Natural Compounds of Salvia L. Genus and Molecular Mechanism of Their Biological Activity
Abstract
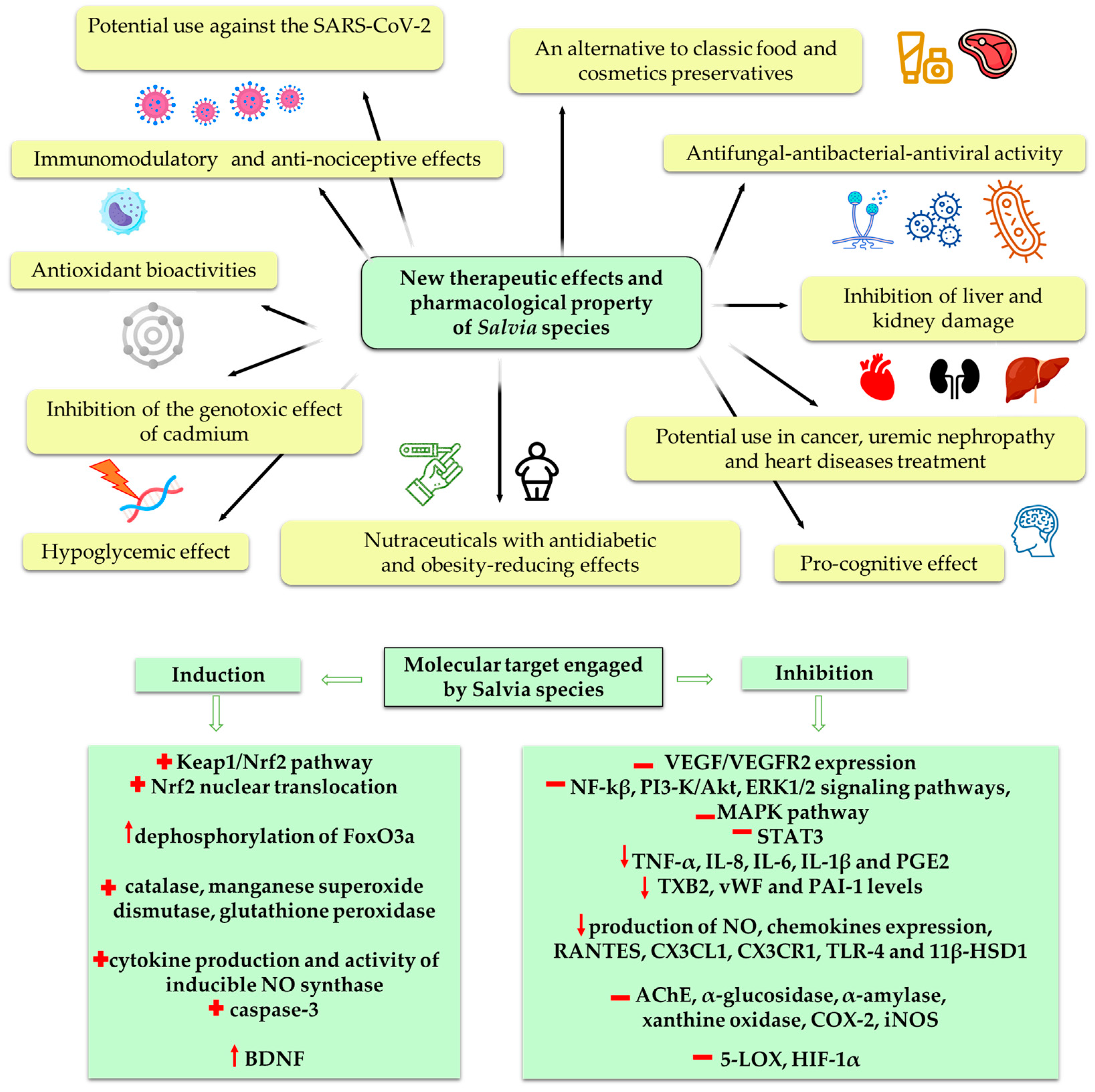
:1. Introduction
2. General Characteristics of the Genus
Kazakhstani Species of Sage
3. Main Biologically Active Compounds of Sage
4. Pharmacological Properties
4.1. The Impact on Immune Cells and Cytokines Production
The Impact on Blood Cells
4.2. Anti-Inflammatory Activity
4.3. Antioxidant Activity
4.4. Hepatoprotective Activity
4.5. Cognitive Effects
4.6. Antiangiogenic Activity
4.7. Anticancer Activity
4.8. Antimicrobial Activity
4.9. Antidiabetic Activity
5. Nephroprotective Properties
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species Name (Part Used) | Distribution in Kazakhstan (Floral Area) | Ecology, Habitats | Information about the Content of Biologically Active Compounds | Therapeutic Effect | References |
|---|---|---|---|---|---|
| Salvia aethiopis L. (aerial part) | Chu-Ili Mountains (26), Kyrgyz Alatau (27), Karatau (28) | Steppes, meadow slopes of steppe mountains | Alkaloids, tannins, flavonoids, triterpenoids, quinones, essential and fatty oils | Antibacterial, antifungal, antihyperhidrosis | [25,37,38] |
| Salvia macrosiphon Boiss. (whole plant) | Western Tien Shan (29) | Foothills, gravelly and loess, rocky slopes, valleys and bottoms of dried rivers | Essential oil, coumarins, flavonoids, quinones, sterols, diterpenes | Antibacterial, repellent, expectorant, cardiotonic | [25,37,38] |
| Salvia deserta Schangin (aerial part) | Tobolsk—Ishim (2), Irtysh (3), Semipalatinsk forest (4), Kokchetau (5), near Caspian (6), Aktobe (7), Mugojary (7a), Turgay (9), western and eastern Melkosopochnik (10, 11), Zaisan (12), Balkhash—Alakul (18), Kyzyl—Kum (20), Turkestan (21), Altay (22), Tarbagatay (23), Dzungarian Alatau (24), Trans—Ili Kungei Alatau (25), Chu—Ili Mountains (26), Karatau (28), Western Tien Shan (29). | Steppe zone, steppe mountain slopes, forest edges, river banks, often as weeds near roads, housing, fields | Organic acids, alkaloids, tannins, flavonoids, phenolic carboxylic acids and their derivatives, quinones, essential and fatty oils, vitamins | Raw materials for the production of quinones, antibacterial | [25,37,38] |
| Salvia sclarea L. (aerial part) | Chu—Ili Mountains (26), Karatau (28), Western Tien Shan (29) | Gravelly, rocky mountain slopes, gorges and valleys | Organic acids, alkaloids, tannins, flavonoids, phenolic carboxylic acids and their derivatives, quinones, essential and fatty oils, vitamins | Anti-inflammatory, antispasmodic, tonic, diuretic, antiseptic, wound healing | [25,37,38] |
| Salvia stepposa Schost. (whole plant) | Tobolsk—Ishim (2), Irtysh (3), Semipalatinsk forest (4), Kokchetau (5), near Caspian (6), Aktobe (7), Mugojary (7a), Turgay (9), Western and Eastern Melkosopochnik (10,11), Altay (22) | Steppes, dry steppe meadows | Carbohydrate, quinones, fatty oil | Antibacterial, antifungal | [25,37,38,39] |
| Salvia trautvetteri Regel (whole plant) | Karatau (28), Talas Alatau (29). Endem | Steppe, rocky and gravelly mountain slopes | Flavonoids, quinones | Antiprotozoal, bacteriostatic | [25,37,38,39,40,41] |
| Salvia virgata Jacg. | Western Tien Shan (29) | Meadow, mountain slopes and lawns, edges of walnut and deciduous forests, often as weeds | No data available | No data available | [25] |
| Salvia verticillata L. (whole plant) | Tobolsk—Ishim (2) | Stony scree, pine forests, dry elevated places, stony-clay soil, often as a weed | Carbohydrate, terpenoids, steroids, coumarins, tannins, flavonoids, anthocyanins, quinones, essential and fatty oils | Hemostatic, astringent, wound healing | [25,37,38] |
References
- Da Cunha, L.; Tizziani, T.; Souza, G.B.; Moreira, M.A.; Neto, J.; dos Santos, C.; de Carvalho, M.G.; Dalmarco, E.M.; Turqueti, L.B.; Scotti, M.T.; et al. Natural products with tandem anti-inflammatory, immunomodulatory and anti-SARS-CoV/2 effects: A drug discovery perspective against SARS-CoV-2. Curr. Med. Chem. 2022, 29, 2530–2564. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, B.; Chen, Y.; Wang, Q.; Ahmed, A.F.; Zhang, Y.; Kang, W. The immunomodulatory effects of active ingredients from Nigella sativa in RAW264.7 cells through NF-κB/MAPK signaling pathways. Front. Nutr. 2022, 9, 899797. [Google Scholar] [CrossRef] [PubMed]
- Montuori, E.; de Pascale, D.; Lauritano, C. Recent discoveries on marine organism immunomodulatory activities. Mar. Drugs 2022, 20, 422. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health-promoting properties of common herbs. Am. J. Clin. Nutr. 1999, 70, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef]
- Hao, D.C.; Xiao, P.G. Genomics and evolution in traditional medicinal plants: Road to a healthier life. Evol. Bioinform. 2015, 11, 197–212. [Google Scholar] [CrossRef]
- Huang, C.F.; Lin, S.S.; Liao, P.H.; Young, S.C.; Yang, C.C. The immunopharmaceutical effects and mechanisms of herb medicine. Cell Mol. Immunol. 2008, 5, 23–31. [Google Scholar] [CrossRef]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Choudhury, A.; Singh, P.A.; Bajwa, N.; Dash, S.; Bisht, P. Pharmacovigilance of herbal medicines: Concerns and future prospects. J. Ethnopharmacol. 2023, 309, 116383. [Google Scholar] [CrossRef]
- Meng, T.; Zhang, Y.; Wang, J.; Leo, C.H.; Li, Z.; Zhang, J.; Gao, K.; He, Q. Editorial: Efficacy and mechanism of herbal medicines and their functional compounds in preventing and treating cardiovascular diseases and cardiovascular disease risk factors. Front. Pharmacol. 2023, 14, 1236821. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Mobasheri, L.; Rakhshandeh, H.; Rahimi, V.B.; Najafi, Z.; Askari, V.R. Edible herbal medicines as an alternative to common medication for sleep disorders: A review article. Curr. Neuropharmacol 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, C.; Chen, Y.; Yu, S.; Liu, Y.; Yu, S.; Jiang, L.; Jin, C.; Wang, X.; Zhang, P.; et al. Herbal/natural compounds resist hallmarks of brain aging: From molecular mechanisms to therapeutic strategies. Antioxidants 2023, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Chaughule, R.S.; Barve, R.S. Role of herbal medicines in the treatment of infectious diseases. Vegetos 2023, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.; Leal, A.L.A.B.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Irtegun, K.S.; Fidan, H.S.; Yener, I.; Mete, N.; Ertas, A.; Topcu, G.; Kolak, U. Investigation of cytotoxic and apoptotic effects of 63 compounds obtained from Salvia species: Promising anticancer agents. J. Food Biochem. 2022, 46, e14226. [Google Scholar] [CrossRef]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef]
