GSK2606414 Sensitizes ABCG2-Overexpressing Multidrug-Resistant Colorectal Cancer Cells to Chemotherapeutic Drugs
Abstract
:1. Introduction
2. Methods and Materials
2.1. Cell Culture and Reagents
2.2. Cytotoxicity Assay
2.3. Drug Accumulation Assay
2.4. Western Blot Assay
2.5. Docking Analysis
2.6. Statistical Analysis
3. Results
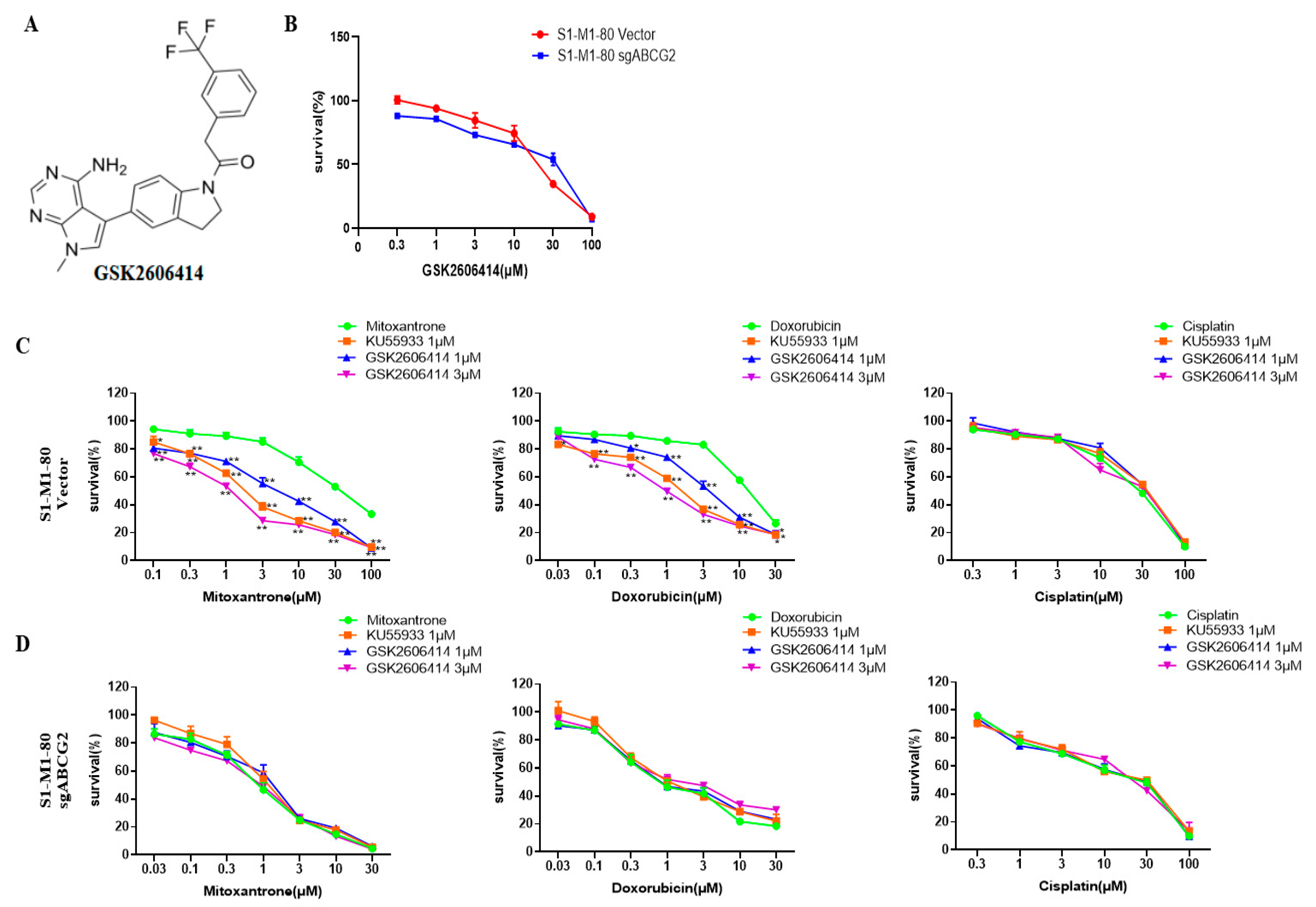
3.1. GSK2606414 Sensitizes ABCG2-Overexpressing Colorectal Cancer Cells to ABCG2-Substrate Chemotherapeutic Drugs
3.2. GSK2606414 Augments the Intracellular Levels of ABCG2 Substrates in ABCG2-Overexpressing Colorectal Cancer Cells
3.3. GSK2606414 Does Not Affect the Protein Expression of ABCG2 in Colorectal Cancer Cells and Binding Model of GSK2606414 with ABCG2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musyuni, P.; Bai, J.; Sheikh, A.; Vasanthan, K.S.; Jain, G.K.; Abourehab, M.A.S.; Lather, V.; Aggarwal, G.; Kesharwani, P.; Pandita, D. Precision medicine: Ray of hope in overcoming cancer multidrug resistance. Drug Resist. Updat. 2022, 65, 100889. [Google Scholar] [CrossRef] [PubMed]
- Yalcin-Ozkat, G. Molecular Modeling Strategies of Cancer Multidrug Resistance. Drug Resist. Updat. 2021, 59, 100789. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Ross, D.D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X.B. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. B 2019, 9, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Dei, S.; Braconi, L.; Romanelli, M.N.; Teodori, E. Recent advances in the search of BCRP- and dual P-gp/BCRP-based multidrug resistance modulators. Cancer Drug Resist. 2019, 2, 710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, S.; Zhang, W.; Shi, Z. Polymorphisms of ABCG2 and its impact on clinical relevance. Biochem. Biophys. Res. Commun. 2018, 503, 408–413. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Fan, J.; To, K.K.W.; Chen, Z.S.; Fu, L. ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist. Updat. 2023, 66, 100905. [Google Scholar] [CrossRef]
- Palshof, J.A.; Cederbye, C.N.; Hogdall, E.V.S.; Poulsen, T.S.; Linnemann, D.; Nygaard, S.B.; Stenvang, J.; Christensen, I.J.; Jensen, B.V.; Pfeiffer, P.; et al. ABCG2 Protein Levels and Association to Response to First-Line Irinotecan-Based Therapy for Patients with Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 5027. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, H.H.; Lin, J.K.; Lin, C.C.; Yang, S.H.; Li, A.F.; Chen, W.S.; Chang, S.C. Expression of ABCG2 associated with tumor response in metastatic colorectal cancer patients receiving first-line FOLFOX therapy–Preliminary evidence. Int. J. Biol. Markers 2013, 28, 182–186. [Google Scholar] [CrossRef]
