Effect of CYP2C19 Pharmacogenetic Testing on Predicting Citalopram and Escitalopram Tolerability and Efficacy: A Retrospective, Longitudinal Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Eligible Patients
2.3. Classification of Side Effects and Main Outcomes
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Distribution and Nature of Drug Events
3.3. Associations between Side Effects and Covariates
3.4. CYP 2C19 Phenotype as Predictor of Latent-Class Membership
4. Discussion
- The unavailability of the patients’ volumes of distribution, which inversely affect the drug concentration;
- No information on comedications with potential interactions with Escitalopram;
- The absence of exact information on the diagnosis and treatment outcomes, including the patients’ side effects, adherence, comorbidities, renal/liver function, Escitalopram continuation, and timing of drug switching.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.-U. Twelve month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012, 21, 169–184. [Google Scholar] [CrossRef]
- Maity, N.; Ghosal, M.K.; Gupta, A.; Sil, A.; Chakraborty, S.; Chatterjee, S. Clinical effectiveness and safety of escitalopram and desvenlafaxine in patients of depression with anxiety: A randomized, open-label controlled trial, Indian. J. Pharmacol. 2014, 46, 433–437. [Google Scholar]
- Ng, C.; Sarris, J.; Singh, A.; Bousman, C.; Byron, K.; Peh, L.H.; Smith, D.J.; Tan, C.H.; Schweitzer, I. Pharmacogenetic polymorphisms and response to escitalopram and venlafaxine over 8 weeks in major depression. Hum. Psychopharmacol. 2013, 28, 516–522. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowitz, B.; McGrath, P.J.; et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatr. 2006, 163, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Middleton, H.; Shaw, I.; Hull, S.; Feder, G. NICE guidelines for the management of depression. BMJ 2005, 330, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Uckun, Z.; Baskak, B.; Ozel-Kizil, E.T.; Ozdemir, H.; Ozguven, H.D.; Suzen, H.S. The impact of CYP2C19 polymorphisms on citalopram metabolism in patients with major depressive disorder. J. Clin. Pharm. Therapeut. 2015, 40, 672–679. [Google Scholar] [CrossRef]
- Spina, E.; de Leon, J. Clinical applications of CYP genotyping in psychiatry. J. Neural Transm. 2015, 122, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Groshan, K.; Blakely, R.D.; Richelson, E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997, 340, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Tan, K.; Tan, H.C.; Huan, P.T.; Li, B.; Phua, Q.H.; Lee, H.K.; Lee, C.H.; Low, A.; Becker, R.C.; et al. CYP2C19 and PON1 polymorphisms regulating clopidogrel bioactivation in Chinese, Malay and Indian subjects. Pharmacogenomics 2012, 13, 533–542. [Google Scholar] [CrossRef]
- Scott, S.A.; Sangkuhl, K.; Shuldiner, A.R.; Hulot, J.S.; Thorn, C.F.; Altman, R.B.; Klein, T.E. PharmGKB summary: Very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet. Genom. 2012, 22, 159–165. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yuan, Z.; Liu, Y.; Zhang, J.; Yan, H.; Shen, L.; Luo, X.; Zhang, Y. Correlation between cytochrome P450 2C19 genetic polymorphism and treatment response to escitalopram in panic disorder. Pharmacogenet. Genom. 2017, 27, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.K.; Bishop, J.R.; Sangkuhl, K.; Müller, D.J.; Ji, Y.; Leckband, S.G.; Leeder, J.S.; Graham, R.L.; Chiulli, D.L.; LLerena, A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015, 98, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.M. SSRIs versus tricyclic antidepressants in depressed patients: A meta-analysis of efficacy and tolerability. Depress. Anxiety 1998, 7 (Suppl. S1), 11–17. [Google Scholar] [CrossRef]
- Rosen, R.C.; Lane, R.G.; Menza, M. Effects of SSRIs on sexual function: A critical review. J. Clin. Psychopharmacol. 1999, 19, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.J.; Begg, E.J.; Winter, A.C.; Sainsbury, R. Incidence and risk factors for hyponatraemia following treatment with fluoxetine or paroxetine in elderly people. Br. J. Clin. Pharmacol. 1999, 47, 211–217. [Google Scholar] [CrossRef]
- Bürkner, P.C. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 November 2022).