- Xia, F.; Wu, C.Y.; Yang, X.W.; Li, X.; Xu, G. Diterpenoids from the roots of Salvia yunnanensis. Nat. Prod. Bioprospect. 2015, 5, 307–312. [Google Scholar] [CrossRef]
- Petruzzello, M. List of plants in the family Lamiaceae. Encyclopedia Britannica. 2021. Available online: https://www.britannica.com/topic/list-of-plants-in-the-family-Lamiaceae-2035853 (accessed on 6 January 2023).
- Kudryashev, S.N. Materials for the study of the sage of Central Asia. In Proceedings of the Plant Resources Sector of the Committee of Sciences of the UzSSR, Tashkent, Uzbekistan; 1937; Volume 3, pp. 1–35. [Google Scholar]
- Turdiboev, O.A.; Shormanova, A.A.; Sheludyakova, M.B.; Akbarov, F.; Drew, B.T.; Celep, F. Synopsis of the Central Asian Salvia species with identification key. Phytotaxa 2022, 543, 20. [Google Scholar] [CrossRef]
- Khassanov, F.O. Conspectus Florae Asiae Mediae, 11th ed.; Science Publishers: Tashkent, Uzbekistan, 2015; Volume 11, pp. 154–156. [Google Scholar]
- Li, W.; Tojibaev, K.S.; Hisoriev, H.; Shomurodov, K.F.; Luo, M.; Feng, Y.; Ma, K. Mapping Asia plants: Current status of floristic information for Central Asian flora. GECCO 2020, 24, e01220. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, N.V. Flora of Kazakhstan. Almaty 1964, 515. [Google Scholar]
- Kasimu, R.; Wang, X.; Wang, X.; Hu, J.; Wang, X.; Mu, Y. Antithrombotic effects and related mechanisms of Salvia deserta Schang. root EtOAc extracts. Sci. Rep. 2018, 8, 17753. [Google Scholar] [CrossRef] [PubMed]
- Savona, G.; Bruno, M.; Rodríguez, B.; Marco, J.L. Triterpenoids from Salvia deserta. Phytochem. 1987, 26, 3305–3308. [Google Scholar] [CrossRef]
- Tezuka, Y.; Kasimu, R.; Li, J.X.; Basnet, P.; Tanaka, K.; Namba, T.; Kadota, S. Constituents of roots of Salvia deserta Schang. (Xinjiang-Danshen). ChemInform 2010, 29. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Jokic, S.; Molnar, M.; Jasic, M.; Babic, J.; Jukic, H.; Banjari, I. Bioactive profile of various Salvia officinalis L. preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef]
- Búfalo, J.; Cantrell, C.L.; Jacob, M.R.; Schrader, K.K.; Tekwani, B.L.; Kustova, T.S.; Ali, A.; Boaro, C.S. Antimicrobial and antileishmanial activities of diterpenoids isolated from the roots of Salvia deserta. Planta Med. 2015, 82, 131–137. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topcu, G.; Sönmez, U.; Eris, C. Terpenoids from Salvia nemorosa. Phytochemistry 1994, 35, 1065–1067. [Google Scholar] [CrossRef]
- Naderi, N.; Akhavan, N.; Aziz Ahari, F.; Zamani, N.; Kamalinejad, M.; Shokrzadeh, M.; Ahangar, N.; Motamedi, F. Effects of hydroalcoholic extract from Salvia verticillata on pharmacological models of seizure, anxiety and depression in mice. Iran. J. Pharm. Res. 2011, 10, 535–545. [Google Scholar]
- Barjaktarevic, A.R.; Cirovic, T.; Arsenijevic, N.; Volarevic, V. Antioxidant, antimicrobial and cytotoxic activities of Salvia verticillata L. extracts. Indian. J. Pharm. Sci. 2021, 83, 1280–1287. [Google Scholar] [CrossRef]
- Srivedavyasasri, R.; White, M.B.; Kustova, T.S.; Gemejiyeva, N.G.; Cantrell, C.L.; Ross, S.A. New tetranorlabdanoic acid from aerial parts of Salvia aethiopis. Nat. Prod. Res. 2018, 32, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Chukalina, O.N.; Darbaeva, T.E. Salvia aethiopis L. in West-Kazakhstan region. Mordovian Univ. Biol. Sci. Bull. 2013, 3-4, 145–146. [Google Scholar]
- Nurmahanova, A.; Ibisheva, N.; Kurbatova, N.; Atabayeva, S.; Seilkhan, A.; Tynybekov, B.; Abidkulova, K.; Childibaeva, A.; Akhmetova, A.; Sadyrova, G. Comparative anatomical and morphological study of three populations of Salvia aethiopis L. growing in the Southern Balkhash region. J. Ecol. Eng. 2023, 24, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Grudzinskaya, L.M.; Gemedzhieva, N.G.; Nelina, N.V.; Karzhaubekova, Z. Annotated List of Medicinal Plants of Kazakhstan; Reference Publication: Almaty, Kazakhstan, 2014; pp. 93–94. [Google Scholar]
- Sokolov, P.D. Plant Resources of the USSR. Flowering Plants, Their Chemical Composition, Use; Families Hippuridaceae—Lobeliaceae; Nauka: St. Petersburg, Russia, 1991; pp. 72–83. [Google Scholar]
- Levaya, Y.K.; Atazhanova, G.A. Chemical composition and pharmacological activity of certain types of sage. In Compilation of Scientific Works from 60th International Scientific Conference of Eurasian Scientific Association “Modern Concepts of Scientific Research”; ENO: Moscow, Russia, 2020; pp. 75–78. [Google Scholar]
- Abdulina, S.A. List of Vascular Plants of Kazakhstan; Kamelin, R.V., Ed.; Institute of Botany and Phytointroduction: Almaty, Kazakhstan, 1999; Volume 112, 187p. [Google Scholar]
- Baytenov, M.S. Flora of Kazakhstan: Vol. 2. Generic Composition of Flora; Gylym: Almaty, Kazakhstan, 2001; Volume 181, 280p. [Google Scholar]
- Grudzinskaya, L.; Gemejiyeva, N.; Karzhaubekova, Z.; Nelina, N. Botanical coverage of the leading families of medicinal flora of Kazakhstan. BIO Web Conf. 2021, 31, 00007. [Google Scholar] [CrossRef]
- Saparbaeva, N.A. Distribution and diversity of plant endemic species ridge Jungar Alatau. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2017, 4, 43–50. [Google Scholar]
- Levaya, Y.K.; Atazhanova, G.A. Distribution of some species of Salvia stepposa Des.-Shost. and Salvia sclarea L. in the Republic of Kazakhstan. Pharm. Kazakhstan 2019, 12, 22–28. [Google Scholar]
- Levaya, Y.K.; Atazhanova, G.A. Marketing analysis of the Kazakhstani pharmaceutical market of drugs containing sage. Vestnik KazNMU. 2020, 1, 546–548. [Google Scholar]
- SK-PHARMACY LLP. Report on the Results of the First Half of 2022: Uninterrupted Provision of Medicines and Medical Products within the Framework of Creating a Fairer and Healthier Kazakhstan, Approved by the SK-Pharmacy LLP Supervisory Board on August 26, 2022 (Protocol No. 7); SK Pharmacy LLP: Nur-Sultan, Kazakhstan, 2022; p. 17. [Google Scholar]
