Intracellular Major Histocompatibility Complex Class II and C-X-C Motif Chemokine Ligand 10-Expressing Neutrophils Indicate the State of Anti-Tumor Activity Induced by Bacillus Calmette–Guérin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Peripheral Blood and Urine Collection
2.2. Flow Cytometric Analysis
2.3. Antibodies
2.4. Comprehensive Analysis of RNA
2.5. Mouse Abdominal Tumor Model
2.6. Statistical Analysis
3. Results
3.1. BCG Therapy Did Not Induce MDSC Production in Peripheral Blood
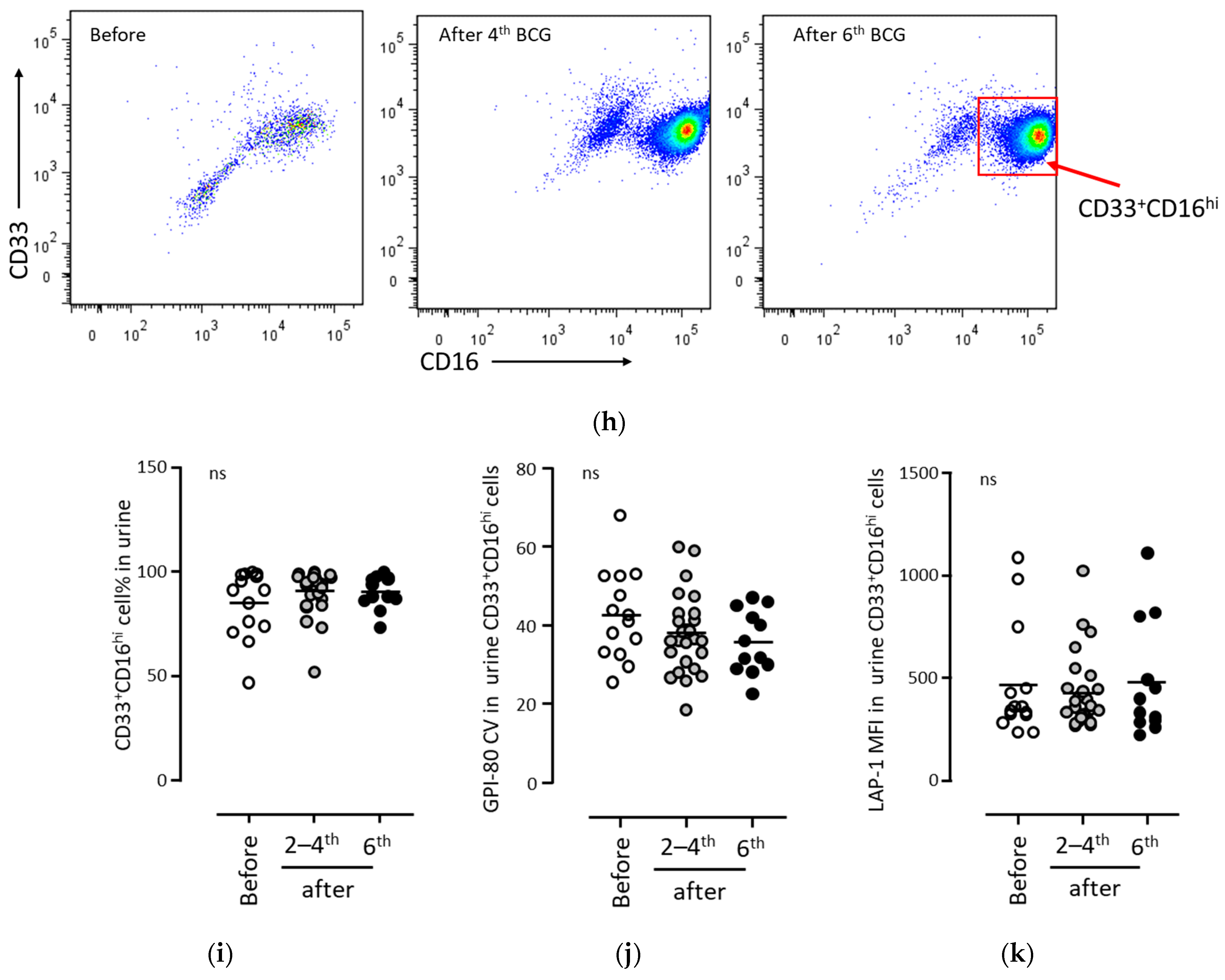