- Liu, H.G.; Pan, Y.F.; You, J.; Wang, O.C.; Huang, K.T.; Zhang, X.H. Expression of ABCG2 and its significance in colorectal cancer. Asian Pac. J. Cancer Prev. 2010, 11, 845–848. [Google Scholar] [PubMed]
- Allen, J.D.; van Loevezijn, A.; Lakhai, J.M.; van der Valk, M.; van Tellingen, O.; Reid, G.; Schellens, J.H.; Koomen, G.J.; Schinkel, A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 2002, 1, 417–425. [Google Scholar] [PubMed]
- Rabindran, S.K.; He, H.; Singh, M.; Brown, E.; Collins, K.I.; Annable, T.; Greenberger, L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998, 58, 5850–5858. [Google Scholar] [PubMed]
- Liu, K.; Chen, Y.; Shi, X.B.; Xing, Z.H.; He, Z.J.; Wang, S.T.; Li, Y.C.; Liu, W.J.; Zhang, P.W.; Yu, Z.Z.; et al. Inhibiting the Activity of ABCG2 by KU55933 in Colorectal Cancer. Recent. Pat. Anticancer Drug Discov. 2022, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.C.; Chen, Y.; Shi, X.B.; Xing, Z.H.; He, Z.J.; Wang, S.T.; Liu, W.J.; Zhang, P.W.; Yu, Z.Z.; et al. AZ32 Reverses ABCG2-Mediated Multidrug Resistance in Colorectal Cancer. Front. Oncol. 2021, 11, 680663. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tiwari, A.K.; Shukla, S.; Robey, R.W.; Singh, S.; Kim, I.W.; Bates, S.E.; Peng, X.; Abraham, I.; Ambudkar, S.V.; et al. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011, 71, 3029–3041. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tiwari, A.K.; Shukla, S.; Robey, R.W.; Kim, I.W.; Parmar, S.; Bates, S.E.; Si, Q.S.; Goldblatt, C.S.; Abraham, I.; et al. Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478. Biochem. Pharmacol. 2009, 77, 781–793. [Google Scholar] [CrossRef]
- Alves, R.; Goncalves, A.C.; Jorge, J.; Almeida, A.M.; Sarmento-Ribeiro, A.B. Combination of Elacridar with Imatinib Modulates Resistance Associated with Drug Efflux Transporters in Chronic Myeloid Leukemia. Biomedicines 2022, 10, 1158. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, Y.; Wei, M.N.; Chen, Y.; Qiu, J.G.; Jiang, Q.W.; Yang, Y.; Zheng, D.W.; Qin, W.M.; Huang, J.R.; et al. Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer. Cancer Lett. 2017, 386, 100–109. [Google Scholar] [CrossRef]
- Chen, X.; Gong, L.; Ou, R.; Zheng, Z.; Chen, J.; Xie, F.; Huang, X.; Qiu, J.; Zhang, W.; Jiang, Q.; et al. Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752. Gynecol. Oncol. 2016, 140, 537–544. [Google Scholar] [CrossRef]