- Linzer, D.A.; Lewis, J.B. poLCA: An R Package for Polytomous Variable Latent Class Analysis. J. Stat. Softw. 2011, 42, 1–29. Available online: https://www.jstatsoft.org/v42/i10/ (accessed on 1 November 2022). [CrossRef]
- Jukić, M.M.; Opel, N.; Ström, J.; Carrillo-Roa, T.; Miksys, S.; Novalen, M.; Renblom, A.; Sim, S.C.; Peñas-Lledó, E.M.; Courtet, P.; et al. Elevated CYP2C19 expression is associated with depressive symptoms and hippocampal homeostasis impairment. Mol. Psychiatry 2016, 22, 1155–1163. [Google Scholar] [CrossRef]
- Jukić, M.M.; Haslemo, T.; Molden, E.; Ingelman-Sundberg, M. Impact of CYP2C19 Genotype on Escitalopram Exposure and Therapeutic Failure: A Retrospective Study Based on 2,087 Patients. Am. J. Psychiatry 2018, 175, 463–470. [Google Scholar] [CrossRef]
- Fabbri, C.; Tansey, K.E.; Perlis, R.H.; Hauser, J.; Henigsberg, N.; Maier, W.; Mors, O.; Placentino, A.; Rietschel, M.; Souery, D.; et al. Effect of cytochrome CYP2C19 metabolizing activity on antidepressant response and side effects: Meta-analysis of data from genome-wide association studies. Eur. Neuropsychopharmacol. 2018, 28, 945–954. [Google Scholar] [CrossRef]
- Rudberg, I.; Mohebi, B.; Hermann, M.; Refsum, H.; Molden, E. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin. Pharmacol. Ther. 2008, 83, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.I.; Byrne, E.M.; Mitchell, B.L.; Wray, N.R.; Lind, P.A.; Licinio, J.; Medland, S.E.; Martin, N.G.; Hickie, I.B.; Rentería, M.E. Impact of CYP2C19 metaboliser status on SSRI response: A retrospective study of 9500 participants of the Australian Genetics of Depression Study. Pharmacogenom. J. 2022, 22, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, M.; Fabbri, C.; Kasper, S.; Zohar, J.; Souery, D.; Montgomery, S.; Albani, D.; Forloni, G.; Ferentinos, P.; Rujescu, D.; et al. Metabolizing status of CYP2C19 in response and side effects to medications for depression: Results from a naturalistic study. Eur. Neuropsychopharmacol. 2022, 56, 100–111. [Google Scholar] [CrossRef] [PubMed]
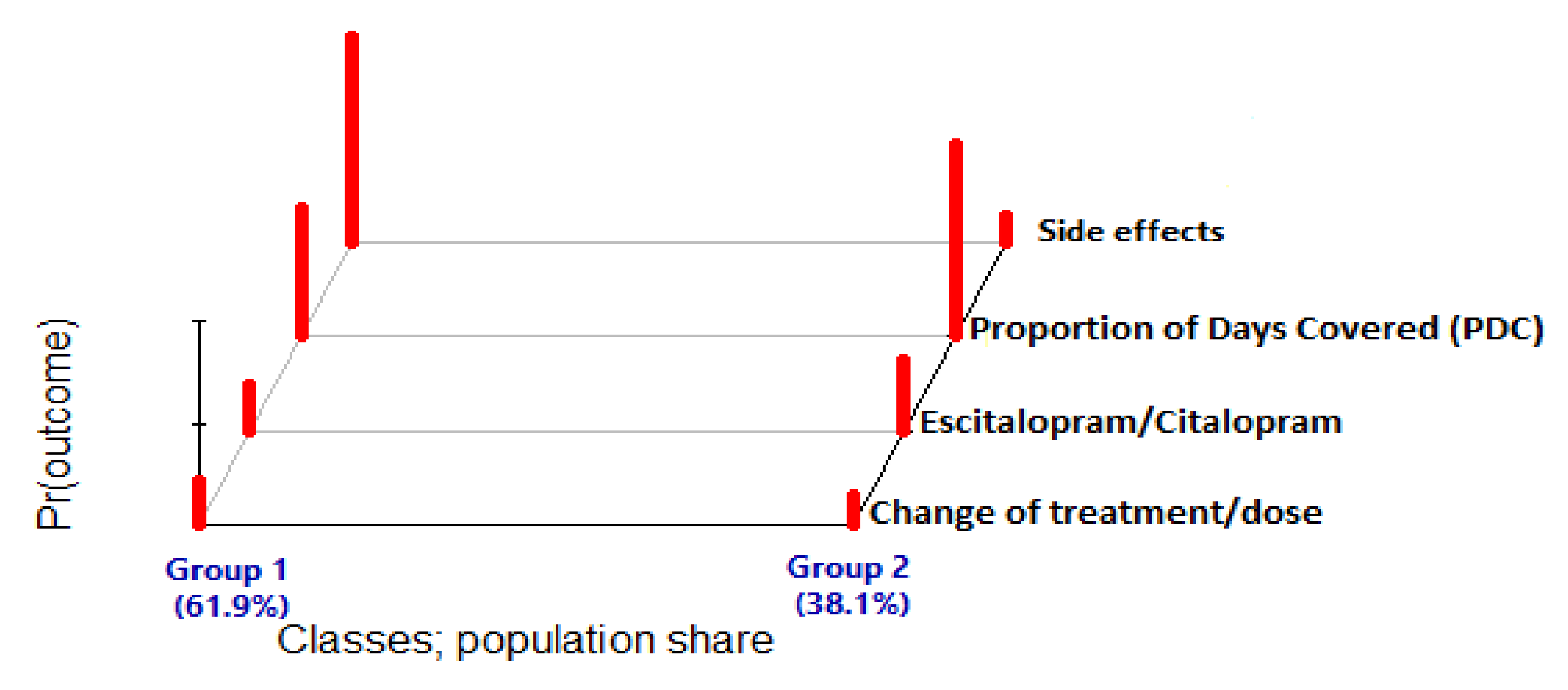
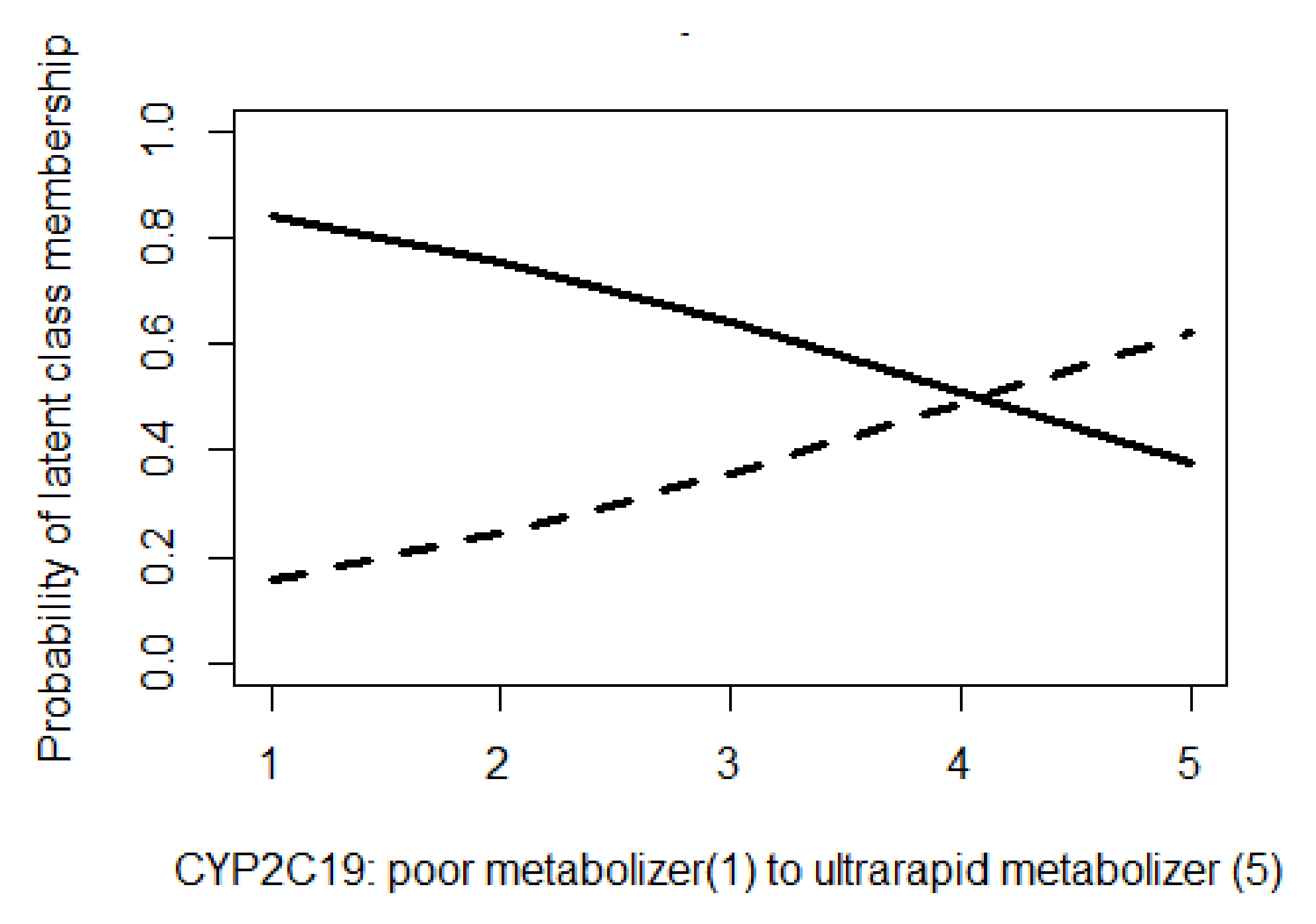
| Poor Metabolizers (n = 5) | Intermediate Metabolizers (n = 63) | Normal Metabolizers (n = 127) | Rapid Metabolizers (n = 76) | Ultrarapid Metabolizers (n = 13) | Total (n = 284) | Reported Result | Patient Information and History |
|---|---|---|---|---|---|---|---|
| 66 (15.9) | 65 (14.1) | 65 (11.8) | 66 (12.4) | 64 (16.3) | 66 (12.7) | Mean (SD) | Age |
| 30 (5.7) | 29.6 (5.5) | 30.1 (5.5) | 30.6 (5.9) | 29.2 (5.05) | 30 (5.62) | Mean (SD) | Body mass index |
| 3 (60) | 19 (30.1) | 38 (30) | 25 (32.9) | 3 (23) | 88 (31) | Males (n (%)) | Gender |
| 2 (40) | 44 (69.9) | 89 (70) | 51 (67.1) | 10 (77) | 196 (69) | Females (n (%)) | |
| 5 (100) | 59 (93.6) | 114 (89.7) | 66 (86.8) | 11 (84.6) | 255 (89.8) | Jewish (n (%)) | Race ¶ |
| 0 (0) | 4 (6.4) | 13 (10.2) | 10 (13.2) | 2 (15.4) | 29 (10.2) | Arabs (n (%)) | |
| 4 (80) | 59 (93.6) | 124 (97.6) | 74 (97.4) | 13 (100) | 274 (96.5) | City (n (%)) | Settlement |
| 1 (20) | 4 (6.4) | 3 (2.4) | 2 (2.6) | - | 10 (3.5) | Village (n (%)) | |
| 4 (2–9) | 6 (3–8) | 6 (0–10) | 5 (3–9) | 5 (4–7) | 6 (0–10) | Median (CI) | Socio Economic Index |
| 2 (40) | 17 (27) | 45 (35.4) | 24 (31.6) | 3 (23) | 91 (32%) | n (%) | Smoking |
| 4 (80) | 45 (71.4) | 94 (74) | 54 (71) | 10 (77) | 207 (72.8) | Escitalopram (n (%)) | Drugs |
