Correlations between MSH2 and MSH6 Concentrations in Different Biological Fluids and Clinicopathological Features in Colorectal Adenocarcinoma Patients and Their Contribution to Fast and Early Diagnosis of Colorectal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Description
2.2. Materials and Reagents
2.3. Instrumentation
2.4. Stochastic Method
3. Results
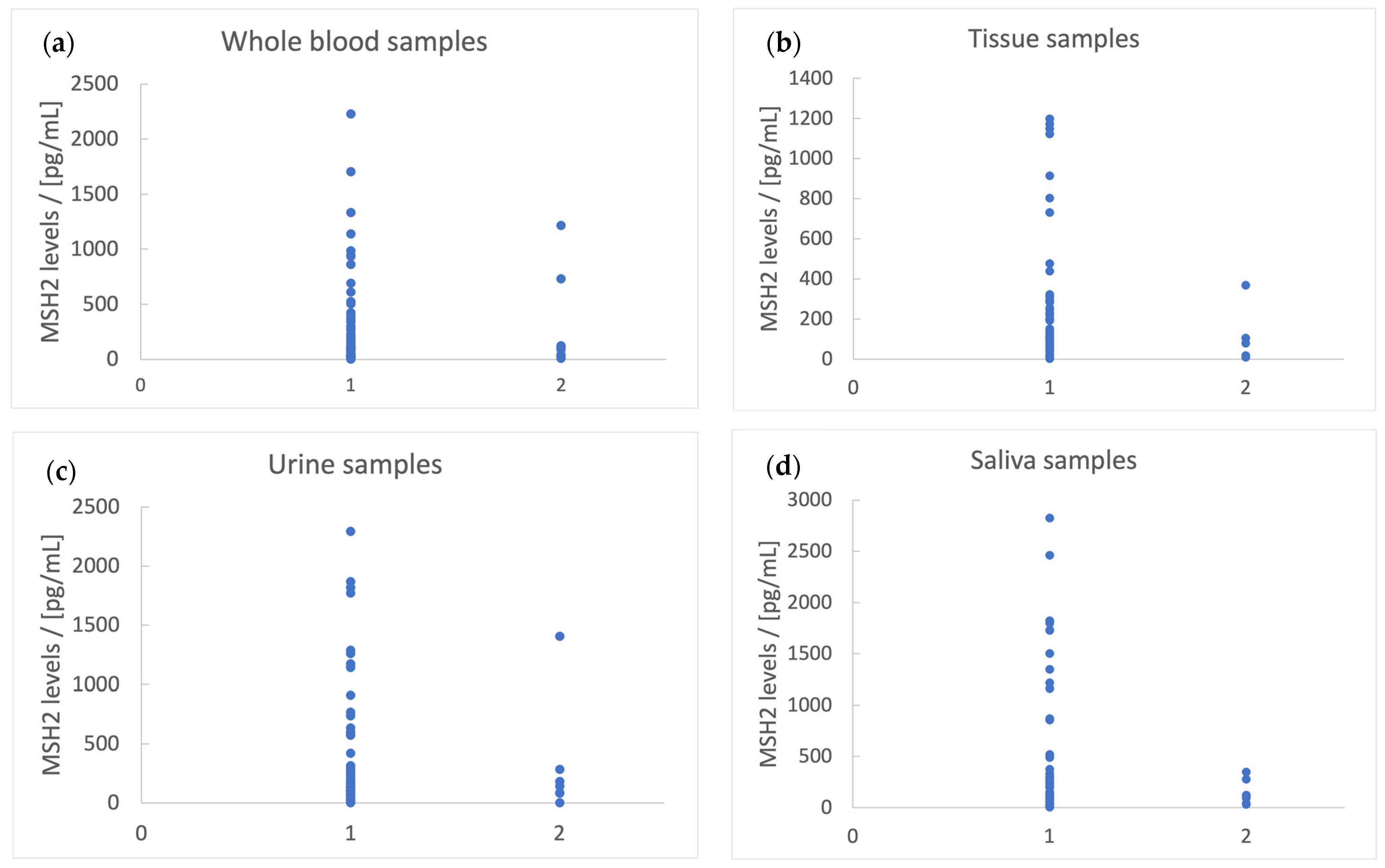
- r1 = [X]whole blood/[X]urine.
- r2 = [X]whole blood/[X]saliva.
- S = [X]saliva + 2 × [X]urine.