- Overview of the Kazakhstani Healthcare System: Results of 2021; Obzor Kazahstanskoj Sistemy Zdravoohranenija: Itogi 2021 Goda. 2022. Available online: https://primeminister.kz/ru/news/reviews/obzor-kazahstanskoy-sistemy-zdravoohraneniya-itogi-2021-goda-1933931 (accessed on 6 January 2023).
- Grudzinskaya, L.M.; Gemejiyeva, N.G.; Karzhaubekova, Z. The Kazakhstan medicinal flora survey in a leading families volume. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2020, 4, 39–51. [Google Scholar] [CrossRef]
- Alkhateeb, H.; Bonen, A. Thujone, a component of medicinal herbs, rescues palmitate-induced insulin resistance in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, 804–812. [Google Scholar] [CrossRef]
- De Sousa, D.P. Analgesic-like activity of essential oils constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef]
- Siveen, K.S.; Kuttan, G. Augmentation of humoral and cell mediated immune responses by thujone. Int. Immunopharmacol. 2011, 11, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochem. 2002, 59, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Kostic, M.; Kitic, D.; Petrovic, M.B.; Jevtovic-Stoimenov, T.; Jovic, M.; Petrovic, A.; Zivanovic, S. Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J. Ethnopharmacol. 2017, 199, 52–59. [Google Scholar] [CrossRef] [PubMed]
- El Gabbas, Z.; Bezza, K.; Laadraoui, J.; Laaradia, M.A.; Kebbou, A.; Oufquir, S.; Boukhira, A.; Aboufatima, R.; Chait, A. Salvia officinalis, rosmarinic and caffeic acids attenuate neuropathic pain and improve function recovery after sciatic nerve chronic constriction in mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 1702378. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Michel, C.; Stettmaier, K.; Lu, Y.; Foo, L.Y. Antioxidant mechanisms of polyphenolic caffeic acid oligomers, constituents of Salvia officinalis. Biol. Res. 2004, 37, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, R.; Manoharan, S. Anti-tumor activity of rosmarinic acid in 7,12-dimethylbenz(a)anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. Indian J. Exp. Biol. 2012, 50, 187–194. [Google Scholar] [PubMed]
- Xu, Y.; Jiang, Z.; Ji, G.; Liu, J. Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 2010, 76, 956–962. [Google Scholar] [CrossRef]
- Huang, S.S.; Zheng, R.L. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006, 239, 271–280. [Google Scholar] [CrossRef]
- Seo, S.; Oh, S.; Shin, Y.; Jung, S.; Kim, Y. Reduction of body weight by rutin is associated with an increase of brown adipose tissue mitochondrial biogenesis in high-fat diet induced obese rat (LB430). FASEB J. 2014, 28, LB430. [Google Scholar] [CrossRef]
- Seo, S.; Lee, M.-S.; Chang, E.; Shin, Y.; Oh, S.; Kim, I.-H.; Kim, Y. Rutin increases muscle mitochondrial biogenesis with AMPK activation in high-fat diet-induced obese rats. Nutrients 2015, 7, 8152–8169. [Google Scholar] [CrossRef]
- Oliveira, K.B.; Palu, E.; Weffort-Santos, A.M.; Oliveira, B.H. Influence of rosmarinic acid and Salvia officinalis extracts on melanogenesis of B16F10 cells. Rev. Bras. Farmacogn. 2013, 23, 249–258. [Google Scholar] [CrossRef]
- Xing, Y.; Cai, L.; Yin, T.P.; Chen, Y.; Yu, J.; Wang, Y.R.; Ding, Z.T. Improving the antioxidant activity and enriching salvianolic acids by the fermentation of Salvia miltiorrhizae with Geomyces luteus. J. Zhejiang Univ. Sci. B 2016, 17, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, P.; Xu, S.; Xu, W.; Xu, W.; Chu, K.; Lu, J. Biological activities of salvianolic acid B from Salvia miltiorrhiza on type 2 diabetes induced by high-fat diet and streptozotocin. Pharm. Biol. 2015, 53, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.H.; Hong, C.Y. Salvianolic acids: Small compounds with multiple mechanisms for cardiovascular protection. J. Biomed. Sci. 2011, 18, 30. [Google Scholar] [CrossRef]
- Feng, Y.; You, Z.; Yan, S.; He, G.; Chen, Y.; Gou, X.; Peng, C. Antidepressant-like effects of salvianolic acid B in the mouse forced swim and tail suspension tests. Life Sci. 2012, 90, 1010–1014. [Google Scholar] [CrossRef]
- Braida, D.; Capurro, V.; Zani, A.; Rubino, T.; Vigano, D.; Parolaro, D. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br. J. Pharmacol. 2009, 157, 844–853. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Kalaycıoglu, Z.; Uzascı, S.; Dirmenci, T.; Bedia Erim, F. α-glucosidase enzyme inhibitory effects and ursolic and oleanolic acid contents of fourteen Anatolian Salvia species. J. Pharm. Biomed. Anal. 2018, 155, 284–287. [Google Scholar] [CrossRef]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Zhao, J.; Lou, J.; Mou, Y.; Li, P.; Wu, J.; Zhou, L. Diterpenoid tanshinones and phenolic acids from cultured hairy roots of Salvia miltiorrhiza Bunge and their antimicrobial activities. Molecules 2011, 16, 2259–2267. [Google Scholar] [CrossRef]
- Jedinak, A.; Muckova, M.; Kost’alova, D.; Maliar, T.; Masterova, I. Antiprotease and antimetastatic activity of ursolic acid isolated from Salvia officinalis. Z. Naturforsch C J. Biosci. 2006, 61, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Nasirzadeh, A.R.; Motaffaf, M. Comparison of Salvia officinalis L. essential oil and antifungal agents against Candida species. J. Pharm. Technol. Drug Res. 2012, 1, 7. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. essential oils: Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-bdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed. Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, J.; Ristic, M.; Kschonsek, J.; Westphal, A.; Mihailovic, M.; Filipovic, V.; Bohm, V. Comparison of chemical profile and antioxidant capacity of seeds and oils from Salvia sclarea and Salvia officinalis. Chem. Biodivers. 2017, 14, e1700344. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Amarowicz, R.; Kandeil, A.; Ali, M.A.; Ibrahim, E.A. Antiviral activity of Lavandula angustifolia L. and Salvia officinalis L. essential oils against avian influenza H5N1 virus. J. Agric. Sci. Food Res. 2021, 4, 100135. [Google Scholar] [CrossRef]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Bakir, D.; Akdeniz, M.; Ertas, A.; Yilmaz, M.A.; Yener, I.; Firat, M.; Kolak, U. A GC-MS method validation for quantitative investigation of some chemical markers in Salvia hypargeia Fisch. & C.A. Mey. of Turkey: Enzyme inhibitory potential of ferruginol. J. Food Biochem. 2020, 44, e13350. [Google Scholar] [CrossRef]