| 1 (20) | 18 (28.6) | 33 (26) | 22 (29) | 3 (23) | 77 (27.2) | Citalopram (n (%)) | |
| - | 28 (44.4) | 45 (35.4) | 29 (38.1) | 3 (23) | 105 (37) | n (%) | Diabetes |
| - | 22 (35) | 39 (30.7) | 32 (42.1) | 4 (30.7) | 97 (34.1) | n (%) | Hypertension |
| - | 4 (6.4) | - | 9 (11.8) | 1 (7.7) | 22 (7.7) | n (%) | Congestive heart failure |
| - | 12 (19) | 11 (8.7) | 24 (31.6) | - | 76 (26.8) | n (%) | Hyperlipidemia |
| - | 16 (25.4) | 16 (12.6) | 18 (23.7) | 2 (15.4) | 52 (18.3) | n (%) | Coronary artery disease |
| - | 5 (8) | 9 (7.1) | 9 (11.8) | 2 (15.4) | 25 (8.8) | n (%) | Asthma/COPD Ϯ |
| - | - | 1 (0.8) | 4 (5.3) | 1 (7.7) | 6 (2.1) | n (%) | Epilepsy/seizures |
| 2 (40) | 8 (12.7) | 13 (10.2) | 3 (3.9) | 3 (23) | 29 (10.2) | n (%) | Hypothyroidism |
| - | 1 (1.6) | 10 (7.9) | 6 (7.9) | - | 17 (6) | n (%) | CVA/TIA Ϯ |
| 1 (20) | 7 (11.1) | 10 (7.9) | 5 (6.6) | 4 (30.7) | 27 (9.5) | n (%) | Osteoporosis |
| - | 10 (15.9) | 8 (6.3) | 12 (15.8) | 2 (15.4) | 32 (11.3) | n (%) | GERD Ϯ |
| 1 (20) | 5 (8) | 8 (6.3) | - | 1 (7.7) | 25 (8.8) | n (%) | Chronic kidney disease |
| 2 (40) | 36 (57.1) | 68 (53.5) | 48 (63.2) | 8 (61.5) | 156 | >80 mL/min | Creatinine clearance—mL/min ⱡ |
| 2 (40) | 23 (36.5) | 50 (39.4) | 19 (25) | 4 (30.7) | 98 | 50–80 mL/min | |
| 1 (20) | 4 (6.4) | 7 (5.5) | 8 (10.5) | 1 (7.7) | 27 | 30–49 mL/min | |
| - | - | 2 (1.6) | 1 (1.3) | - | 3 | <30 mL/min | |
| 2 (40) | 3 (4.8) | 13 (10.2) | 8 (10.5) | 4 (30.7) | 30 (10.6) | n (%) | Atrial fibrillation |
| - | 10 (15.9) | 15 (11.8) | 10 (13.2) | 3 (23) | 38 (13.4) | n (%) | Anxiety |
| - | - | 7 (5.5) | - | - | 7 (2.5) | n (%) | Schizophrenia |
