Dapagliflozin Ameliorates Neural Damage in the Heart and Kidney of Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
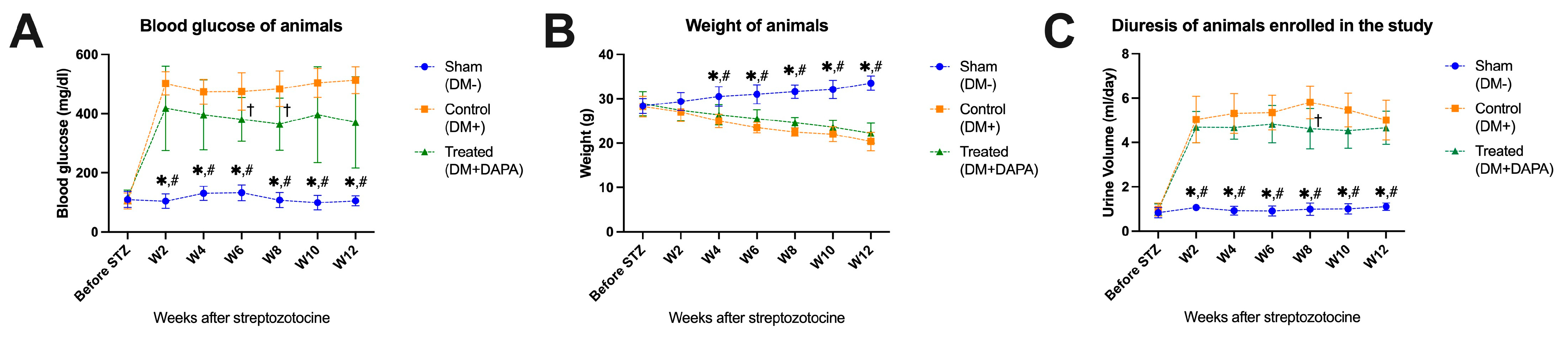
- The sham group (DM−), receiving 0.9% NaCl, without streptozotocin. Diabetes did not occur in this group of animals. We evaluated the clinical changes in this group (body weight, blood sugar, and diuresis), as well as changes in the nervous system in the heart and kidneys of mice in the absence of diabetes.
- The control group (DM+), into which a single dose of STZ, at a dose of 150 mg/kg body weight, was injected intraperitoneally. The animals in this group did not receive any therapeutic protocol. In this group of animals, biological parameters (such as weight, diuresis, polydipsia, and polyphagia) were monitored, and structural features of the nervous system at the level of the heart and kidneys were also evaluated.
- The treated group with DM+ and dapagliflozin treatment (DM+DAPA). In these animals, after diabetes was induced by means of intraperitoneal injection of STZ and verified through the observation of elevated blood glucose levels, the therapy was given as 10 mg dapagliflozin/kg body weight administered via gastric gavage. The therapy was applied daily for 12 weeks from the onset of diabetes mellitus.
2.2. Tissue Preparation and Histological Analysis
2.3. Statistical Analysis