- Fronza, M.; Murillo, R.; Ślusarczyk, S.; Adams, M.; Hamburger, M.; Heinzmann, B.; Laufer, S.; Merfort, I. In vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem. 2011, 19, 4876–4881. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topcu, G.; Eri, C.; Sönmez, U.; Kartal, M.; Kurucu, S.; Bozok-Johansson, C. Terpenoids from Salvia sclarea. Phytochem. 1994, 36, 971–974. [Google Scholar] [CrossRef]
- Devansh, M. Salvia officinalis Linn.: Relevance to modern research drive. Inven. Impact Planta Act. 2012, 4, 203–207. [Google Scholar]
- Sienkiewicz, M.; Głowacka, A.; Poznańska-Kurowska, K.; Kaszuba, A.; Urbaniak, A.; Kowalczyk, E. The effect of clary sage oil on staphylococci responsible for wound infections. Postepy Dermatol. Alergol. 2015, 32, 21–26. [Google Scholar] [CrossRef]
- Gavyar, P.H.H.; Amiri, H. Chemical composition of essential oil and antioxidant activity of an endemic species from Iran. J. Essent. Oil Bear. Plants. 2018, 21, 1138–1145. [Google Scholar] [CrossRef]
- El Hadri, A.; del Río, M.Á.G.; Sanz, J. Cytotoxic activity of α-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An. R. Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Maache, S.; Zbadi, L.; Ghouizi, A.E.; Soulo, N.; Saghrouchni, H.; Siddique, F.; Sitotaw, B.; Salamatullah, A.M.; Nafidi, H.A.; Bourhia, M.; et al. Antioxidant and antimicrobial effects of essential oils from two salvia species with in vitro and in silico analysis targeting 1AJ6 and 1R4U proteins. Sci. Rep. 2023, 13, 14038. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martinez, M.; Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. Major selected monoterpenes alpha-pinene and 1,8-cineole found in Salvia lavandulifolia (spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Pavic, V.; Jakovljevic, M.; Molnar, M.; Jokic, S. Extraction of carnosic acid and carnosol from sage (Salvia officinalis L.) leaves by supercritical fluid extraction and their antioxidant and antibacterial activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Kuehnl, S.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz, O.; Koeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J. Pharmacol. Exp. Ther. 2012, 342, 169–176. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Pace, S.; Chini, M.G.; Bisio, A.; Romussi, G.; Pieretti, S.; Werz, O.; Koeberle, A.; Mascolo, N.; et al. Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br. J. Pharmacol. 2017, 174, 1497–1508. [Google Scholar] [CrossRef]
- Nicolella, H.D.; Fernandes, G.; Ozelin, S.D.; Rinaldi-Neto, F.; Ribeiro, A.B.; Furtado, R.A.; Senedese, J.M.; Esperandim, T.R.; Veneziani, R.C.S.; Tavares, D.C. Manool, a diterpene from Salvia officinalis, exerts preventive effects on chromosomal damage and preneoplastic lesions. Mutagenesis 2021, 31, 177–185. [Google Scholar] [CrossRef]
- Nicolella, H.D.; de Oliveira, P.F.; Munari, C.C.; Costa, G.F.; Moreira, M.R.; Veneziani, R.C.; Tavares, D.C. Differential effect of manool—A diterpene from Salvia officinalis, on genotoxicity induced by methyl methanesulfonate in V79 and HepG2 cells. Food Chem. Toxicol. 2014, 72, 8–12. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.F.; Munari, C.C.; Nicolella, H.D.; Veneziani, R.C.; Tavares, D.C. Manool, a Salvia officinalis diterpene, induces selective cytotoxicity in cancer cells. Cytotechnology 2016, 68, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- Đurović, S.; Micić, D.; Pezo, L.; Radić, D.; Bazarnova, J.G.; Smyatskaya, Y.A.; Blagojević, S. The effect of various extraction techniques on the quality of sage (Salvia officinalis L.) essential oil, expressed by chemical composition, thermal properties and biological activity. Food Chem. X 2022, 13, 100213. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Mecacci, G.; Nardi, V.; Cervelli, C.; Nardoni, S.; Mancianti, F.; Ebani, V.V.; Giannecchini, S.; Pistelli, L. Volatiles and antifungal-antibacterial-antiviral activity of South African Salvia spp. essential oils cultivated in uniform conditions. Molecules 2021, 26, 2826. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, J.; Zheng, M.; Zou, R.; Zhou, Y.; Zhu, M. Tanshinone IIA protects against subclinical lipopolysaccharide induced cardiac fibrosis in mice through inhibition of NADPH oxidase. Int. Immunopharmacol. 2018, 60, 59–63. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.; Lu, Y.; Li, L.; Abdolmaleky, H.; Blackburn, G.L.; Zhou, J. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int. J. Cancer 2011, 129, 1042–1052. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, critical pharmacological components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Poliwoda, A.; Petecka, M.; Buslovych, O.; Shlyapnikov, V.A.; Wieczorek, P.P. Antioxidant phenolic compounds in Salvia officinalis L. and Salvia sclarea L. Ecol. Chem. Eng. S 2018, 25, 133–142. [Google Scholar] [CrossRef]
- Privitera, G.; Luca, T.; Castorina, S.; Passanisi, R.; Ruberto, G.; Napoli, E. Anticancer activity of Salvia officinalis essential oil and its principal constituents against hormone-dependent tumour cells. Asian Pac. J. Trop. Biomed. 2019, 9, 24–28. [Google Scholar] [CrossRef]
- Schwager, J.; Richard, N.; Fowler, A.; Seifert, N.; Raederstorff, D. Carnosol and related substances modulate chemokine and cytokine production in macrophages and chondrocytes. Molecules 2016, 21, 465. [Google Scholar] [CrossRef]
- Wang, L.C.; Wei, W.H.; Zhang, X.W.; Liu, D.; Zeng, K.W.; Tu, P.F. An integrated proteomics and bioinformatics approach reveal the anti-inflammatory mechanism of carnosic acid. Front. Pharmacol. 2018, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Schwertassek, U.; Seydel, A.; Weber, K.; Hauschildt, S.; Lehmann, J. Therapeutic efficacy of a combined sage and bitter apple phytopharmaceutical in chronic DSS-induced colitis. Sci. Rep. 2017, 7, 14214. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, D.; Lou, H.; Sun, L.; Ji, J. Evaluation of the anti-inflammatory activities of tanshinones isolated from Salvia miltiorrhiza var. alba roots in THP-1 macrophages. J. Ethnopharmacol. 2016, 188, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Cappello, M.S.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.R.; Tundis, R. New insights into the antioxidant and anti-inflammatory effects of italian Salvia officinalis leaf and flower extracts in lipopolysaccharide and tumor-mediated inflammation models. Antioxidants 2021, 10, 311. [Google Scholar] [CrossRef]