- C1-located tumors are associated with r1 > 1.5, S > 500 pg/mL, r2 < 0.75, and [MSH2]whole blood > 50 pg/mL;
- C2 + C3-located tumors are associated with r1 < 1.5, S > 500 pg/mL, r2 > 0.75, and [MSH2]whole blood > 50 pg/mL;
- C4-located tumors are associated with r1 < 1.5, S < 500 pg/mL, r2 < 0.75, and [MSH2]whole blood > 50 pg/mL;
- C5-located tumors are associated with r1 < 1.5, S < 500 pg/mL, r2 < 0.75, and [MSH2]whole blood < 50 pg/mL;
- C6-located tumors are associated with r1 < 1.5, S > 500 pg/mL, r2 < 0.75, and [MSH2]whole blood > 50 pg/mL;
- Vegetant gross aspect is associated with [MSH2]whole blood < 150 pg/mL;
- Mucus presence is associated with [MSH2]whole blood < 125 pg/mL and [MSH2]saliva < 140 pg/mL;
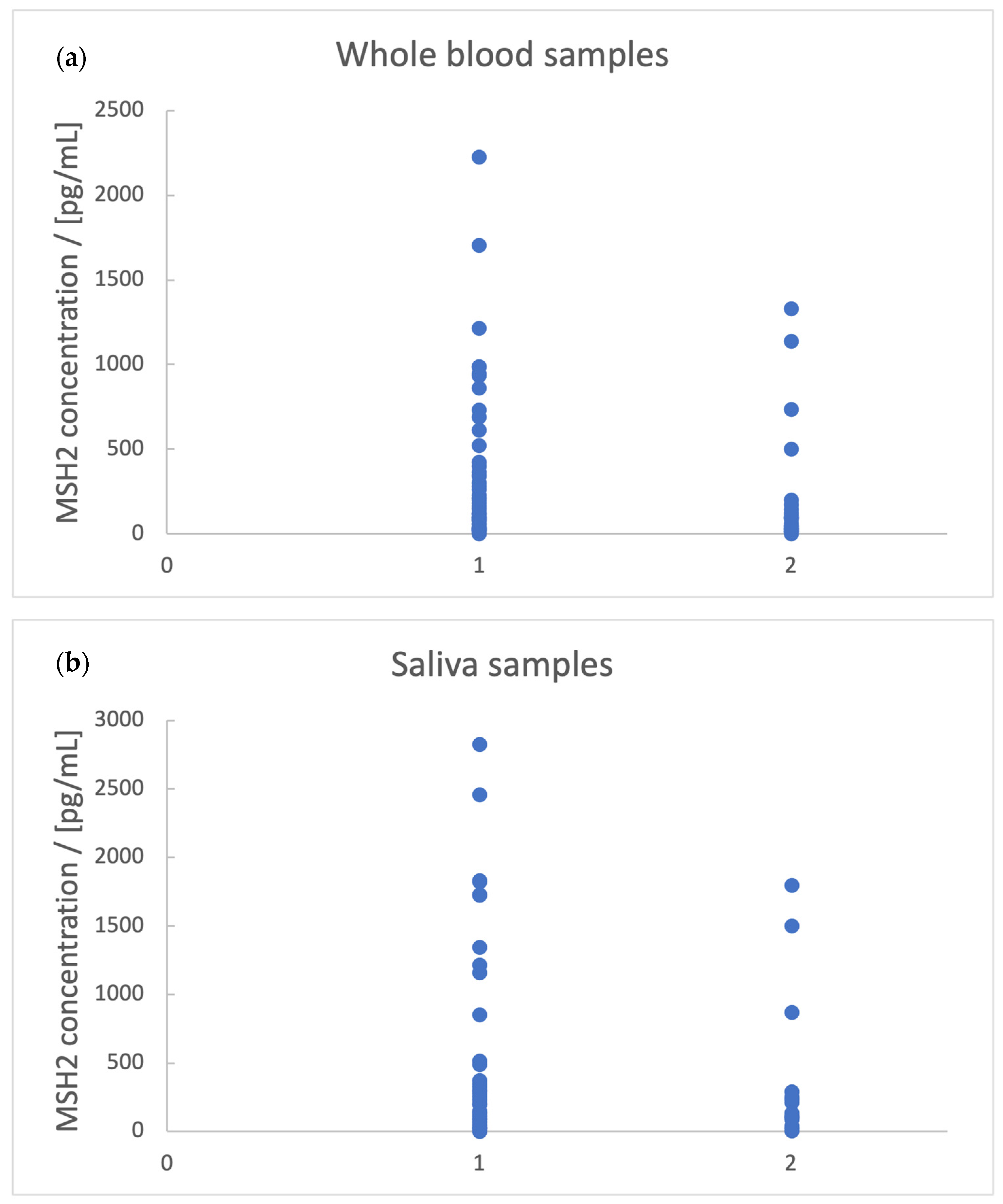
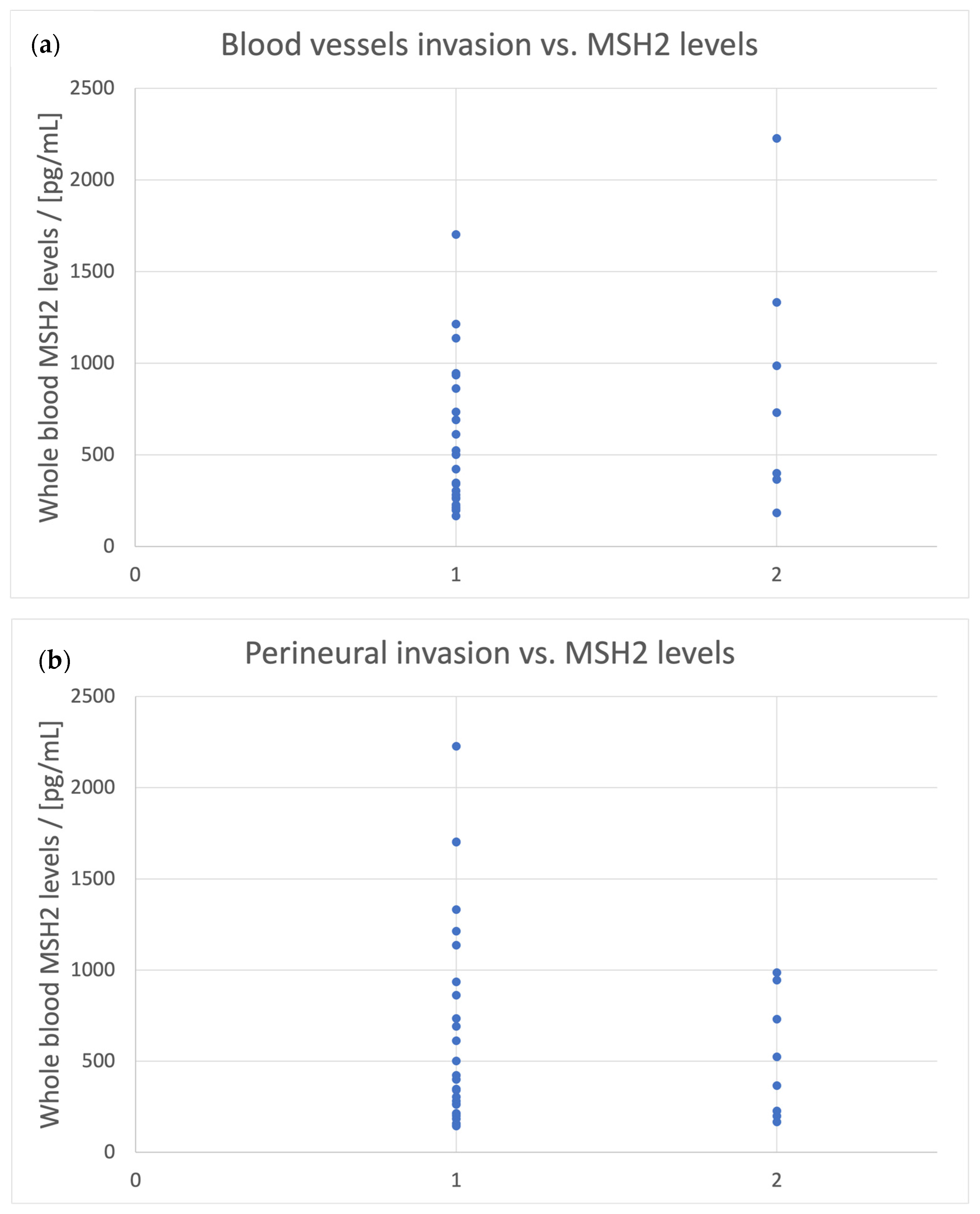
- Blood vessel invasion is associated with [MSH2]whole blood < 160 pg/mL;
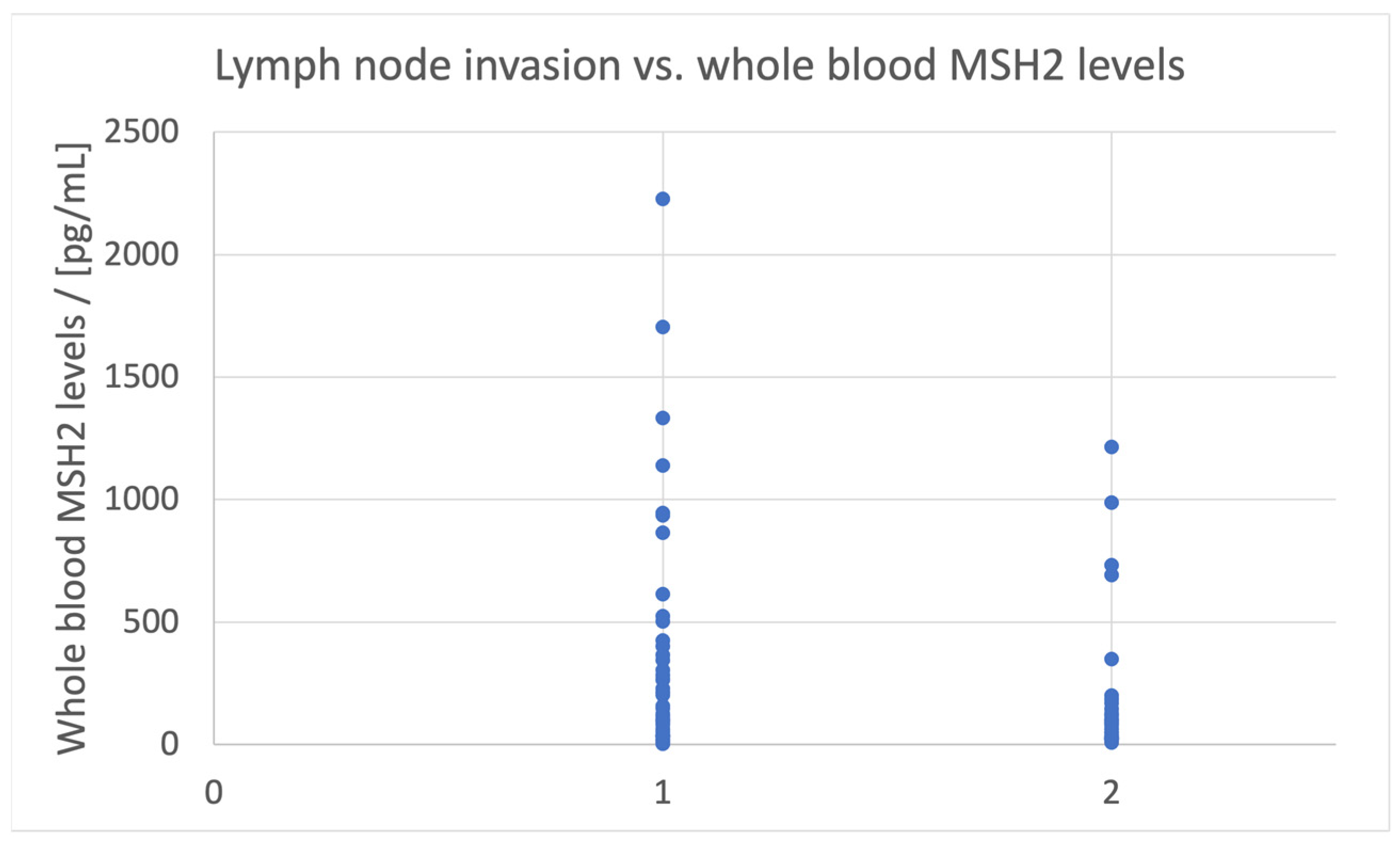
- Lymph vessel invasion is associated with [MSH2]whole blood < 125 pg/mL;