3. Results
3.1. Effect of Dapagliflozin on Blood Glucose Level, Body Weight, and Diuresis
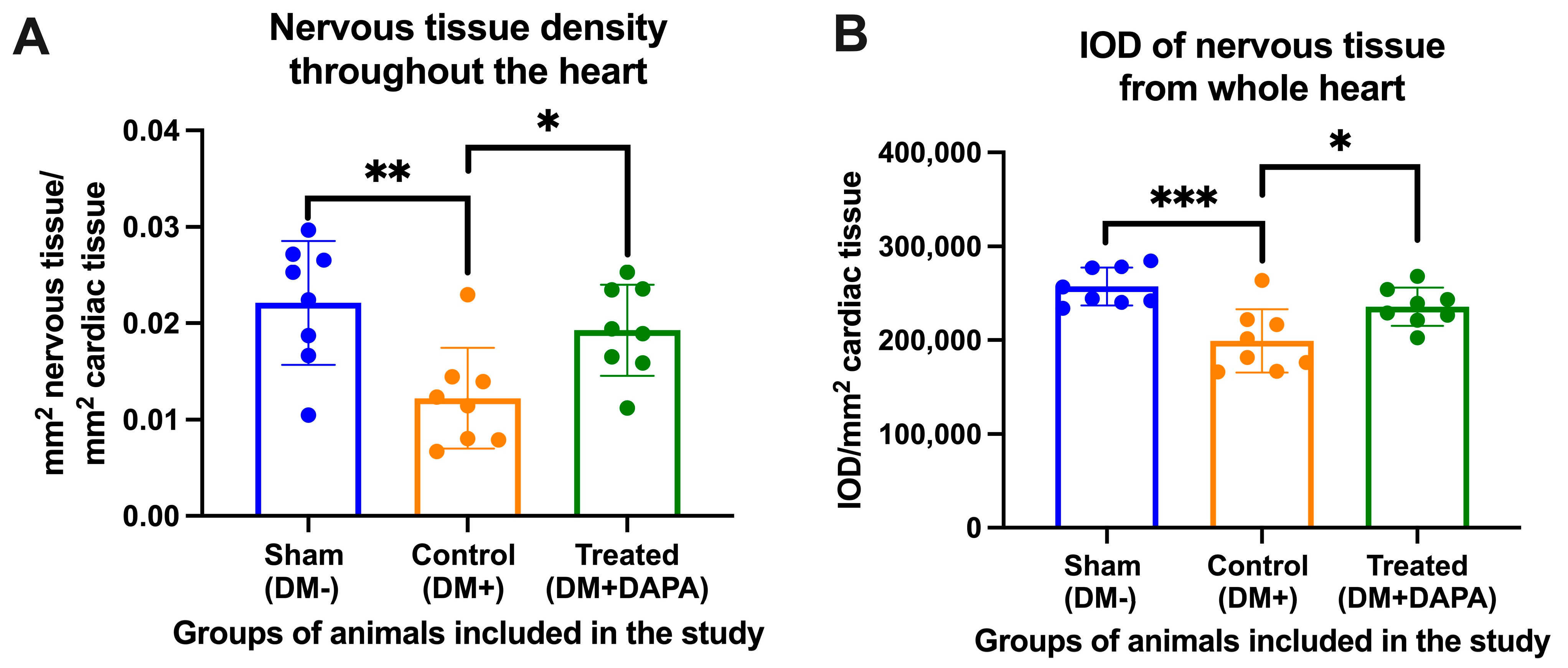
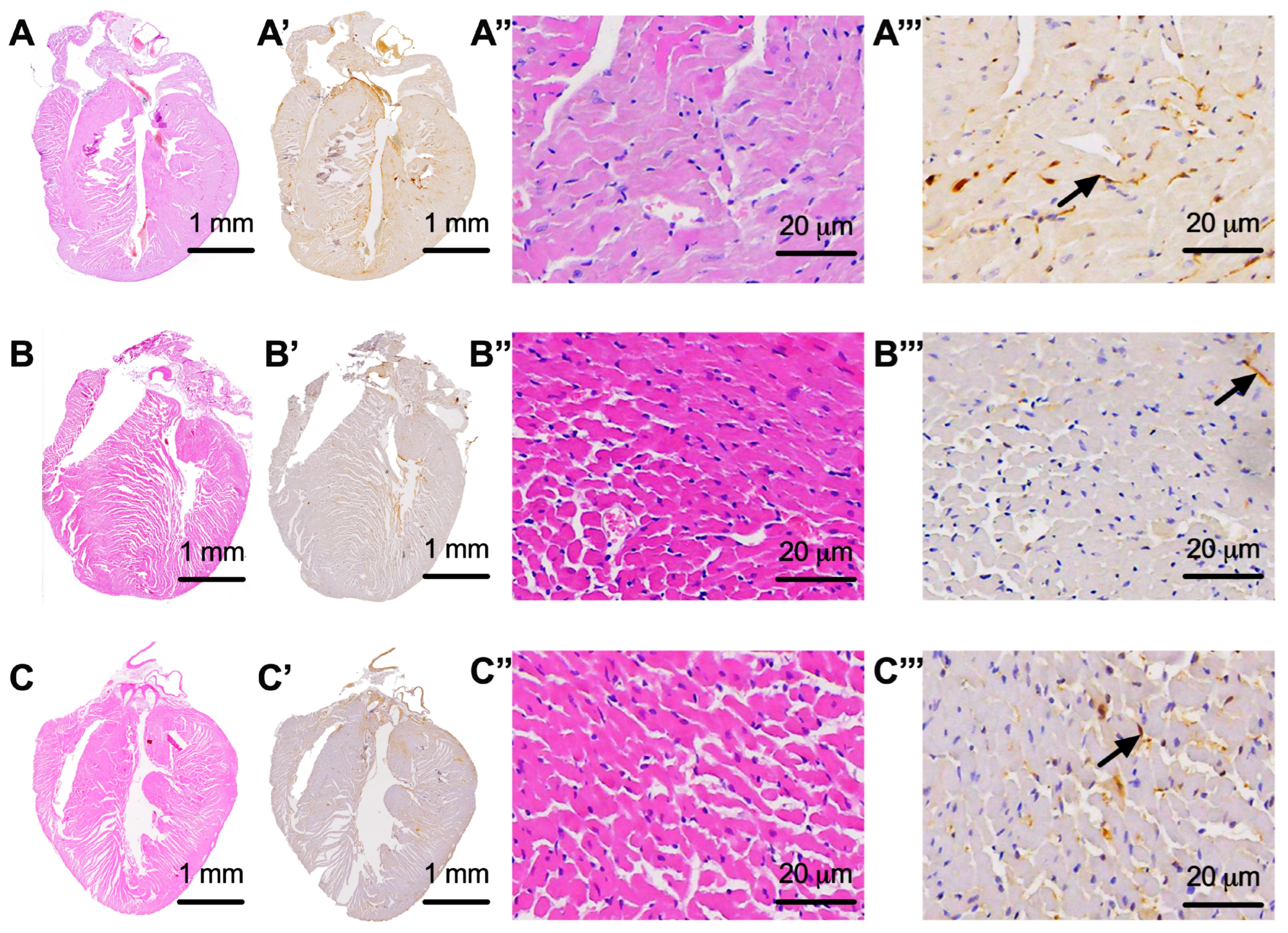
3.2. Assessment of Whole-Heart Nervous Tissue
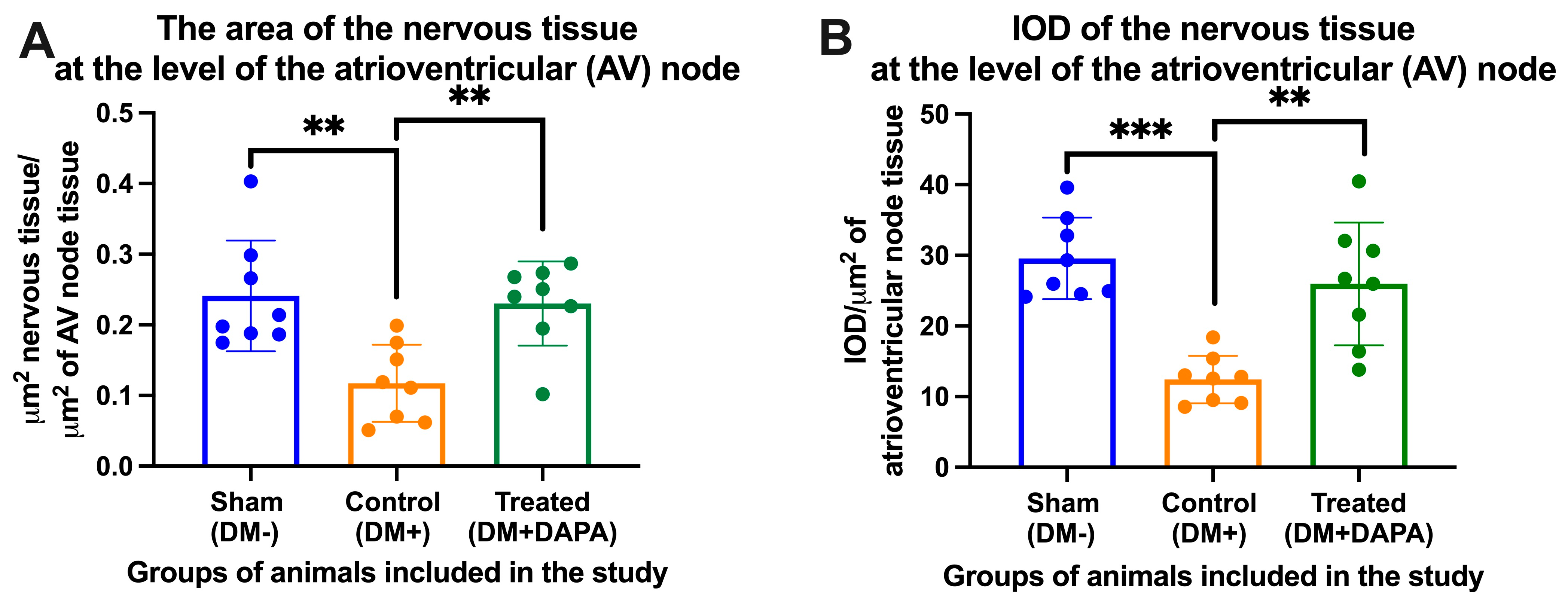
3.3. Assessment of Nervous Tissue at the Level of the Atrioventricular Node
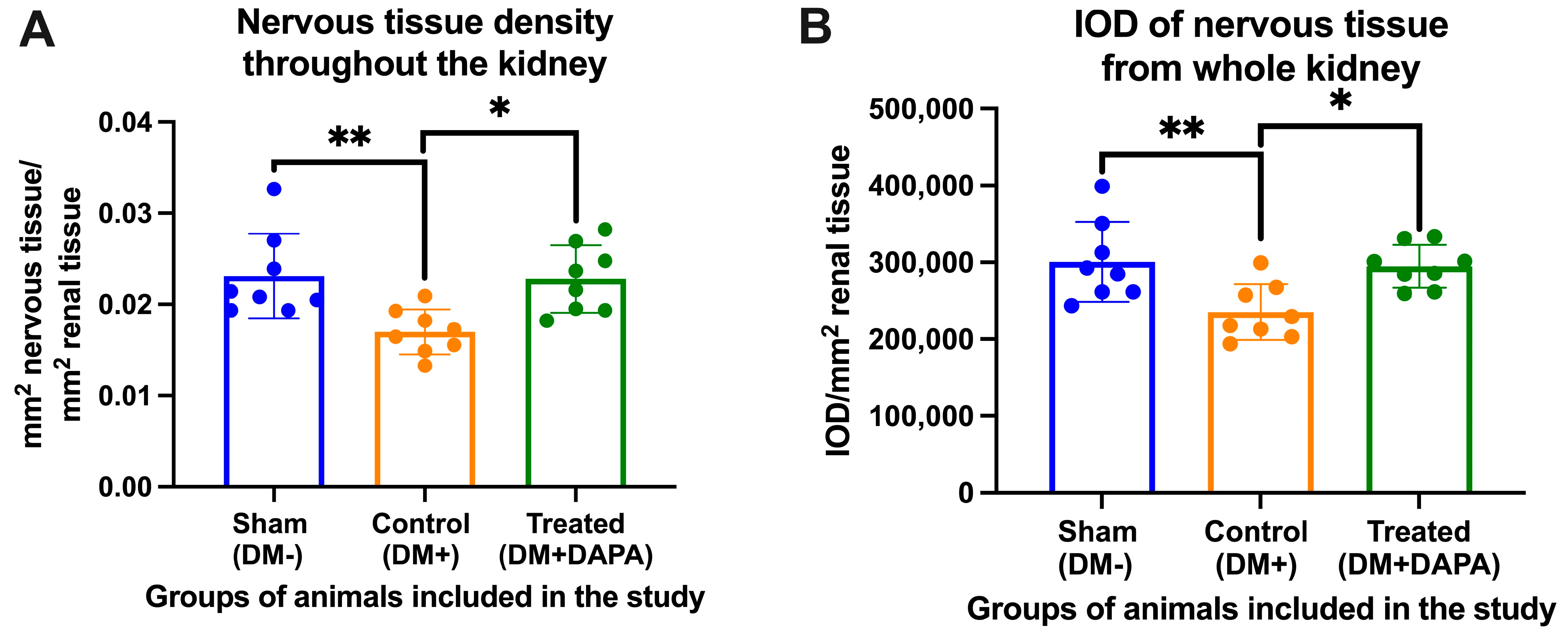
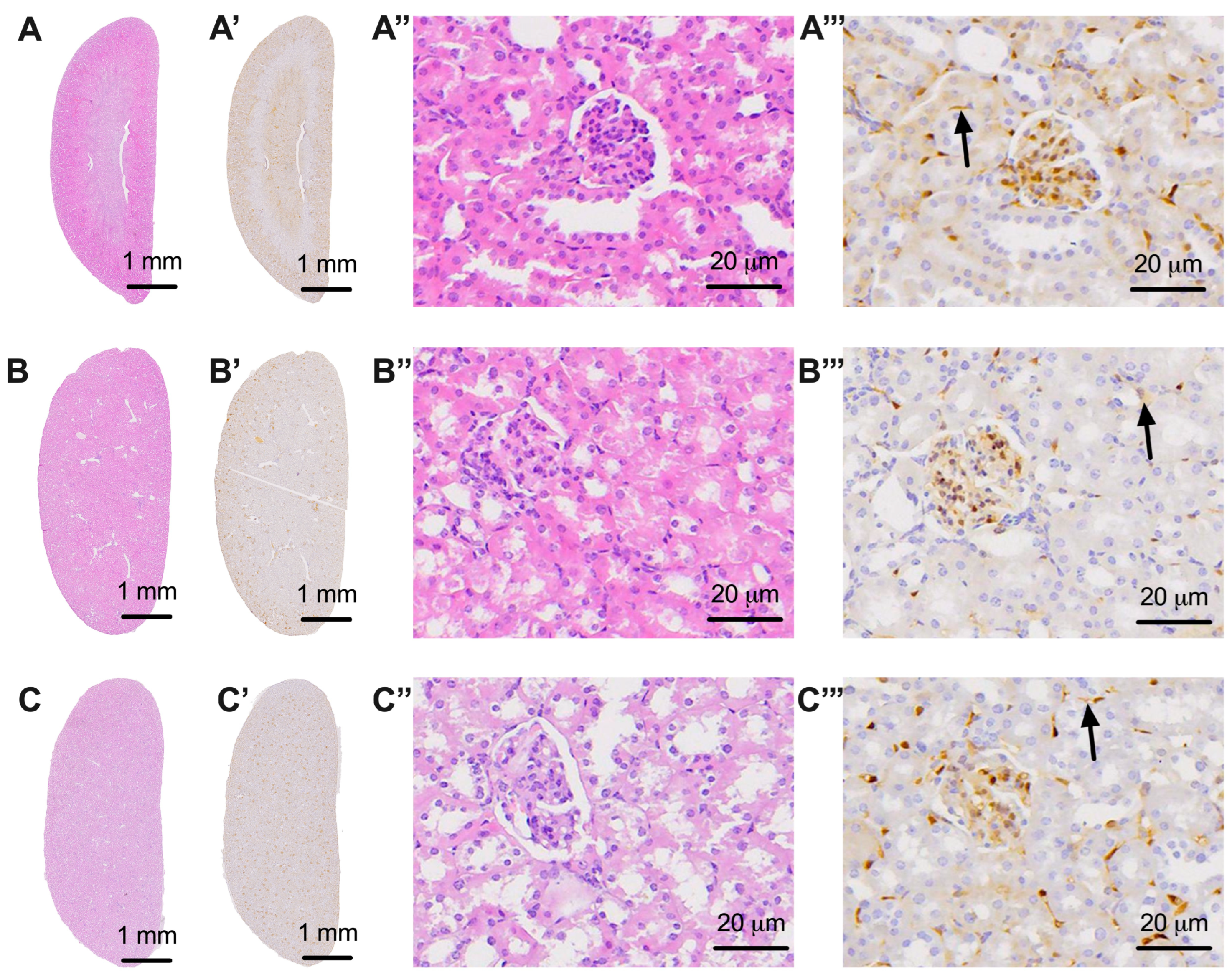
3.4. Evaluation of Nervous Tissue at the Level of the Kidneys
4. Discussion
Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, D.; Kar, D.; Khunti, K.; Davies, M.J.; Scott, A.R.; Walker, J.; Tesfaye, S. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet. Diabetes Endocrinol. 2019, 7, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Seewaldt-Becker, E.; Macha, S.; Hantel, S.; Pinnetti, S.; Seman, L.; Woerle, H.J. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Plosker, G.L. Dapagliflozin: A review of its use in type 2 diabetes mellitus 2012. Drugs 2012, 72, 2289–2312. [Google Scholar] [CrossRef]
- Lamos, E.M.; Younk, L.M.; Davis, S.N. Canagliflozin, an inhibitor of sodium-glucose cotransporter 2, for the treatment of type 2 diabetes mellitus. Expert Opin. Drug Metab. Toxicol. 2013, 9, 763–775. [Google Scholar] [CrossRef]
- Scott, L.J. Empagliflozin: A review of its use in patients with type 2 diabetes mellitus. Drugs 2014, 74, 1769–1784. [Google Scholar] [CrossRef]