- Al-Ezzy, R.M.; Al-Samarrae, K.; Ad’haih, A.H. Effect of sage (Salvia officinalis) aqueous extract on mitotic index in albino male mice. Res. J. Biotechnol. 2010, 4, 1. [Google Scholar] [CrossRef]
- Shin, J.; Kim, O.K.; Kim, S.; Bae, D.; Lee, J.; Park, J.; Jun, W. Immunomodulatory effect of a Salvia plebeia R. aqueous extract in forced swimming exercise-induced mice. Nutrients 2020, 12, 2260. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Firmino, J.P.; Reyes-Lopez, F.E.; Andree, K.B.; Gonzalez-Silvera, D.; Esteban, M.A.; Tort, L.; Quintela, J.C.; Pinilla-Rosas, J.M.; Vallejos-Vidal, E.; et al. The growth promoting and immunomodulatory effects of a medicinal plant leaf extract obtained from Salvia officinalis and Lippia citriodora in gilthead seabream (Sparus aurata). Aquaculture 2020, 524, 735291. [Google Scholar] [CrossRef]
- Salomon, R.; Reyes-López, F.E.; Tort, L.; Firmino, J.P.; Sarasquete, C.; Ortiz-Delgado, J.B.; Quintela, J.C.; Pinilla-Rosas, J.M.; Vallejos-Vidal, E.; Gisbert, E. Medicinal plant leaf extract from sage and lemon verbena promotes intestinal immunity and barrier function in gilthead seabream (Sparus aurata). Front. Immunol. 2021, 12, 670279. [Google Scholar] [CrossRef]
- Revajova, V.; Pistl, J.; Levkut, M.; Marcin, A.; Levkutova, M. Influence of oregano and Salvia extracts on lymphocyte subpopulation and functional activity of blood phagocytes and lymphocytes in chickens. Food Agric. Immunol. 2010, 21, 307–316. [Google Scholar] [CrossRef]
- Margetts, G.; Kleidonas, S.; Zaibi, N.S.; Zaibi, M.S.; Edwards, K.D. Evidence for anti-inflammatory effects and modulation of neurotransmitter metabolism by Salvia officinalis L. BMC Complement. Med. Ther. 2022, 22, 131. [Google Scholar] [CrossRef]
- Jurca, T.; Baldea, I.; Filip, G.A.; Olteanu, D.; Clichici, S.; Pallag, A.; Vicaş, L.; Marian, E.; Micle, O.; Crivii, C.B.; et al. A phytocomplex consisting of Tropaeolum majus L. and Salvia officinalis L. extracts alleviates the inflammatory response of dermal fibroblasts to bacterial lipopolysaccharides. Oxid. Med. Cell Longev. 2020, 2020, 8516153. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, B.; Movahhedkhah, S.; Seidavi, A.; Imranul Haq, Q.M.; Kadim, I.; Laudadio, V.; Mazzei, D.; Tufarelli, V. Effect of sage (Salvia officinalis L.) aqueous leaf extract on performance, blood constituents, immunity response and ileal microflora of broiler chickens. Agroforest Syst. 2020, 94, 1179–1187. [Google Scholar] [CrossRef]
- Dal Pra, V.; Bisol, L.B.; Detoni, S.; Denti, M.; Grando, J.; Pollo, C.; Pasquali, T.R.; Hoffmann, A.E.; Mazutti, M.A.; Macedo, S.M.D. Anti-inflammatory activity of fractionated extracts of Salvia officinalis. J. Appl. Pharm. Sci. 2011, 01, 67–71. [Google Scholar]
- Melo, G.A.; Fonseca, J.P.; Oliveira Farinha, T.; Pinho, R.J.; Damião, M.J.; Grespan, R.; da Silva, E.L.; Bersani-Amado, C.A.; Nakamura Cuman, R.K. Anti-inflammatory activity of Salvia officinalis L. J. Med. Plant Res. 2012, 6, 4934–4939. [Google Scholar] [CrossRef]
- Kolac, U.K.; Ustuner, M.C.; Tekin, N.; Ustuner, D.; Colak, E.; Entok, E. The anti-inflammatory and antioxidant effects of Salvia officinalis on lipopolysaccharide-induced inflammation in rats. J. Med. Food. 2017, 20, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Kammoun El Euch, S.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- El Jery, A.; Hasan, M.; Rashid, M.M.; Al Mesfer, M.K.; Danish, M.; Ben Rebah, F. Phytochemical characterization, and antioxidant and antimicrobial activities of essential oil from leaves of the common sage Salvia officinalis L. from Abha, Saudi Arabia. Asian Biomed. Res. Rev. News. 2020, 14, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Bonaccini, L.; Karioti, A.; Bergonzi, M.C.; Bilia, A.R. Effects of Salvia miltiorrhiza on CNS neuronal injury and degeneration: A plausible complementary role of tanshinones and depsides. Planta Med. 2015, 81, 1003–1016. [Google Scholar] [CrossRef]
- Capurso, A.; Crepaldi, G.; Capurso, C. Benefits of Mediterranean Diet in Elderly Patients; Practical Issues in Geriatrics; Springer Nature eBook: Berlin/Heidelberg, Germany, 2018; p. 445. [Google Scholar]
- Zhussupova, A.; Zhumaliyeva, G.; Ogay, V.; Issabekova, A.; Ross, S.A.; Zhusupova, G.E. Immunomodulatory effects of plant extracts from Salvia deserta Schang. and Salvia sclarea L. Plants 2022, 11, 2690. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.Y.; Chung, S.W.; Kim, S.H.; Ry, S.Y.; Kim, T.S. Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology 2000, 49, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Lee, Y.R.; Lee, Y.H.; Oh, S.T.; Gerelchuluun, T.; Kim, B.H.; Kim, Y.; Yun, Y.P.; Song, S.; Lee, C.K. Synergistic activation of monocytes by polysaccharides isolated from Salicornia herbacea and interferon-gamma. J. Ethnopharmacol. 2007, 111, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, J.; Song, P.; Wang, X.; Shi, W. Effects of Salvia miltiorrhiza polysaccharides on lipopolysaccharide-induced inflammatory factor release in RAW264.7 cells. J. Interferon Cytokine Res. 2018, 38, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Salvia (sage): A review of its potential cognitive-enhancing and protective effects. Drugs R D 2017, 17, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chang, Y.C.; Hu, W.L.; Hung, Y.C. Oxidative stress and Salvia miltiorrhiza in aging-associated cardiovascular diseases. Oxid. Med. Cell Longev. 2016, 118, 4797102. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Salvia species as nutraceuticals: Focus on antioxidant, antidiabetic and anti-obesity properties. Appl. Sci. 2021, 11, 9365. [Google Scholar] [CrossRef]
- Gong, J.; Ju, A.; Zhou, D.; Li, D.; Zhou, W.; Geng, W.; Li, B.; Li, L.; Liu, Y.; He, Y.; et al. Salvianolic acid Y: A new protector of PC12 cells against hydrogen peroxide-induced injury from Salvia officinalis. Molecules 2015, 20, 683–692. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, W.; Mu, Y.P.; Zhang, H.; Wang, X.N.; Zhao, C.Q.; Chen, J.M.; Liu, P. Pharmacological Effects of Salvianolic Acid B Against Oxidative Damage. Front. Pharmacol. 2020, 11, 572373. [Google Scholar] [CrossRef]