- Eckenstaler, R.; Benndorf, R.A. 3D structure of the transporter ABCG2-What’s new? Br. J. Pharmacol. 2020, 177, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Liao, M. ABCG2 transports anticancer drugs via a closed-to-open switch. Nat. Commun. 2020, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Manolaridis, I.; Jackson, S.M.; Taylor, N.M.I.; Kowal, J.; Stahlberg, H.; Locher, K.P. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 2018, 563, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Axten, J.M.; Medina, J.R.; Feng, Y.; Shu, A.; Romeril, S.P.; Grant, S.W.; Li, W.H.; Heerding, D.A.; Minthorn, E.; Mencken, T.; et al. Discovery of 7-methyl-5-(1-[3-(trifluoromethyl)phenyl]acetyl-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 2012, 55, 7193–7207. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ge, Y.; Dong, J.; Wang, H.; Zhao, T.; Wang, X.; Liu, J.; Gao, S.; Shi, L.; Yang, S.; et al. BZW1 Facilitates Glycolysis and Promotes Tumor Growth in Pancreatic Ductal Adenocarcinoma through Potentiating eIF2alpha Phosphorylation. Gastroenterology 2022, 162, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Sun, X.; Jin, F.; Xiao, D.; Li, H.; Sun, H.; Wang, Y.; Lu, Y.; Liu, J.; Huang, C.; et al. PERK-eIF2alpha-ERK1/2 axis drives mesenchymal-endothelial transition of cancer-associated fibroblasts in pancreatic cancer. Cancer Lett. 2021, 515, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Dastghaib, S.; Shojaei, S.; Mostafavi-Pour, Z.; Sharma, P.; Patterson, J.B.; Samali, A.; Mokarram, P.; Ghavami, S. Simvastatin Induces Unfolded Protein Response and Enhances Temozolomide-Induced Cell Death in Glioblastoma Cells. Cells 2020, 9, 2339. [Google Scholar] [CrossRef]
- Goyal, H.; Sharma, R.; Lamba, D.; Kaur, J. Folic acid depletion along with inhibition of the PERK arm of endoplasmic reticulum stress pathway promotes a less aggressive phenotype of hepatocellular carcinoma cells. Mol. Cell. Biochem. 2023, 478, 2057–2068. [Google Scholar] [CrossRef]
- Moon, H.S.; Kim, B.; Gwak, H.; Suh, D.H.; Song, Y.S. Autophagy and protein kinase RNA-like endoplasmic reticulum kinase (PERK)/eukaryotic initiation factor 2 alpha kinase (eIF2alpha) pathway protect ovarian cancer cells from metformin-induced apoptosis. Mol. Carcinog. 2016, 55, 346–356. [Google Scholar] [CrossRef]
- He, C.; Xia, J.; Gao, Y.; Chen, Z.; Wan, X. Chlorin A-mediated photodynamic therapy induced apoptosis in human cholangiocarcinoma cells via impaired autophagy flux. Am. J. Transl. Res. 2020, 12, 5080. [Google Scholar]