| 22.2 (± 2.5) | 22.6 (± 0.9) | 22.5 (± 0.53) | 22.2 (± 0.76) | 20.1 (± 1.2) | 22.3 (± 0.37) | Mean (± SE) | AST Ϯ |
| 23 (± 2.5) | 19 (± 1.0) | 20 (± 0.8) | 19 (± 1.04) | 15 (± 1.3) | 19 (± 0.52) | Mean (± SE) | ALT Ϯ |
| 21 (± 2.1) | 28 (± 2.7) | 34.4 (± 2.4) | 36.1 (± 5.2) | 23.7 (± 3.1) | 32.7 (± 1.9) | Mean (± SE) | GGT Ϯ |
| 1.17 (± 0.3) | 0.8 (± 0.04) | 0.89 (± 0.05) | 0.92 (± 0.06) | 0.81 (± 0.07) | 0.89 (± 0.03) | Mean (± SE) | Creatinine |
| Phenotype | Genotype | Patients (n (%)) |
|---|---|---|
| Extensive/normal metabolizers | CYP2C19*1/*1 | 127 (44.7) |
| Intermediate metabolizers | CYP2C19*1/*2 | 46 (16.2) |
| CYP2C19*2/*17 | 17 (5.9) | |
| Poor metabolizers | CYP2C19*2/*2 | 5 (1.8) |
| Rapid metabolizers | CYP2C19*1/*17 | 76 (26.7) |
| Ultrarapid metabolizers | CYP2C19*17/*17 | 13 (4.6) |
| System Adverse Reaction | Total Cohort (n = 284) (n (%)) | Escitalopram (n = 207) (n (%)) | Citalopram (n = 77) (n (%)) | Median Time to Event (Weeks) | |
|---|---|---|---|---|---|
| Gastrointestinal (n = 261) | Abdominal pain | 127 (48.7) | 94 (36.0) | 33 (12.7) | 4 (2–7) |
| Constipation | 60 (23.0) | 56 (21.4) | 4 (1.5) | ||
| Diarrhea | 44 (16.8) | 34 (13.0) | 10 (3.8) | ||
| Nausea and vomiting | 30 (11.5) | 26 (10.0) | 4 (1.5) | ||
| Nervous system (n = 455) | Dizziness | 53 (11.6) | 37 (8.1) | 16 (3.5) | 13 (9–18) |
| Headache | 65 (14.3) | 55 (12.1) | 10 (2.2) | ||
| Insomnia | 96 (21.1) | 77 (16.9) | 19 (4.2) | ||
| Anxiety | 105 (23.1) | 75 (16.5) | 30 (6.6) | ||
| Agitation | 17 (3.7) | 16 (3.5) | 1 (0.2) | ||
| Vertigo | 22 (4.8) | 15 (3.3) | 7 (1.5) | ||
| Fatigue | 37 (8.1) | 21 (4.6) | 16 (3.5) | ||
| Drowsiness | 43 (9.4) | 28 (6.1) | 15 (3.3) | ||
| Depressive episode | 17 (3.7) | 9 (2.0) | 8 (1.7) | ||