- Pereira, O.R.; Catarino, M.D.; Afonso, A.F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis Decoctions: Antioxidant activities and inhibition of carbohydrate and lipid metabolic enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Eldeeb, H.M.; Khan, R.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Sajid, M.S.M.; Aly, M.S.A.; Ahmad, A.M.; Abdellatif, A.A.H.; Eid, S.Y.; et al. Sage, Salvia officinalis L.; constituents, hepatoprotective activity, and cytotoxicity evaluations of the essential oils obtained from fresh and differently timed dried herbs: A comparative analysis. Molecules 2021, 26, 5757. [Google Scholar] [CrossRef] [PubMed]
- Doğan, M.; Akıcı, N.; Diken, M.E.; Doğan, S.; Yilmaz Kardas, B.; Dirmenci, T. Biological activities of some Salvia species. Z. Naturforsch C J. Biosci. 2021, 77, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J. Agric. Food Chem. 2002, 50, 1845–1851. [Google Scholar] [CrossRef]
- Mot, M.D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.M.; Copolovici, L.; Copolovici, D.M.; et al. Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Skalicka-Woźniak, K.; Mihai, C.-T.; Gradinaru, A.C.; Mandici, A.; Ciocarlan, N.; Miron, A.; Aprotosoaie, A.C. Chemical Profile and Bioactivity Evaluation of Salvia Species from Eastern Europe. Antioxidants 2023, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Koubaa, F.G.; Chaabane, M.; Turki, M.; Ayadi, F.M.; El Feki, A. Anti-oxidant and hepatoprotective effects of Salvia officinalis essential oil against vanadium-induced oxidative stress and histological changes in the rat liver. Environ. Sci. Pollut. Res. Int. 2021, 28, 11001–11015. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Hamza, A.A. Hepatoprotective effects of Hibiscus, Rosmarinus and Salvia on azathioprine-induced toxicity in rats. Life Sci. 2005, 77, 266–278. [Google Scholar] [CrossRef]
- Babault, N.; Noureddine, A.; Amiez, N.; Guillemet, D.; Cometti, C. Acute Effects of Salvia Supplementation on Cognitive Function in Athletes During a Fatiguing Cycling Exercise: A Randomized Cross-Over, Placebo-Controlled, and Double-Blind Study. Front. Nutr. 2021, 8, 771518. [Google Scholar] [CrossRef]
- Edwards, K.D.; Dubberke, A.; Meyer, N.; Kugel, S.; Hellhammer, J. Assessment of the Effects of a Sage (Salvia officinalis) Extract on Cognitive Performance in Adolescents and Young Adults. medRxiv 2021. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, M.Q.; Wang, W.; Liu, L.T.; Zhou, L.M.; Miao, S.K.; Wan, L.H. Compound danshen tablet ameliorated abeta 25-35-induced spatial memory impairment in mice via rescuing imbalance between cytokines and neurotrophins. BMC Complement. Altern. Med. 2014, 14, 23. [Google Scholar] [CrossRef]
- Bowling, H.; Bhattacharya, A.; Klann, E.; Chao, M.V. Deconstructing brain-derived neurotrophic factor actions in adult brain circuits to bridge an existing informational gap in neuro-cell biology. Neural Regen. Res. 2016, 11, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Dinel, A.L.; Lucas, C.; Guillemet, D.; Layé, S.; Pallet, V.; Joffre, C. Chronic supplementation with a mix of Salvia officinalis and Salvia lavandulaefolia improves Morris water maze learning in normal adult C57Bl/6J mice. Nutrients 2020, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Jackson, P.A.; Spittlehouse, B.; Heffernan, T.; Guillemet, D.; Kennedy, D.O. The acute and chronic cognitive effects of a sage extract: A randomized, placebo controlled study in healthy humans. Nutrients 2021, 13, 218. [Google Scholar] [CrossRef]
- Keshavarz, M.; Mostafaie, A.; Mansouri, K.; Bidmeshkipour, A.; Motlagh, H.R.; Parvaneh, S. In vitro and ex vivo antiangiogenic activity of Salvia officinalis. Phytother. Res. 2010, 24, 1526–1531. [Google Scholar] [CrossRef]
- Ahmed, O.H. Antiangiogenic effect of Salvia officinalis. Int. J. Psychosoc. Rehabil. 2020, 24, 2535–2543. [Google Scholar] [CrossRef]
- Zihlif, M.; Afifi, F.; Abu-Dahab, R.; Abdul Majid, A.M.; Sumrein, H.; Saleh, M.M.; Nassar, Z.D.; Naffa, R. The antiangiogenic activities of ethanolic crude extracts of four Salvia species. BMC Complement. Altern. Med. 2013, 13, 358. [Google Scholar] [CrossRef]
- Dat, N.T.; Jin, X.; Lee, J.H.; Lee, D.; Hong, Y.S.; Lee, K.; Kim, Y.H.; Lee, J.J. Abietane diterpenes from Salvia miltiorrhiza inhibit the activation of hypoxia-inducible factor-1. J. Nat. Prod. 2007, 70, 1093–1097. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, Y.S.; Kim, J.H.; Kim, T.I.; Li, W.; Oh, T.W.; Jeon, C.H.; Kim, S.J.; Chung, H.S. Anticancer effect of Salvia plebeia and its active compound by improving T-cell activity via blockade of PD-1/PD-L1 interaction in humanized PD-1 mouse model. Front. Immunol. 2020, 11, 598556. [Google Scholar] [CrossRef]
- Ezema, C.A.; Ezeorba, T.P.C.; Aguchem, R.N.; Okagu, I.U. Therapeutic benefits of Salvia species: A focus on cancer and viral infection. Heliyon 2022, 8, e08763. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sher, H.F.; Chen, H.W.; Liu, C.C.; Chen, C.H.; Lin, C.S.; Yang, P.C.; Tsay, H.-S.; Chen, J.J. Anticancer effects of tanshinone I in human non-small cell lung cancer. Mol. Cancer Ther. 2008, 7, 3527–3538. [Google Scholar] [CrossRef]
- Xie, J.; Liu, J.; Liu, H.; Liang, S.; Lin, M.; Gu, Y.; Liu, T.; Wang, D.; Ge, H.; Mo, S.L. The antitumor effect of tanshinone IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on the human non-small cell lung cancer A549 cell line. Acta Pharm. Sin. B 2015, 5, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Hassan, Z.M.; Mohammadi, M.; Habibi, Z.; Sohrabi, N.; Bayanolhagh, S. Sclareol modulates the Treg intra-tumoral infiltrated cell and inhibits tumor growth in vivo. Cell Immunol. 2010, 263, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, T.; Cai, P. Sclareol inhibits cell proliferation and sensitizes cells to the antiproliferative effect of bortezomib via upregulating the tumor suppressor caveolin-1 in cervical cancer cells. Mol. Med. Rep. 2017, 15, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Rozalski, M.; Kuzma, L.; Krajewska, U.; Wysokinska, H. Cytotoxic and proapoptotic activity of diterpenoids from in vitro cultivated Salvia sclarea roots. Studies on the leukemia cell lines. Z. Naturforsch C J. Biosci. 2006, 61, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Balaei-Kahnamoei, M.; Eftekhari, M.; Ardekani, M.R.S.; Akbarzadeh, T.; Saeedi, M.; Jamalifar, H.; Safavi, M.; Sam, S.; Zhalehjoo, N.; Khanavi, M. Phytochemical constituents and biological activities of Salvia macrosiphon Boiss. BMC Chem. 2021, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Beheshti-Rouy, M.; Azarsina, M.; Rezaie-Soufi, L.; Alikhani, M.Y.; Roshanaie, G.; Komaki, S. The antibacterial effect of sage extract (Salvia officinalis) mouthwash against Streptococcus mutans in dental plaque: A randomized clinical trial. Iran. J. Microbiol. 2015, 7, 173–177. [Google Scholar]