- Tumor deposit presence is associated with [MSH2]saliva < 125 pg/mL, [MSH2]whole blood < 125 pg/mL, and [MSH2]urine < 150 pg/mL.
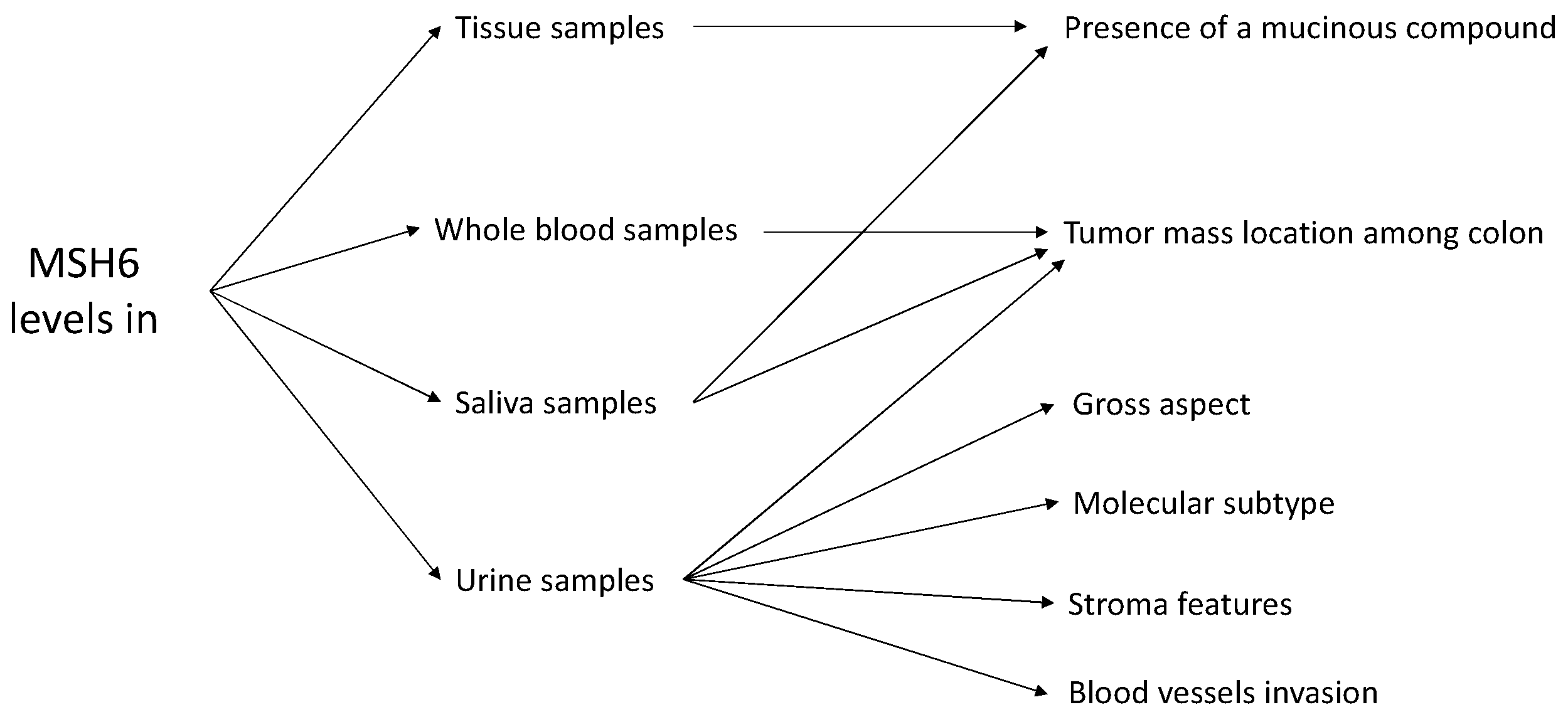
- C2 + C3-located tumors are associated with [MSH6]urine > 250 pg/mL, S > 600 pg/mL, r2 = 0.3–1.2, and [MSH6]whole blood = 130–300 pg/mL;
- C5-located tumors are associated with r1 < 0.5, S > 1500 pg/mL, r2 < 0.83, and [MSH6]whole blood > 130 pg/mL;
- C1 + C4 + C6 cannot be differentiated with high probability using only MSH6 levels;