- Wu, M.S.; Chien, C.C.; Jargalsaikhan, G.; Ilsan, N.A.; Chen, Y.C. Activation of PERK Contributes to Apoptosis and G(2)/M Arrest by Microtubule Disruptors in Human Colorectal Carcinoma Cells (double dagger). Cancers 2019, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Chien, C.C.; Chang, J.; Chen, Y.C. Pro-apoptotic effect of haem oxygenase-1 in human colorectal carcinoma cells via endoplasmic reticular stress. J. Cell. Mol. Med. 2019, 23, 5692–5704. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Z.; Wang, C.; Zhou, H.; Liu, W.; Kanchana, K.; Dai, X.; Zou, P.; Gu, J.; Cai, L.; et al. Curcumin derivative WZ35 efficiently suppresses colon cancer progression through inducing ROS production and ER stress-dependent apoptosis. Am. J. Cancer Res. 2017, 7, 275. [Google Scholar] [PubMed]
- Wu, W.S.; Chien, C.C.; Chen, Y.C.; Chiu, W.T. Protein Kinase RNA-Like Endoplasmic Reticulum Kinase-Mediated Bcl-2 Protein Phosphorylation Contributes to Evodiamine-Induced Apoptosis of Human Renal Cell Carcinoma Cells. PLoS ONE 2016, 11, e0160484. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chien, C.C.; Wu, M.S.; Chen, Y.C. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine 2016, 23, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Zhang, J.; Dang, B.; Li, H.; Shen, H.; Li, X.; Wang, Z. PERK Pathway Activation Promotes Intracerebral Hemorrhage Induced Secondary Brain Injury by Inducing Neuronal Apoptosis Both In Vivo and In Vitro. Front. Neurosci. 2018, 12, 111. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, X.Y.; Zhou, Z.W.; Bai, H.; Shi, L.; Yang, Y.X.; Zhou, S.F.; Zhang, X.C. The combination of digoxin and GSK2606414 exerts synergistic anticancer activity against leukemia in vitro and in vivo. Biofactors 2017, 43, 812–820. [Google Scholar] [CrossRef]
- Bagratuni, T.; Patseas, D.; Mavrianou-Koutsoukou, N.; Liacos, C.I.; Sklirou, A.D.; Rousakis, P.; Gavriatopoulou, M.; Terpos, E.; Tsitsilonis, O.E.; Trougakos, I.P.; et al. Characterization of a PERK Kinase Inhibitor with Anti-Myeloma Activity. Cancers 2020, 12, 2864. [Google Scholar] [CrossRef]
- Ghaddar, N.; Wang, S.; Woodvine, B.; Krishnamoorthy, J.; van Hoef, V.; Darini, C.; Kazimierczak, U.; Ah-Son, N.; Popper, H.; Johnson, M.; et al. The integrated stress response is tumorigenic and constitutes a therapeutic liability in KRAS-driven lung cancer. Nat. Commun. 2021, 12, 4651. [Google Scholar] [CrossRef]
- Alasiri, G.; Jiramongkol, Y.; Zona, S.; Fan, L.Y.; Mahmud, Z.; Gong, G.; Lee, H.J.; Lam, E.W. Regulation of PERK expression by FOXO3: A vulnerability of drug-resistant cancer cells. Oncogene 2019, 38, 6382–6398. [Google Scholar] [CrossRef]
