 15, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Uchino, Y.; Uchino, M.; Yokoi, N.; Dogru, M.; Kawashima, M.; Okada, N.; Inaba, T.; Tamaki, S.; Komuro, A.; Sonomura, Y.; et al. Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol. 2014, 132, 985–992. [Google Scholar] [CrossRef]
- Lau, O.C.; Samarawickrama, C.; Skalicky, S.E. P2Y2 receptor agonists for the treatment of dry eye disease: A review. Clin. Ophthalmol. 2014, 8, 327–334. [Google Scholar]
- Nakamura, M.; Imanaka, T.; Sakamoto, A. Diquafosol ophthalmic solution for dry eye treatment. Adv. Ther. 2012, 29, 579–589. [Google Scholar] [CrossRef]
- Ali, A.S.; Ajaz, A. The role of mucin-educated platelet activation in tumor invasiveness: An unfolding concern in the realm of cancer biology. BioMedicine 2017, 7, 21. [Google Scholar] [CrossRef]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choo, J.; Liao, C.-H.; Tseng, C.-L.; Chen, J.-L.; Cheng, H.-C.; Liang, C.-L.; Juo, S.-H.H. Inhibition of microRNA-328 Increases Ocular Mucin Expression and Conjunctival Goblet Cells. Biomedicines 2023, 11, 287. https://doi.org/10.3390/biomedicines11020287
Choo J, Liao C-H, Tseng C-L, Chen J-L, Cheng H-C, Liang C-L, Juo S-HH. Inhibition of microRNA-328 Increases Ocular Mucin Expression and Conjunctival Goblet Cells. Biomedicines. 2023; 11(2):287. https://doi.org/10.3390/biomedicines11020287
Chicago/Turabian StyleChoo, Jackson, Chun-Huei Liao, Ching-Li Tseng, Jiunn-Liang Chen, Huey-Chuan Cheng, Chung-Ling Liang, and Suh-Hang Hank Juo. 2023. "Inhibition of microRNA-328 Increases Ocular Mucin Expression and Conjunctival Goblet Cells" Biomedicines 11, no. 2: 287. https://doi.org/10.3390/biomedicines11020287
APA StyleChoo, J., Liao, C.-H., Tseng, C.-L., Chen, J.-L., Cheng, H.-C., Liang, C.-L., & Juo, S.-H. H. (2023). Inhibition of microRNA-328 Increases Ocular Mucin Expression and Conjunctival Goblet Cells. Biomedicines, 11(2), 287. https://doi.org/10.3390/biomedicines11020287