- Vegetant and ulcero-vegetant gross aspect is associated with [MSH6]urine < 220 pg/mL;
- Mucus presence is associated with [MSH6]tissue > 250 pg/mL and [MSH6]saliva > 250 pg/mL;
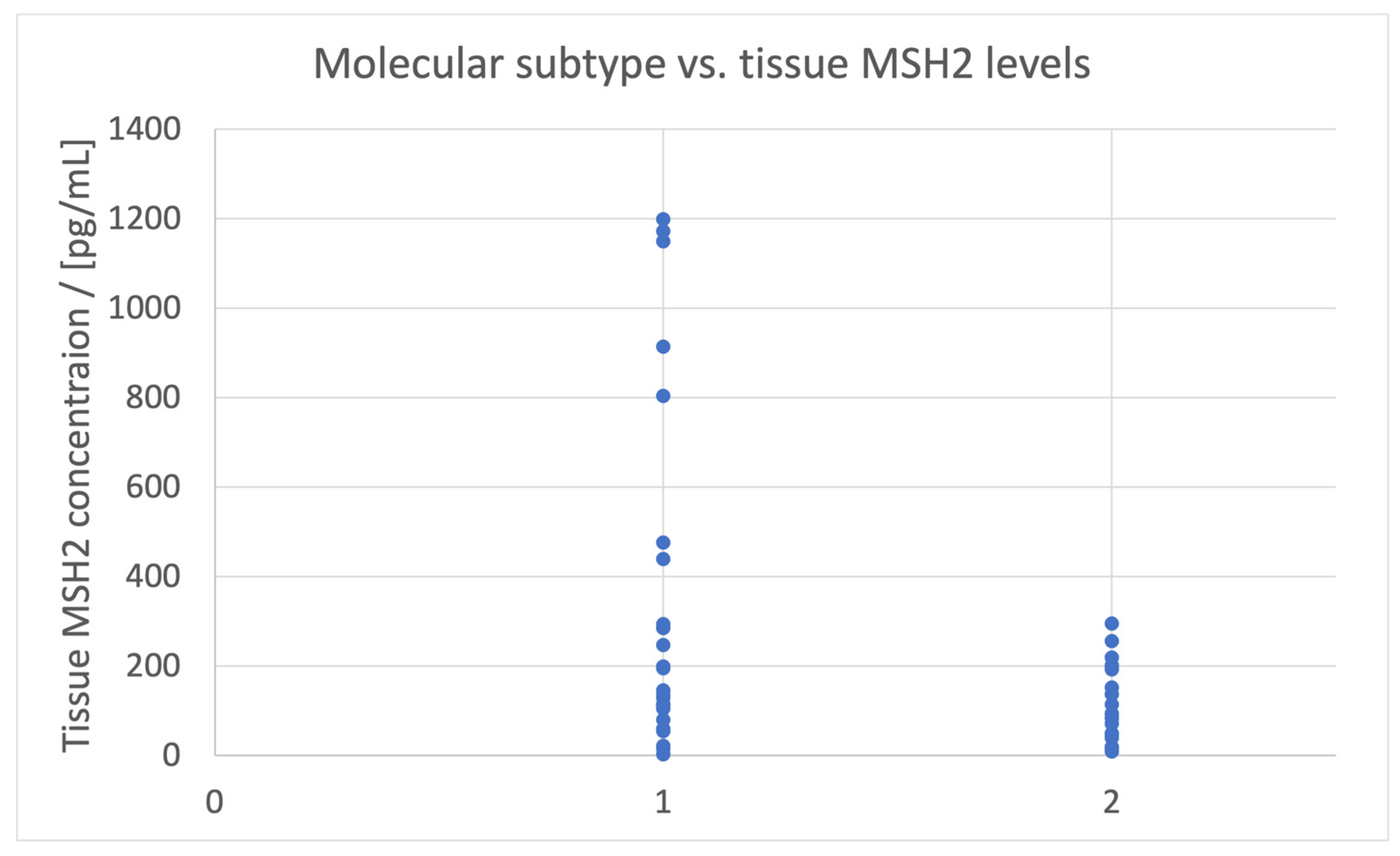
- The presence of an epithelial subtype compound is related to [MSH6]urine < 200 pg/mL;
- Stroma features are related to urine MSH6 levels as [MSH6]urine > 300 pg/mL involves a hyalinized stroma and [MSH6]urine < 250 pg/mL involves mainly inflammatory stroma;
- Blood vessel invasion is associated with [MSH6]urine > 450 pg/mL.