- McLaughlin, M.; Pedersen, M.; Roulstone, V.; Bergerhoff, K.F.; Smith, H.G.; Whittock, H.; Kyula, J.N.; Dillon, M.T.; Pandha, H.S.; Vile, R.; et al. The PERK Inhibitor GSK2606414 Enhances Reovirus Infection in Head and Neck Squamous Cell Carcinoma via an ATF4-Dependent Mechanism. Mol. Ther. Oncolytics 2020, 16, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Halliday, M.; Molloy, C.; Radford, H.; Verity, N.; Axten, J.M.; Ortori, C.A.; Willis, A.E.; Fischer, P.M.; Barrett, D.A.; et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 2013, 5, 206ra138. [Google Scholar] [CrossRef] [PubMed]
- Ounallah-Saad, H.; Sharma, V.; Edry, E.; Rosenblum, K. Genetic or pharmacological reduction of PERK enhances cortical-dependent taste learning. J. Neurosci. 2014, 34, 14624–14632. [Google Scholar] [CrossRef] [PubMed]
- Grande, V.; Ornaghi, F.; Comerio, L.; Restelli, E.; Masone, A.; Corbelli, A.; Tolomeo, D.; Capone, V.; Axten, J.M.; Laping, N.J.; et al. PERK inhibition delays neurodegeneration and improves motor function in a mouse model of Marinesco-Sjogren syndrome. Hum. Mol. Genet. 2018, 27, 2477–2489. [Google Scholar] [CrossRef] [PubMed]
- Mercado, G.; Castillo, V.; Soto, P.; Lopez, N.; Axten, J.M.; Sardi, S.P.; Hoozemans, J.J.M.; Hetz, C. Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson’s disease. Neurobiol. Dis. 2018, 112, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Radford, H.; Moreno, J.A.; Verity, N.; Halliday, M.; Mallucci, G.R. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol. 2015, 130, 633–642. [Google Scholar] [CrossRef]
- Gundu, C.; Arruri, V.K.; Sherkhane, B.; Khatri, D.K.; Singh, S.B. GSK2606414 attenuates PERK/p-eIF2alpha/ATF4/CHOP axis and augments mitochondrial function to mitigate high glucose induced neurotoxicity in N2A cells. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100087. [Google Scholar] [CrossRef]
- Guo, J.; Ren, R.; Sun, K.; Yao, X.; Lin, J.; Wang, G.; Guo, Z.; Xu, T.; Guo, F. PERK controls bone homeostasis through the regulation of osteoclast differentiation and function. Cell Death Dis. 2020, 11, 847. [Google Scholar] [CrossRef]
- Kim, M.J.; Min, S.H.; Shin, S.Y.; Kim, M.N.; Lee, H.; Jang, J.Y.; Kim, S.W.; Park, K.S.; Jung, H.S. Attenuation of PERK enhances glucose-stimulated insulin secretion in islets. J. Endocrinol. 2018, 236, 125–136. [Google Scholar] [CrossRef]
- Chintha, C.; Carlesso, A.; Gorman, A.M.; Samali, A.; Eriksson, L.A. Molecular modeling provides a structural basis for PERK inhibitor selectivity towards RIPK1. RSC Adv. 2019, 10, 367–375. [Google Scholar] [CrossRef]