| Endocrine and metabolic (n = 89) | Weight loss | 17 (19.1) | 12 (13.5) | 5 (5.6) | 27 (17–39) |
| Loss of appetite | 2 (2.2) | 1 (1.1) | 1 (1.1) | ||
| Sexual dysfunction | 8 (9.0) | 5 (5.6) | 3 (3.4) | ||
| Weight gain | 47 (52.8) | 32 (36.0) | 15 (16.8) | ||
| Hypoglycemia | 6 (6.7) | 4 (4.5) | 2 (2.2) | ||
| Hyponatremia and SIADH | 9 (10.1) | 6 (6.7) | 3 (3.4) | ||
| Genitourinary (n = 45) | Urinary frequency | 28 (62.2) | 25 (55.5) | 3 (6.7) | 21 (14–29) |
| Urinary retention | 17 (37.7) | 2 (4.4) | 15 (33.3) | ||
| Neuromuscular and skeletal (n = 58) | Parkinsonism | 10 (17.2) | 9 (15.5) | 1 (1.7) | 38 (29–49) |
| Tardive dyskinesia | 2 (3.4) | 1 (1.7) | 1 (1.7) | ||
| Muscle weakness | 2 (3.4) | 1 (1.7) | 1 (1.7) | ||
| Tremor | 18 (31.0) | 13 (22.4) | 5 (8.6) | ||
| Myalgia and myositis | 26 (44.8) | 16 (27.6) | 10 (17.2) | ||
| Cardiovascular system (n = 58) | Orthostatic hypotension | 8 (13.8) | 5 (8.6) | 3 (5.2) | 28 (21–36) |
| Palpitation and tachycardia | 27 (46.5) | 16 (27.6) | 11 (18.9) | ||
| Syncope | 18 (31.0) | 12 (20.7) | 6 (10.3) | ||
| Prolonged QT | 5 (8.6) | 3 (5.2) | 2 (3.4) | ||
| Ophthalmic (n = 47) | Visual disturbance | 30 (63.8) | 19 (9.2) | 11 (14.3) | 47 (36–59) |
| Blurred vision | 17 (36.1) | 10 (17.2) | 7 (14.9) | ||
| Dermatologic (n = 19) | Skin rash | 19 (100) | 13 (68.4) | 6 (31.6) | 52 (38–64) |
| Escitalopram/Citalopram | Poor Metabolizers (n = 5) | Intermediate Metabolizers (n = 63) | Normal Metabolizers (n = 127) | Rapid Metabolizers (n = 76) | Ultrarapid Metabolizers (n = 13) |
|---|---|---|---|---|---|
| Gastrointestinal | 8 | 90 | 107 | 45 | 11 |
| Nervous system | 26 | 114 | 232 | 75 | 8 |