4. Discussion
4.1. MSH2 and Its Clinicopathological Features
4.2. MSH6 and Its Clinicopathological Features
4.3. The Practical Use of the Developed Criteria Using Coding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedberg, E.C. DNA Repair; W.H. Freeman: New York, NY, USA, 1985; Volume 38, pp. 305–319. [Google Scholar]
- Friedberg, E.C. The enzymology of DNA repair. Mutat. Res. 1990, 236, 145–314. [Google Scholar] [CrossRef]
- Modrich, P. Methyl-directed DNA mismatch correction. J. Biol. Chem. 1969, 264, 6597–6600. [Google Scholar] [CrossRef]
- Modrich, P. Mechanisms and biological effects of mismatch repair. Ann. Rev. Genet. 1991, 25, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Su, S.S.; Modrich, P. Escherichia coli mutS-encoded protein binds to mismatched DNA base pairs. Proc. Nat. Acad. Sci. USA 1986, 83, 5057–5061. [Google Scholar] [CrossRef] [PubMed]
- Grilley, M.; Welsh, K.M.; Su, S.; Modrich, P. Isolation and characterization of the Escherichia coli mutL gene product. J. Biol. Chem. 1989, 264, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.; Clark, S.; Modrich, P. Strand-specific mis-match correction in nuclear extracts of human and Drosophila melanogaster cell lines. Proc. Nat. Acad. Sci. USA 1990, 87, 5831–5837. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.C.; Roberts, J.D.; Kunkel, T.A. Heteroduplex repair in extracts of human Hela cells. J. Biol. Chem. 1991, 266, 3744–3751. [Google Scholar] [CrossRef]
- Reenan, R.; Kolodner, R.D. Isolation and characterization of two Saccharomyces cerevisiae genes encoding homologues of the bacterial HexA and MutS mismatch repair proteins. Genetics 1992, 732, 963–973. [Google Scholar] [CrossRef]
- Fishel, R.; Lescoe, M.K.; Rao, M.; Copeland, N.G.; Jenkins, N.A.; Garber, J.; Kane, M.; Kolodner, R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 1993, 75, 1027–1038. [Google Scholar] [CrossRef]
- Fishel, R.; Ewel, A.; Lee, S.; Lescoe, M.K.; Griffith, J. Binding of mismatched microsatellite DNA sequences by the human MSH2 protein. Science 1994, 266, 1403–1405. [Google Scholar] [CrossRef]
- Obmolova, G.; Ban, C.; Hsieh, P.; Yang, W. Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature 2000, 407, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.H.; Perrakis, A.; Enzlin, J.H.; Winterwerp, H.H.; de Wind, N.; Sixma, T.K. The crystal structure of DNA mismatch repair protein MutS binding to a GxT mismatch. Nature 2000, 407, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Sakato, M.; De Rocco, V.; Yamane, K.; Du, C.; Erie, D.A.; Hingorani, M.; Hsieh, P. Biochemical analysis of the human mismatch repair proteins hMutSα MSH2(G674A)-MSH6 and MSH2-MSH6(T1219D). J. Biol. Chem. 2012, 287, 9777–9791. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, R.; Wang, X.W.; Linke, S.P.; Sengupta, S.; Hickson, I.D.; Pedrazzi, G.; Perrera, C.; Stagljar, I.; Littman, S.J.; et al. The mismatch DNA repair heterodimer, hMSH2/6, regulates BLM helicase. Oncogene 2004, 23, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Ma, Z.; McIlhatton, M.A.; Fishel, R.; Dong, Z. hMSH2 Recruits ATR to DNA Damage Sites for Activation during DNA Damage-induced Apoptosis. J. Biol. Chem. 2011, 286, 10411–10418. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Kinsella, T.J. A novel role for DNA mismatch repair and the autophagic processing of chemotherapy drugs in human tumor cells. Autophagy 2007, 3, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Bermudez, A.; Royle, N.J. Deficiency in DNA mismatch repair increases the rate of telomere shortening in normal human cells. Human Mutat. 2011, 32, 939–946. [Google Scholar] [CrossRef]
- Lastella, P.; Surdo, N.C.; Resta, N.; Guanti, G.; Stella, A. In silico and in vivo splicing analysis of MLH1 and MSH2 missense mutations shows exon and tissue-specific effects. BMC Genom. 2006, 7, 243. [Google Scholar] [CrossRef]
- Nikitin, A.G.; Chudakova, D.A.; Enikeev, R.F.; Sakaeva, D.; Druzhkov, M.; Shigapova, L.H.; Brovkina, O.I.; Shagimardanova, E.I.; Gusev, O.A.; Gordiev, M.G. Lynch syndrome germline mutations in breast cancer: Next generation sequencing case-control study of 1263 participants. Front. Oncol. 2020, 10, 666. [Google Scholar] [CrossRef]