3.2. BCG Therapy Did Not Induce MDSC Production in Urine
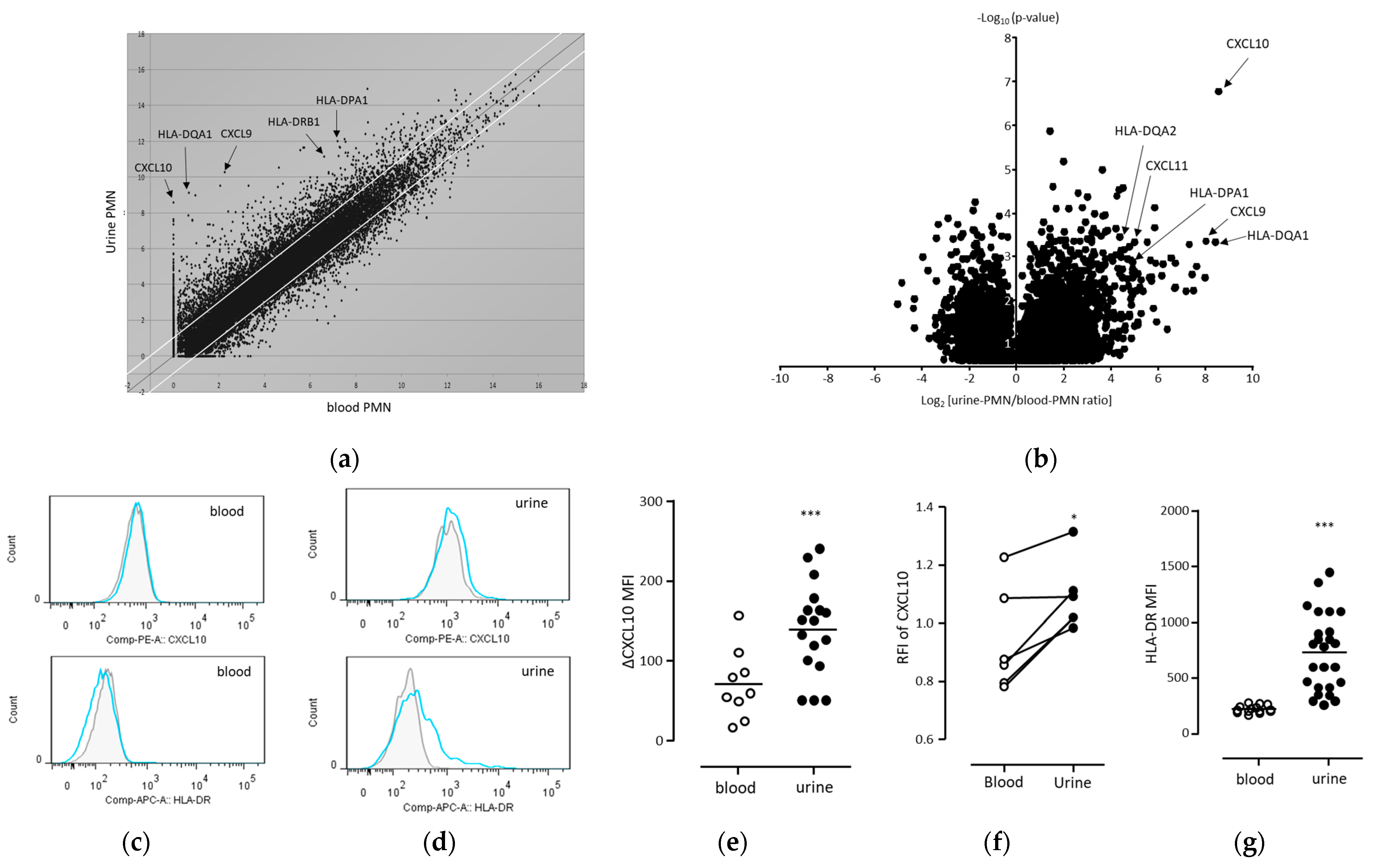
3.3. Intracellular Expression of CXCL10 and HLA-DR Was Enhanced in Human Urinary