- Rojas-Rivera, D.; Delvaeye, T.; Roelandt, R.; Nerinckx, W.; Augustyns, K.; Vandenabeele, P.; Bertrand, M.J.M. When PERK inhibitors turn out to be new potent RIPK1 inhibitors: Critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ. 2017, 24, 1100–1110. [Google Scholar] [CrossRef]
- Mahameed, M.; Wilhelm, T.; Darawshi, O.; Obiedat, A.; Tommy, W.S.; Chintha, C.; Schubert, T.; Samali, A.; Chevet, E.; Eriksson, L.A.; et al. The unfolded protein response modulators GSK2606414 and KIRA6 are potent KIT inhibitors. Cell Death Dis. 2019, 10, 300. [Google Scholar] [CrossRef]


| IC50 (μM) ± SD (Fold Reversal) | ||
|---|---|---|
| Compounds | S1-M1-80 Vector | S1-M1-80 sgABCG2 |
| Mitoxantrone | 31.407 ± 2.119 (1.00) | 0.911 ± 0.087 (1.00) |
| +KU55933 1 μM | 2.012 ± 0.067 (15.61) ** | 1.106 ± 0.113 (1.21) |
| +GSK2606414 1 μM | 5.080 ± 1.168 (6.18) ** | 0.904 ± 0.188 (0.99) |
| +GSK2606414 3 μM | 1.289 ± 0.209 (24.36) ** | 0.904 ± 0.183 (0.99) |
| Doxorubicin | 11.920 ± 0.605 (1.00) | 0.776 ± 0.128 (1.00) |
| +KU55933 1 μM | 1.562 ± 0.069 (7.63) ** | 0.919 ± 0.084 (1.18) |
| +GSK2606414 1 μM | 3.403 ± 0.362 (3.50) ** | 0.836 ± 0.084 (1.08) |
| +GSK2606414 3 μM | 1.179 ± 0.122 (10.11) ** | 0.975 ± 0.049 (1.26) |
| Cisplatin | 28.933 ± 0.472 (1.00) | 27.830 ± 1.682 (1.00) |
| +KU55933 1 μM | 28.813 ± 1.259 (1.00) | 28.000 ± 0.616 (1.01) |
| +GSK2606414 1 μM | 28.053 ± 1.766 (1.03) | 26.707 ± 1.499 (0.96) |
| +GSK2606414 3 μM | 27.483 ± 2.283 (1.05) | 27.047 ± 2.213 (0.97) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.-Z.; Xu, B.-Q.; Wang, Y.-Y.; Zhang, P.-W.; Shu, Y.-B.; Shi, Z. GSK2606414 Sensitizes ABCG2-Overexpressing Multidrug-Resistant Colorectal Cancer Cells to Chemotherapeutic Drugs. Biomedicines 2023, 11, 3103. https://doi.org/10.3390/biomedicines11113103
Yu Z-Z, Xu B-Q, Wang Y-Y, Zhang P-W, Shu Y-B, Shi Z. GSK2606414 Sensitizes ABCG2-Overexpressing Multidrug-Resistant Colorectal Cancer Cells to Chemotherapeutic Drugs. Biomedicines. 2023; 11(11):3103. https://doi.org/10.3390/biomedicines11113103
Chicago/Turabian StyleYu, Ze-Zhong, Bu-Qing Xu, Ying-Ying Wang, Peng-Wei Zhang, Yu-Bin Shu, and Zhi Shi. 2023. "GSK2606414 Sensitizes ABCG2-Overexpressing Multidrug-Resistant Colorectal Cancer Cells to Chemotherapeutic Drugs" Biomedicines 11, no. 11: 3103. https://doi.org/10.3390/biomedicines11113103
APA StyleYu, Z.-Z., Xu, B.-Q., Wang, Y.-Y., Zhang, P.-W., Shu, Y.-B., & Shi, Z. (2023). GSK2606414 Sensitizes ABCG2-Overexpressing Multidrug-Resistant Colorectal Cancer Cells to Chemotherapeutic Drugs. Biomedicines, 11(11), 3103. https://doi.org/10.3390/biomedicines11113103