- Staden, R.-I.S.-V.; Bratei, A.A.; Ilie-Mihai, R.-M.; Gheorghe, D.-C.; Tuchiu, B.M.; Gurzu, S. Miniplatforms for screening of biological samples for KRAS and four mismatch repair proteins as new tools for fast screening for gastric and colon cancers. J. Electrochem. Soc. 2023, 170, 057510. [Google Scholar] [CrossRef]
- Caja, F.; Vodickova, L.; Kral, J.; Vymetalkova, V.; Naccarati, A.; Vodicka, P. DNA Mismatch Repair Gene Variants in Sporadic Solid Cancers. Int. J. Mol. Sci. 2020, 21, 5561. [Google Scholar] [CrossRef]
- Hargreaves, V.V.; Shell, S.S.; Mazur, D.J.; Hess, M.T.; Kolodner, R.D. Interaction between the Msh2 and Msh6 nucleotide-binding sites in the Saccharomyces cerevisiae Msh2-Msh6 complex. J. Biol. Chem. 2010, 285, 9301–9310. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Lee, J.H.; Kang, Y.; Lee, S.H.; Hyun, J.W.; Chang, I.Y.; Jun, J.Y.; You, H.J. Mismatch-repair protein MSH6 is associated with Ku70 and regulates DNA double-strand break repair. Nucleic Acids Res. 2011, 39, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.B.; Deterding, L.; Tomer, K.B.; Kunkel, T.A. Multiple functions for the N-terminal region of Msh6. Nucleic Acids Res. 2007, 35, 4114–4123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gradia, S.; Acharya, S.; Fishel, R. The role of mismatched nucleotides in activating the hMSH2-hMSH6 molecular switch. J. Biol. Chem. 2000, 275, 3922–3930. [Google Scholar] [CrossRef] [PubMed]
- Thirumal, K.D.; Susmita, B.; Judith, E.; Priyadharshini, C.J.; George, C.; Zayed, H. Elucidating the role of interacting residues of the MSH2-MSH6 complex in DNA repair mechanism: A computational approach. Adv. Protein Chem. Struct. Biol. 2019, 115, 325–350. [Google Scholar]
- Warren, J.J.; Pohlhaus, T.J.; Changela, A.; Iyer, R.R.; Modrich, P.L.; Beese, L.S. Structure of the human MutSalpha DNA lesion recognition complex. Mol. Cell 2007, 26, 579–592. [Google Scholar] [CrossRef]
- Edelbrock, M.A.; Kaliyaperumal, S.; Williams, K.J. Structural, molecular and cellular functions of MSH2 and MSH6 during DNA mismatch repair, damage signaling and other noncanonical activities. Mutat. Res. 2013, 743–744, 53–66. [Google Scholar] [CrossRef]
- Cederquist, K.; Emanuelsson, M.; Göransson, I.; Holinski-Feder, E.; Müller-Koch, Y.; Golovleva, I.; Grönberg, H. Mutation analysis of the MLH1, MSH2 and MSH6 genes in patients with double primary cancers of the colorectum and the endometrium: A population- based study in northern Sweden. Int. J. Cancer 2004, 109, 370–376. [Google Scholar] [CrossRef]
- Roberts, M.E.; Jackson, S.A.; Susswein, L.R.; Zeinomar, N.; Ma, X.; Marshall, M.L.; Stettner, A.R.; Milewski, B.; Xu, Z.; Solomon, B.D.; et al. MSH6 and PMS2 germ-line pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet. Med. 2018, 20, 1167–1174. [Google Scholar] [CrossRef]
- Wilczak, W.; Rashed, S.; Hube-Magg, C.; Kluth, M.; Simon, R.; Büscheck, F.; Clauditz, T.S.; Grupp, K.; Minner, S.; Tsourlakis, M.C.; et al. Up-regulation of mismatch repair genes MSH6, PMS2 and MLH1 parallels development of genetic instability and is linked to tumor aggressiveness and early PSA recurrence in prostate cancer. Carcinogenesis 2017, 38, 19–27. [Google Scholar] [CrossRef]
- Uraki, S.; Ariyasu, H.; Doi, A.; Takeshima, K.; Morita, S.; Inaba, H.; Furuta, H.; Fukuhara, N.; Inoshita, N.; Nishioka, H.; et al. MSH6/2 and PD-L1 Expressions Are Associated with Tumor Growth and Invasiveness in Silent Pituitary Adenoma Subtypes. Int. J. Mol. Sci. 2020, 21, 2831. [Google Scholar] [CrossRef]
- Lynch, H.T.; de la Chapelle, A. Hereditary colorectal cancer. N. Engl. J. Med. 2003, 348, 919–932. [Google Scholar] [CrossRef]
- Grover, S.; Syngal, S. Genetic testing in gastroenterology: Lynch syndrome. Best. Pract. Res. Clin. Gastroenterol. 2009, 23, 185–196. [Google Scholar] [CrossRef]
- Hampel, H.; Frankel, W.L.; Martin, E.; Arnold, M.; Khanduja, K.; Kuebler, P.; Clendenning, M.; Sotamaa, K.; Prior, T.; Westman, J.A.; et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J. Clin. Oncol. 2008, 26, 5783–5788. [Google Scholar] [CrossRef]
- Aaltonen, L.A.; Salovaara, R.; Kristo, P.; Canzian, F.; Hemminki, A.; Peltomäki, P.; Chadwick, R.B.; Kääriäinen, H.; Eskelinen, M.; Järvinen, H.; et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N. Engl. J. Med. 1998, 338, 1481–1487. [Google Scholar] [CrossRef]