- Nauck, M.A. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des. Dev. Ther. 2014, 8, 1335–1380. [Google Scholar] [CrossRef]
- Piątkowska-Chmiel, I.; Herbet, M.; Gawrońska-Grzywacz, M.; Pawłowski, K.; Ostrowska-Leśko, M.; Dudka, J. Molecular and neural roles of sodium-glucose cotransporter 2 inhibitors in alleviating neurocognitive impairment in diabetic mice. Psychopharmacology 2023, 240, 983–1000. [Google Scholar] [CrossRef]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, X.; Ilyas, I.; Zheng, X.; Luo, S.; Little, P.J.; Kamato, D.; Sahebkar, A.; Wu, W.; Weng, J.; et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: From pharmacology to pre-clinical and clinical therapeutics. Theranostics 2021, 11, 4502–4515. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.; Talanki Manjunatha, R.; Bollu, B.; Jhaveri, S.; Avanthika, C.; Reddy, N.; Saha, T.; Gandhi, F. A Comprehensive Review of Neuronal Changes in Diabetics. Cureus 2021, 13, e19142. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Xu, G.; Song, K.H.; Suzuma, K.; Bonner-Weir, S.; Sharma, A.; Weir, G.C. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J. Biol. Chem. 2001, 276, 31099–31104. [Google Scholar] [CrossRef]