| Endocrine and metabolic | 6 | 39 | 25 | 14 | 5 |
| Genitourinary | 4 | 21 | 12 | 8 | 0 |
| Neuromuscular and skeletal | 1 | 15 | 29 | 12 | 1 |
| Cardiovascular system | 3 | 13 | 35 | 6 | 1 |
| Ophthalmic | 1 | 19 | 22 | 5 | 0 |
| Dermatologic | 4 | 5 | 8 | 2 | 0 |
| Average SE per patient | 10.6 | 5 | 3.7 | 2.2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahajna, M.; Abu Fanne, R.; Berkovitch, M.; Tannous, E.; Vinker, S.; Green, I.; Matok, I. Effect of CYP2C19 Pharmacogenetic Testing on Predicting Citalopram and Escitalopram Tolerability and Efficacy: A Retrospective, Longitudinal Cohort Study. Biomedicines 2023, 11, 3245. https://doi.org/10.3390/biomedicines11123245
Mahajna M, Abu Fanne R, Berkovitch M, Tannous E, Vinker S, Green I, Matok I. Effect of CYP2C19 Pharmacogenetic Testing on Predicting Citalopram and Escitalopram Tolerability and Efficacy: A Retrospective, Longitudinal Cohort Study. Biomedicines. 2023; 11(12):3245. https://doi.org/10.3390/biomedicines11123245
Chicago/Turabian StyleMahajna, Mahmood, Rami Abu Fanne, Matitiahu Berkovitch, Elias Tannous, Shlomo Vinker, Ilan Green, and Ilan Matok. 2023. "Effect of CYP2C19 Pharmacogenetic Testing on Predicting Citalopram and Escitalopram Tolerability and Efficacy: A Retrospective, Longitudinal Cohort Study" Biomedicines 11, no. 12: 3245. https://doi.org/10.3390/biomedicines11123245
APA StyleMahajna, M., Abu Fanne, R., Berkovitch, M., Tannous, E., Vinker, S., Green, I., & Matok, I. (2023). Effect of CYP2C19 Pharmacogenetic Testing on Predicting Citalopram and Escitalopram Tolerability and Efficacy: A Retrospective, Longitudinal Cohort Study. Biomedicines, 11(12), 3245. https://doi.org/10.3390/biomedicines11123245