- Stanciu, G.; Lupsor, S.; Oancea, E.; Mititelu, M. Biological activity of essential sage oil. J. Sci. Arts 2022, 22, 211–218. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–4867. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Badri, W.; Dumas, E.; Ghnimi, S.; Elaissari, A.; Saurel, R.; Gharsallaoui, A. Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview. Appl. Sci. 2021, 11, 5778. [Google Scholar] [CrossRef]
- Djakovic Sekulic, T.; Bozin, B.; Smolinski, A. Chemometric study of biological activities of 10 aromatic Lamiaceae species’ essential oils. J. Chemom. 2016, 30, 188–196. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. The pharmacological effects of Salvia species on the central nervous system. Phytother. Res. 2006, 20, 427–437. [Google Scholar] [CrossRef]
- Rzepa, J.; Wojtal, L.; Staszek, D.; Grygierczyk, G.; Labe, K.; Hajnos, M.; Kowalska, T.; Waksmundzka-Hajnos, M. Fingerprint of Selected Salvia Species by HS-GC-MS Analysis of Their Volatile Fraction. J. Chromatogr. Sci. 2009, 4, 575–580. [Google Scholar] [CrossRef]
- Kumar Singh, V.; Das, S.; Kumar Dwivedy, A.; Kumar Chaudhari, A.; Upadhyay, N.; Dubey, N.K. Assessment of chemically characterized Salvia sclarea L. essential oil and its combination with linalyl acetate as novel plant based antifungal, antiaflatoxigenic and antioxidant agent against herbal drugs contamination and probable mode of action. Nat. Prod. Res. 2019, 35, 782–787. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2023, 24, 5179. [Google Scholar] [CrossRef]
- Levaya, Y.; Atazhanova, G.; Erkenuly, Z.; Boltabaevna, A. Antibacterial activity of ultrasonic extracts of Salvia stepposa growing in Kazakhstan. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2021, 1, 45–50. [Google Scholar] [CrossRef]
- Ultanbekova, G.D.; Mukhataeva, K.A.; Zhusupova, A.I.; Gelani, C.D.; Ibisheva, N.; Nurmakhanova, A.S.; Zhalgasbaeva, M.O.; Sagyndykova, A.A.; Dastan, Z.D. A Survey of Endophytes from the Kazakhstani Species of Salvia aethiopis L.; Salvia stepposa Desshost and Salvia sclarea L. Microbiol. Virol. 2023, 3. Available online: https://cyberleninka.ru/article/n/aza-stan-territoriyasynda-setin-farmaflora-salvia-aethiopis-l-salvia-stepposa-desshost-zh-ne-salvia-sclarea-l-simdikterini (accessed on 16 October 2023).
- Ürgeová, E.; Uváčková, Ľ.; Vaneková, M.; Maliar, T. Antibacterial Potential of Microwave-Assisted Extraction Prepared Hydrolates from Different Salvia Species. Plants 2023, 12, 1325. [Google Scholar] [CrossRef]
- Jeshan, M.; Yousefbeyk, F.; Rahmati, H.; Hosein Shoormeij, A.; Rezazadeh, M.; Zamani, E. Salvia spinosa L. protects against diabetes-induced nephropathy by attenuation of mitochondrial oxidative damage in mice. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 4657514. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats, diabetes & metabolic syndrome. Clin. Res. Rev. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Kanana, F.M.; Maina, M.C.; Kibet, J.M.; Clement, J.M. Hypoglycaemic effects of Salvia officinalis extracts on alloxan-induced diabetic Swiss albino mice. J. Med. Plants Res. 2020, 14, 515–525. [Google Scholar] [CrossRef]
- Lima, C.F.; Azevedo, M.F.; Araujo, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Metformin-like effect of Salvia officinalis (common sage): Is it useful in diabetes prevention? Br. J. Nutr. 2006, 96, 326–333. [Google Scholar] [CrossRef]
- Mocan, A.; Babota, M.; Pop, A.; Fizeșan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical Constituents and Biologic Activities of Sage Species: A Comparison between Salvia officinalis L.; S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) Schur. Antioxidants 2020, 9, 480. [Google Scholar] [CrossRef]
- Behradmanesh, S.; Derees, F.; Rafieian-Kopaei, M. Effect of Salvia officinalis on diabetic patients. J. Ren. Inj. Prev. 2013, 2, 51–54. [Google Scholar] [CrossRef]
- Kianbakht, S.; Dabaghian, F.H. Improved glycemic control and lipid profile in hyperlipidemic type 2 diabetic patients consuming Salvia officinalis L. leaf extract: A randomized placebo. Controlled clinical trial. Complement. Ther. Med. 2013, 21, 441–446. [Google Scholar] [CrossRef]
- Jedidi, S.; Aloui, F.; Selmi, S.; Selmi, H.; Sammari, H.; Ayari, A.; Abbes, C.; Sebai, H. Antioxidant properties of Salvia officinalis decoction extract and mechanism of its protective effects on ethanol-induced liver and kidney injuries. J. Med. Food. 2022, 25, 546–556. [Google Scholar] [CrossRef]
- Ahn, Y.M.; Kim, S.K.; Lee, S.H.; Ahn, S.Y.; Kang, S.W.; Chung, J.H.; Kim, S.D.; Lee, B.C. Renoprotective effect of tanshinone IIA, an active component of Salvia miltiorrhiza, on rats with chronic kidney disease. Phytother. Res. 2010, 24, 1886–1892. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.S.; Lee, S.J.; Lee, B.C. The protective effect of Salvia miltiorrhiza in an animal model of early experimentally induced diabetic nephropathy. J. Ethnopharmacol. 2011, 137, 1409–1414. [Google Scholar] [CrossRef]
- Borkar, P.; Yadav, V.; Tiwari, R.R.; Samarth, R.M. A systematic review of potential candidates of herbal medicine in treatment of chronic kidney disease. Phytomed. Plus 2022, 2, 100361. [Google Scholar] [CrossRef]
- Rashwan, H.M.; Mohammed, H.E.; El-Nekeety, A.A.; Hamza, Z.K.; Abdel-Aziem, S.H.; Hassan, N.S.; Abdel-Wahhab, M.A. Bioactive phytochemicals from Salvia officinalis attenuate cadmium-induced oxidative damage and genotoxicity in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 68498–68512. [Google Scholar] [CrossRef] [PubMed]
- Wannes, W.A.; Tounsi, M.S. Tunisian nephroprotective plants: A review. J. Explor. Res. Pharmacol. 2023, 8, 74–91. [Google Scholar] [CrossRef]
- Hosivandi, S.; Asadi, F.; Salimikia, I. Evaluation of the protective effect of Salvia macrosiphon Boiss on the serum urea and creatinine levels in renal ischemia reperfusion injury. Yafte 2021, 23, 79–88. [Google Scholar]