- Karachalias, N.; Babaei-Jadidi, R.; Ahmed, N.; Thornalley, P.J. Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem. Soc. Trans. 2003, 31 Pt 6, 1423–1425. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; Schmidt, A.M. Arguing for the motion: Yes, RAGE is a receptor for advanced glycation endproducts. Mol. Nutr. Food Res. 2007, 51, 1111–1115. [Google Scholar] [CrossRef]
- Sekido, H.; Suzuki, T.; Jomori, T.; Takeuchi, M.; Yabe-Nishimura, C.; Yagihashi, S. Reduced cell replication and induction of apoptosis by advanced glycation end products in rat Schwann cells. Biochem. Biophys. Res. Commun. 2004, 320, 241–248. [Google Scholar] [CrossRef]
- Uehara, K.; Yamagishi, S.; Otsuki, S.; Chin, S.; Yagihashi, S. Effects of polyol pathway hyperactivity on protein kinase C activity, nociceptive peptide expression, and neuronal structure in dorsal root ganglia in diabetic mice. Diabetes 2004, 53, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Drel, V.R.; Xu, W.; Zhang, J.; Kador, P.F.; Ali, T.K.; Shin, J.; Julius, U.; Slusher, B.; El-Remessy, A.B.; Obrosova, I.G. Poly(ADP-ribose)polymerase inhibition counteracts cataract formation and early retinal changes in streptozotocin-diabetic rats. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Joardar, S.; Manna, P.; Dua, T.K.; Bhattacharjee, N.; Khanra, R.; Bhowmick, S.; Kalita, J.; Saha, A.; Ray, S.; et al. Carnosic Acid, a Natural Diterpene, Attenuates Arsenic-Induced Hepatotoxicity via Reducing Oxidative Stress, MAPK Activation, and Apoptotic Cell Death Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 1421438. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Spallone, V.; Valensi, P. SGLT2 inhibitors and the autonomic nervous system in diabetes: A promising challenge to better understand multiple target improvement. Diabetes Metab. 2021, 47, 101224. [Google Scholar] [CrossRef]
- Matthews, V.B.; Elliot, R.H.; Rudnicka, C.; Hricova, J.; Herat, L.; Schlaich, M.P. Role of the sympathetic nervous system in regulation of the sodium glucose cotransporter 2. J. Hypertens. 2017, 35, 2059–2068. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC. Basic Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Kim, H.K.; Ishizawa, R.; Fukazawa, A.; Wang, Z.; Bezan Petric, U.; Hu, M.C.; Smith, S.A.; Mizuno, M.; Vongpatanasin, W. Dapagliflozin Attenuates Sympathetic and Pressor Responses to Stress in Young Prehypertensive Spontaneously Hypertensive Rats. Hypertension 2022, 79, 1824–1834. [Google Scholar] [CrossRef]
- Basalay, M.V.; Arjun, S.; Davidson, S.M.; Yellon, D.M. The role of parasympathetic mechanisms in the infarct-limiting effect of SGLT2 inhibitor ertugliflozin. bioRxiv 2021. [Google Scholar] [CrossRef]
- Saleh, S.; Hanna, G.; El-Nabi, S.H.; El-Domiaty, H.; Shabaan, A.; Fayez Ewida, S. Dapagliflozin, a sodium glucose cotransporter 2 inhibitors, protects cardiovascular function in type-2 diabetic murine model. J. Genet. 2020, 99, 46. [Google Scholar] [CrossRef] [PubMed]