- Wijnen, J.; de Leeuw, W.; Vasen, H.; van der Klift, H.; Møller, P.; Stormorken, A.; Meijers-Heijboer, H.; Lindhout, D.; Menko, F.; Vossen, S.; et al. Familial endometrial cancer in female carriers of MSH6 germline mutations. Nat. Genet. 1999, 23, 142–144. [Google Scholar] [CrossRef]
- Vasen, H.F.; Mecklin, J.P.; Khan, P.M.; Lynch, H.T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis. Colon. Rectum 1991, 34, 424–425. [Google Scholar] [CrossRef]
- Keutgen, X.M.; Filicori, F.; Lee, S.W.; Zarnegar, R. En bloc right hemicolectomy and pancreaticoduodenectomy for HNPCC with simultaneous mutations of MSH-2 and MSH-6. Int. J. Color. Dis. 2011, 26, 527–529. [Google Scholar] [CrossRef]
- Jang, E.; Chung, D.C. Hereditary colon cancer: Lynch syndrome. Gut Liver 2010, 4, 151–160. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087. [Google Scholar] [CrossRef]
- Wang, S.M.; Jiang, B.; Deng, Y.; Huang, S.L.; Fang, M.Z.; Wang, Y. Clinical significance of MLH1/MSH2 for stage II/III sporadic colorectal cancer. World J. Gastrointest. Oncol. 2019, 11, 1065–1080. [Google Scholar] [CrossRef]
- Arshita, N.; Lestari, R.V.; Hutajulu, S.H.; Ghozali, A.; Paramita, D.K. The Tendency of Having MSH2 and MSH6 Microsatellite Instability among Clinicopathological Features in Patients with Colorectal Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 3147–3152. [Google Scholar] [CrossRef]
- Dong, L.; Zou, S.; Jin, X.; Lu, H.; Zhang, Y.; Guo, L.; Cai, J.; Ying, J. Cytoplasmic MSH2 Related to Genomic Deletions in the MSH2/EPCAM Genes in Colorectal Cancer Patients with Suspected Lynch Syndrome. Front. Oncol. 2021, 11, 627460. [Google Scholar] [CrossRef]

| Feature | Sample/Parameter | Level (pg/mL)/Value of Parameter |
|---|---|---|
| Location | r1 | 1.5 |
| S | 500 | |
| r2 | 0.75 | |
| Whole blood | 50 | |
| Vegetant grossing aspect | Whole blood | 150 |
| Presence of mucus | Whole blood | 125 |
| Saliva | 140 | |
| Blood vessel invasion | Whole blood | 160 |
| Lymph vessel invasion | Whole blood | 125 |
| Tumor deposit presence | Saliva | 125 |
| Whole blood | 125 | |
| Urine | 150 |
| Feature | Sample/Parameter | Level (pg/mL)/Value of Parameter |
|---|---|---|
| Location—C1 + C4 + C6 | r1 | 0.5 |
| S | 1500 | |
| r2 | 0.83 | |
| Whole blood | 130 | |
| Location—C2 + C3 | Urine | 250 |
| S | 600 | |
| r2 | 0.3–1.2 | |
| Whole blood | 130–300 | |
| Location—C5 | r1 | 0.5 |
| S | 1500 | |
| r2 | 0.83 | |
| Whole blood | 130 | |
| Vegetant and ulcero-vegetant grossing aspect | Urine | 220 |
| Presence of mucus | Tissue | 250 |
| Saliva | 250 | |
| Molecular subtype | Urine | 200 |
| Stroma features | Urine | 250 |
| Urine | 300 | |
| Blood vessel invasion | Urine | 450 |
| Criteria | [MSH2]whole blood < 50 pg/mL | r1 > 1.5 | r2 > 0.75 | S > 500 pg/mL |
|---|---|---|---|---|
| High probability | C5 | C1 | C2, C3, C6 | C1, C2, C3 |
| Medium probability | C2, C3, C4, C6 | - | C1, C4 | C4, C6 |
| Low probability | C1 | C2, C3, C4, C5, C6 | C5 | C5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratei, A.A.; Stefan-van Staden, R.-I. Correlations between MSH2 and MSH6 Concentrations in Different Biological Fluids and Clinicopathological Features in Colorectal Adenocarcinoma Patients and Their Contribution to Fast and Early Diagnosis of Colorectal Adenocarcinoma. Biomedicines 2023, 11, 3213. https://doi.org/10.3390/biomedicines11123213
Bratei AA, Stefan-van Staden R-I. Correlations between MSH2 and MSH6 Concentrations in Different Biological Fluids and Clinicopathological Features in Colorectal Adenocarcinoma Patients and Their Contribution to Fast and Early Diagnosis of Colorectal Adenocarcinoma. Biomedicines. 2023; 11(12):3213. https://doi.org/10.3390/biomedicines11123213
Chicago/Turabian StyleBratei, Alexandru Adrian, and Raluca-Ioana Stefan-van Staden. 2023. "Correlations between MSH2 and MSH6 Concentrations in Different Biological Fluids and Clinicopathological Features in Colorectal Adenocarcinoma Patients and Their Contribution to Fast and Early Diagnosis of Colorectal Adenocarcinoma" Biomedicines 11, no. 12: 3213. https://doi.org/10.3390/biomedicines11123213
APA StyleBratei, A. A., & Stefan-van Staden, R.-I. (2023). Correlations between MSH2 and MSH6 Concentrations in Different Biological Fluids and Clinicopathological Features in Colorectal Adenocarcinoma Patients and Their Contribution to Fast and Early Diagnosis of Colorectal Adenocarcinoma. Biomedicines, 11(12), 3213. https://doi.org/10.3390/biomedicines11123213