Neutrophils during BCG Treatment
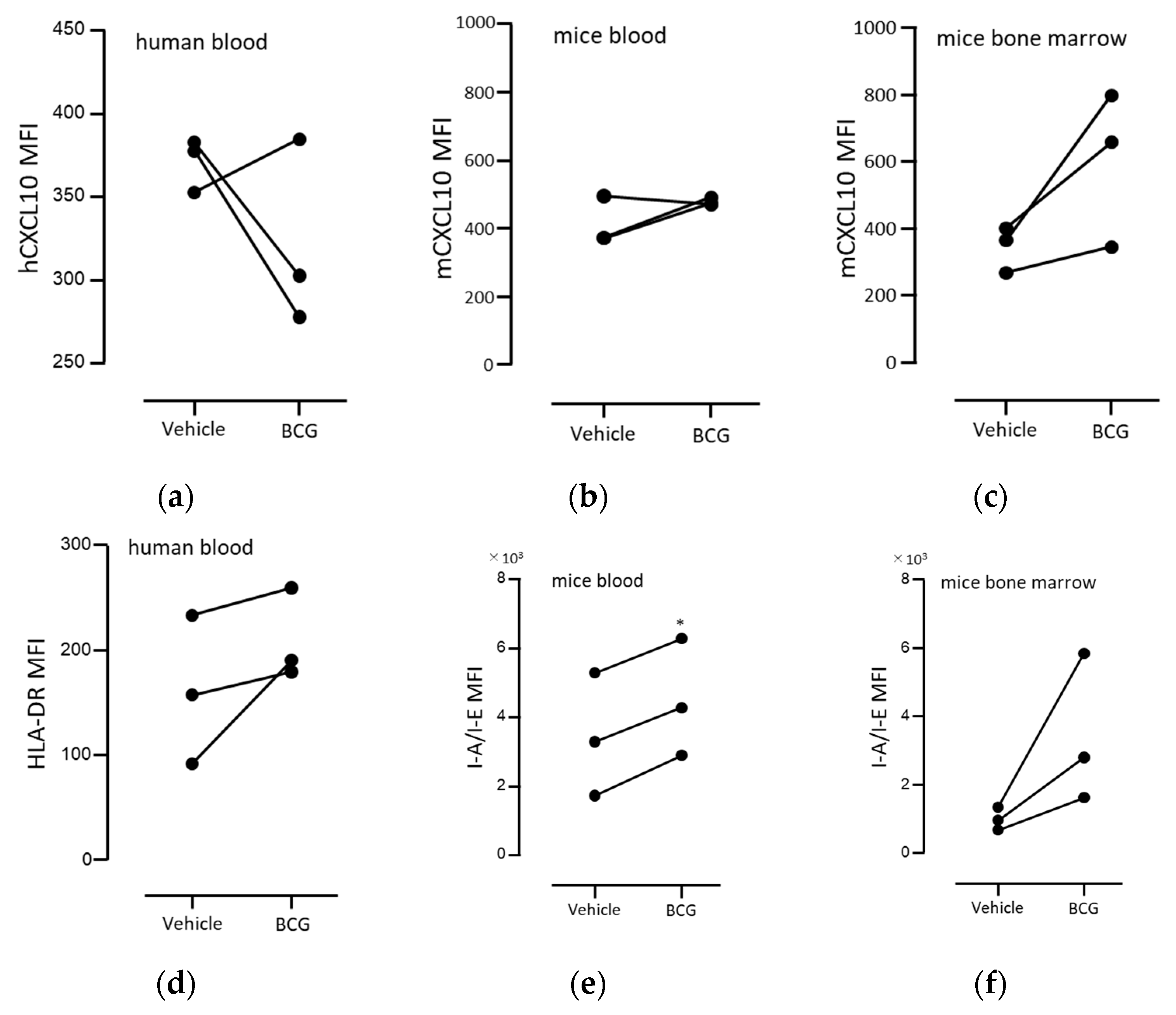
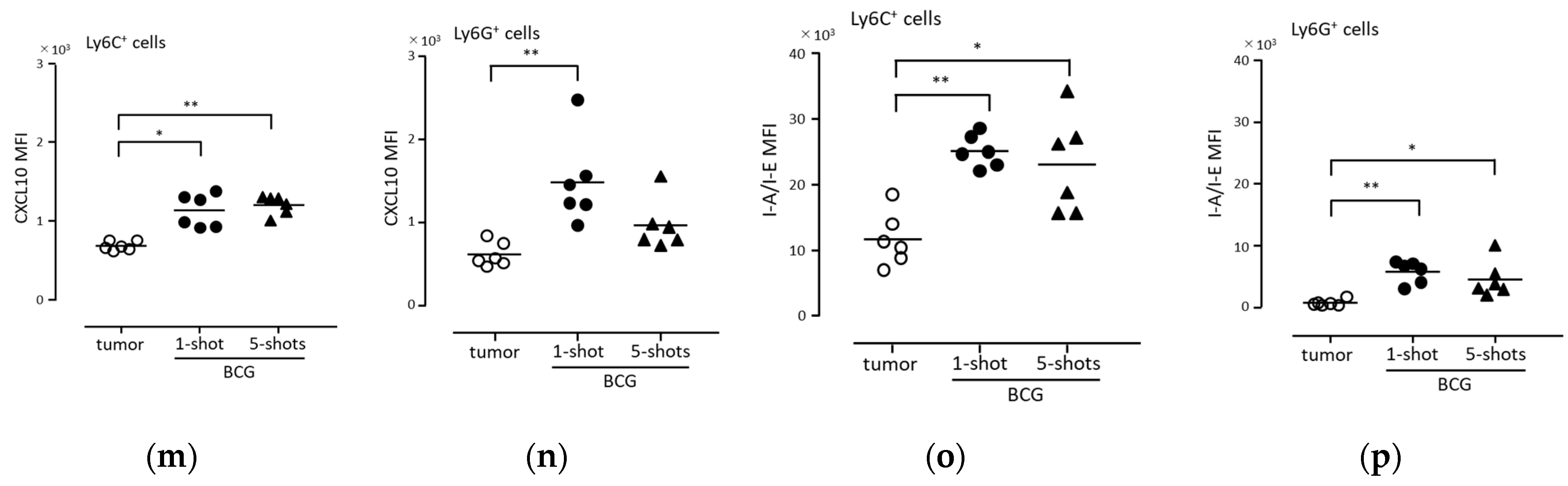
3.4. BCG Administration Induced the Intracellular Expression of