- Farias, R.S.; Silva-Aguiar, R.P.; Teixeira, D.E.; Gomes, C.P.; Pinheiro, A.A.S.; Peruchetti, D.B.; Caruso-Neves, C. Inhibition of SGLT2 co-transporter by dapagliflozin ameliorates tubular proteinuria and tubule-interstitial injury at the early stage of diabetic kidney disease. Eur. J. Pharmacol. 2023, 942, 175521. [Google Scholar] [CrossRef] [PubMed]
- Arcari, L.; Ciavarella, G.M.; Altieri, S.; Limite, L.R.; Russo, D.; Luciani, M.; De Biase, L.; Mené, P.; Volpe, M. Longitudinal changes of left and right cardiac structure and function in patients with end-stage renal disease on replacement therapy. Eur. J. Intern. Med. 2020, 78, 95–100. [Google Scholar] [CrossRef]
- Chang, D.Y.; Li, X.Q.; Chen, M.; Zhao, M.H. Dapagliflozin Ameliorates Diabetic Kidney Disease via Upregulating Crry and Alleviating Complement Over-activation in db/db Mice. Front. Pharmacol. 2021, 12, 729334. [Google Scholar] [CrossRef]
- Chi, P.J.; Lee, C.J.; Hsieh, Y.J.; Lu, C.W.; Hsu, B.G. Dapagliflozin Ameliorates Lipopolysaccharide Related Acute Kidney Injury in Mice with Streptozotocin-induced Diabetes Mellitus. Int. J. Med. Sci. 2022, 19, 729–739. [Google Scholar] [CrossRef]