- Kim, J.K.; Kim, W.J.; Hyun, J.M.; Lee, J.S.; Kwon, J.G.; Seo, C.; Song, M.J.; Choi, C.W.; Hong, S.S.; Park, K.; et al. Salvia plebeia Extract Inhibits Xanthine Oxidase Activity In Vitro and Reduces Serum Uric Acid in an Animal Model of Hyperuricemia. Planta Med. 2017, 83, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, R.; Shang, Y.; Jiao, B.; Huang, C. Lithospermic acid as a novel xanthine oxidase inhibitor has anti-inflammatory and hypouricemic effects in rats. Chem. Biol. Interact. 2008, 176, 137–142. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhou, M.; An, L.; Zhang, P.; Li, P.; Chen, J. Lipophilic extract and tanshinone IIA derived from Salvia miltiorrhiza attenuate uric acid nephropathy through suppressing oxidative stress-activated MAPK pathways. Am. J. Chin. Med. 2020, 48, 1455–1473. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Valizadeh, H.; Asghari, B.; Dinparast, L.; Farimani, M.M.; Bahadori, S. Chemical composition and antimicrobial, cytotoxicity, antioxidant and enzyme inhibitory activities of Salvia spinosa L. J. Funct. Foods 2015, 18, 727–736. [Google Scholar] [CrossRef]
- Hudaib, M.M.; Tawaha, K.A.; Mohammad, M.K.; Assaf, A.M.; Issa, A.Y.; Alali, F.Q.; Aburjai, T.A.; Bustanji, T.K. Xanthine oxidase inhibitory activity of the methanolic extracts of selected Jordanian medicinal plants. Pharmacogn. Mag. 2011, 7, 20–324. [Google Scholar] [CrossRef]
| Compound | Structure | Plant Name | Biological Activity | References |
|---|---|---|---|---|
| Phenolic acids | ||||
| Caffeic acid | 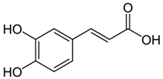 | S. sclarea, S. officinalis, leaves | anti-inflammatory antioxidant, antineuropathic, neuroprotective | [53,54,55] |
| Rosmarinic acid |  | S. sclarea, S. officinalis, leaves | anti-inflammatory, antioxidant, activity on melanogenesis, antineuropathic, neuroprotective | [56,57,58,59,60,61] |
| Salvianolic acids |  | S. miltiorrhiza, S. divinorum | antioxidant, antidiabetic, hepatoprotective, neuroprotective, cardiovascular protection, antidepressant, anxiolytic | [62,63,64,65,66] |
| Terpenoids | ||||
| Oleanolic acid | 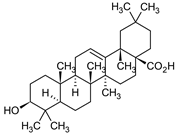 | S.officinalis, leaves S. sclarea, leaves S. virgata, leaves | antimicrobial, anti-inflammatory, antidiabetic, anti-nociceptive | [67,68] |
| Ursolic acid |  | S. officinalis, leaves S. sclarea, leaves S. virgata, leaves | anti-inflammatory, antidiabetic, antiprotease and antimetastatic | [68,69,70,71] |
| 1,8-cineole |  | S. officinalis, aerial part | antifungal, antibacterial, anti-inflammatory | [70,71,72] |
| Borneol | 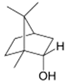 | S. officinalis, aerial part | antifungal, antibacterial | [52,72,73] |
| Thujone | 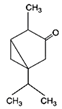 | S. officinalis, aerial part | antifungal, antioxidant, anti-inflammatory, antiviral, antibacterial | [70,71,74,75] |
| Camphor | 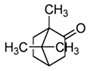 | S. officinalis, aerial part | antioxidant, anti-inflammatory, antiviral, antibacterial, antifungal | [72,74] |
| Ferruginol |  | S. deserta, S. hypargeia, root, S. miltiorrhiza, S. sclarea | antimicrobial, antileishmanial, antioxidant, antielastase, anticholinesterase, antiurease, cytotoxic, antihypertensive | [44,75,76,77,78] |
| Linalyl acetate |  | S. officinalis, leaves S. sclarea | analgesic, antiproliferative, antistaphylococcal | [79,80] |
| Humulene |  | S. sclareopsi, S. officinalis | antioxidant, cytotoxic | [81,82,83,84] |
| Pinene |  | S. lavandulifolia, S. sclarea | antioxidant, antistaphylococcal | [85,86] |
| Carnosic acid |  | S. officinalis, leaves | antioxidant, antimicrobial, anticarcinogenic, anti-inflammatory, antinociceptive | [87,88,89] |
| Carnosol | 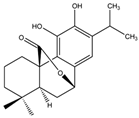 | S. officinalis, leaves | antioxidant, antimicrobial, anticarcinogenic, anti-inflammatory, antinociceptive | [87,88,89] |
| Manool | 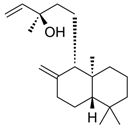 | S. officinalis, leaves, S. sclarea | anti-inflammatory, antigenotoxic, anticarcinogenic, chemopreventive, cytotoxic, antibacterial | [90,91,92] |
| Viridiflorol | 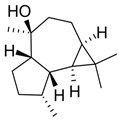 | S. dentata, S. sclareopsis, S. officinalis | antimicrobial, antifungal, antioxidant, cytotoxic | [93,94] |
| Tanshinones | 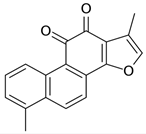 | S. miltiorrhiza, root | antioxidant, antimicrobial, anti-tumor, cardiovascular protective, neuroprotective | [35,95,96,97] |
| Flavonoids | ||||
| Luteolin |  | S. sclarea, S. officinalis | anti-inflammatory, antioxidant | [98] |
| Apigenin | 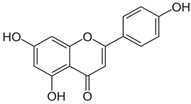 | S. sclarea, S. officinalis | anti-inflammatory, antioxidant, benzodiazepine receptor activity | [5,44] |
| Hispidulin |  | S. plebeia | hepatoprotective, anticancer | [16,45] |
| Quercetin | 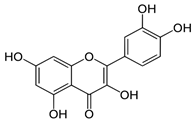 | S. officinalis | antioxidant | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhumaliyeva, G.; Zhussupova, A.; Zhusupova, G.E.; Błońska-Sikora, E.; Cerreto, A.; Omirbekova, N.; Zhunusbayeva, Z.; Gemejiyeva, N.; Ramazanova, M.; Wrzosek, M.; et al. Natural Compounds of Salvia L. Genus and Molecular Mechanism of Their Biological Activity. Biomedicines 2023, 11, 3151. https://doi.org/10.3390/biomedicines11123151
Zhumaliyeva G, Zhussupova A, Zhusupova GE, Błońska-Sikora E, Cerreto A, Omirbekova N, Zhunusbayeva Z, Gemejiyeva N, Ramazanova M, Wrzosek M, et al. Natural Compounds of Salvia L. Genus and Molecular Mechanism of Their Biological Activity. Biomedicines. 2023; 11(12):3151. https://doi.org/10.3390/biomedicines11123151
Chicago/Turabian StyleZhumaliyeva, Gaziza, Aizhan Zhussupova, Galiya E. Zhusupova, Ewelina Błońska-Sikora, Antonella Cerreto, Nargul Omirbekova, Zhazira Zhunusbayeva, Nadezhda Gemejiyeva, Madina Ramazanova, Małgorzata Wrzosek, and et al. 2023. "Natural Compounds of Salvia L. Genus and Molecular Mechanism of Their Biological Activity" Biomedicines 11, no. 12: 3151. https://doi.org/10.3390/biomedicines11123151
APA StyleZhumaliyeva, G., Zhussupova, A., Zhusupova, G. E., Błońska-Sikora, E., Cerreto, A., Omirbekova, N., Zhunusbayeva, Z., Gemejiyeva, N., Ramazanova, M., Wrzosek, M., & Ross, S. A. (2023). Natural Compounds of Salvia L. Genus and Molecular Mechanism of Their Biological Activity. Biomedicines, 11(12), 3151. https://doi.org/10.3390/biomedicines11123151