- Diomedi-Camassei, F.; Ravà, L.; Lerut, E.; Callea, F.; Van Damme, B. Protein gene product 9.5 and ubiquitin are expressed in metabolically active epithelial cells of normal and pathologic human kidney. Nephrol. Dial. Transplant. 2005, 20, 2714–2719. [Google Scholar] [CrossRef][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donoiu, I.; Târtea, G.; Sfredel, V.; Raicea, V.; Țucă, A.M.; Preda, A.N.; Cozma, D.; Vătășescu, R. Dapagliflozin Ameliorates Neural Damage in the Heart and Kidney of Diabetic Mice. Biomedicines 2023, 11, 3324. https://doi.org/10.3390/biomedicines11123324
Donoiu I, Târtea G, Sfredel V, Raicea V, Țucă AM, Preda AN, Cozma D, Vătășescu R. Dapagliflozin Ameliorates Neural Damage in the Heart and Kidney of Diabetic Mice. Biomedicines. 2023; 11(12):3324. https://doi.org/10.3390/biomedicines11123324
Chicago/Turabian StyleDonoiu, Ionuț, Georgică Târtea, Veronica Sfredel, Victor Raicea, Anca Maria Țucă, Alexandra Nicoleta Preda, Dragoş Cozma, and Radu Vătășescu. 2023. "Dapagliflozin Ameliorates Neural Damage in the Heart and Kidney of Diabetic Mice" Biomedicines 11, no. 12: 3324. https://doi.org/10.3390/biomedicines11123324
APA StyleDonoiu, I., Târtea, G., Sfredel, V., Raicea, V., Țucă, A. M., Preda, A. N., Cozma, D., & Vătășescu, R. (2023). Dapagliflozin Ameliorates Neural Damage in the Heart and Kidney of Diabetic Mice. Biomedicines, 11(12), 3324. https://doi.org/10.3390/biomedicines11123324