CXCL10 and HLA-DR in Mouse Peritoneal Exudate Neutrophils
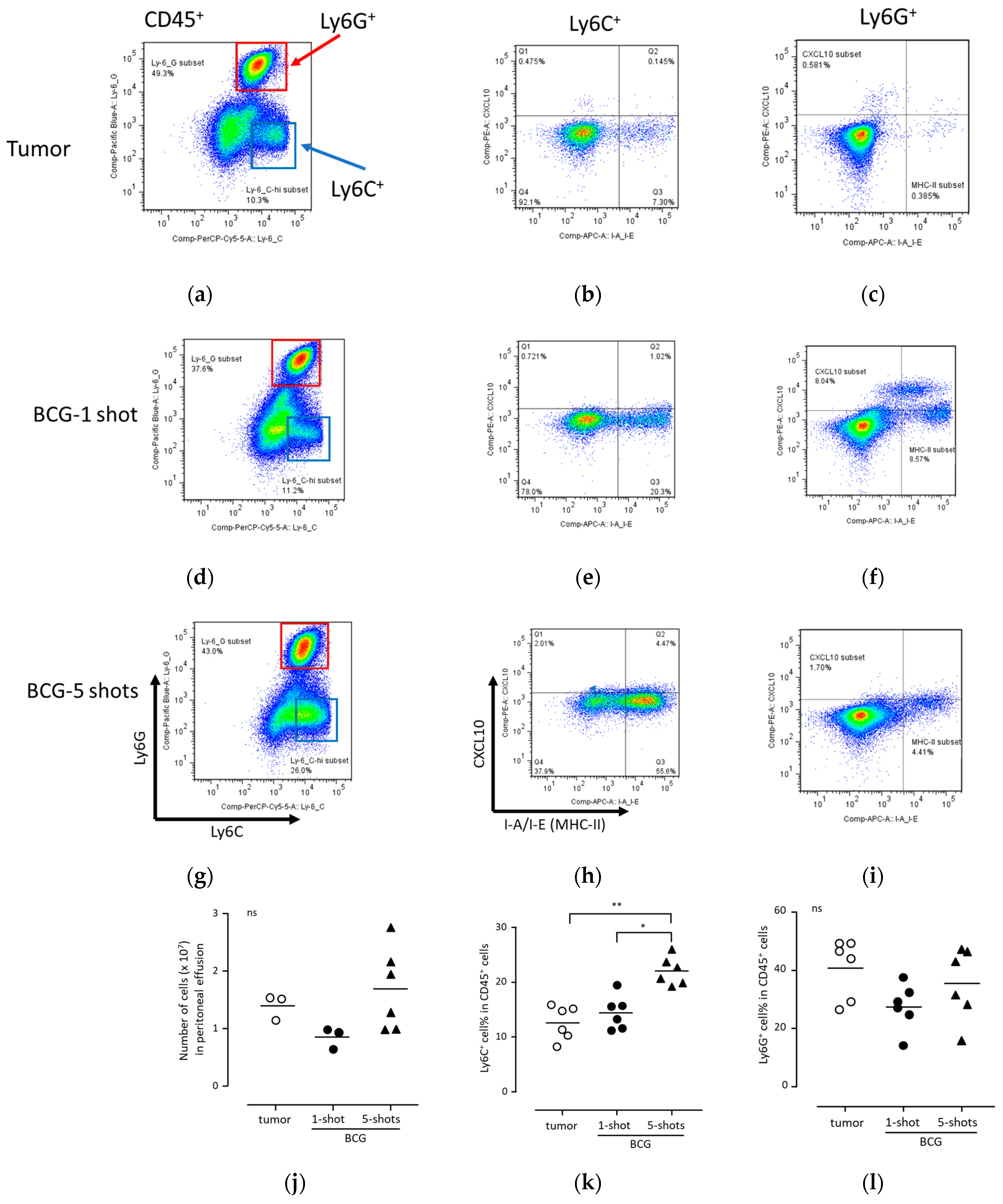
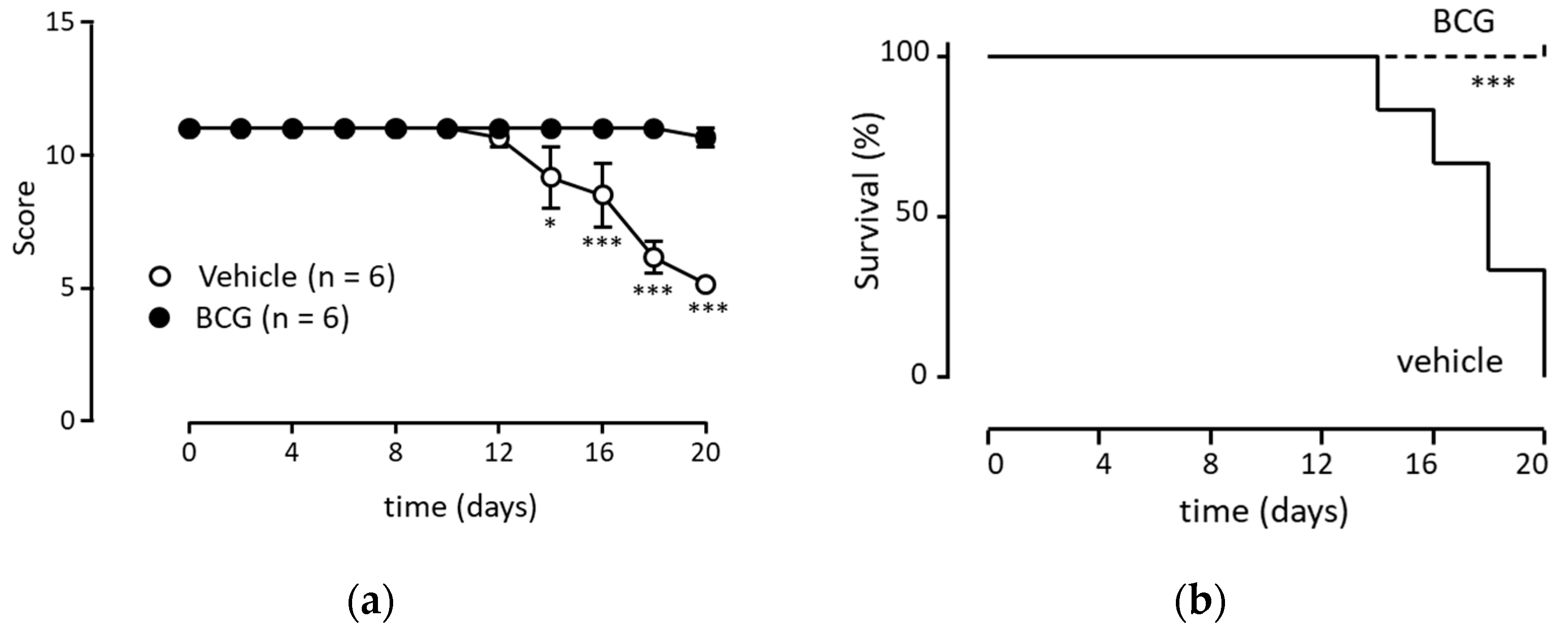
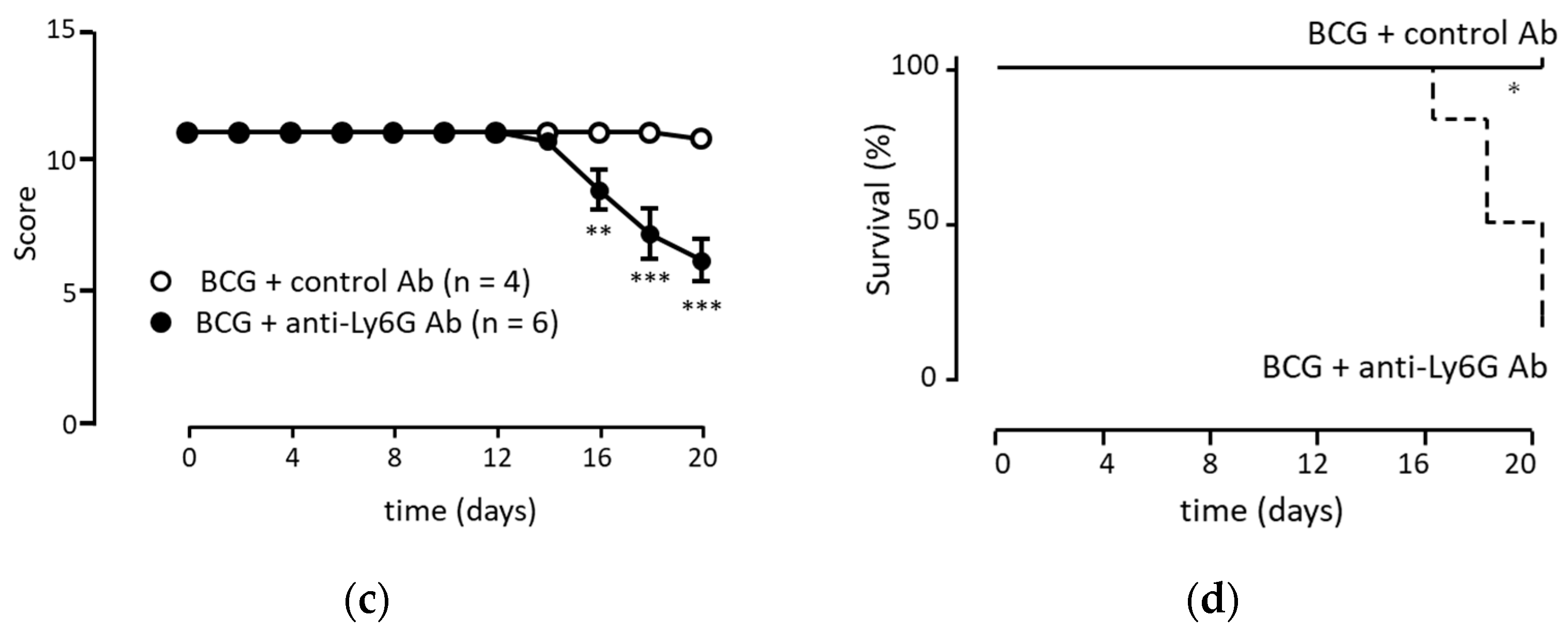
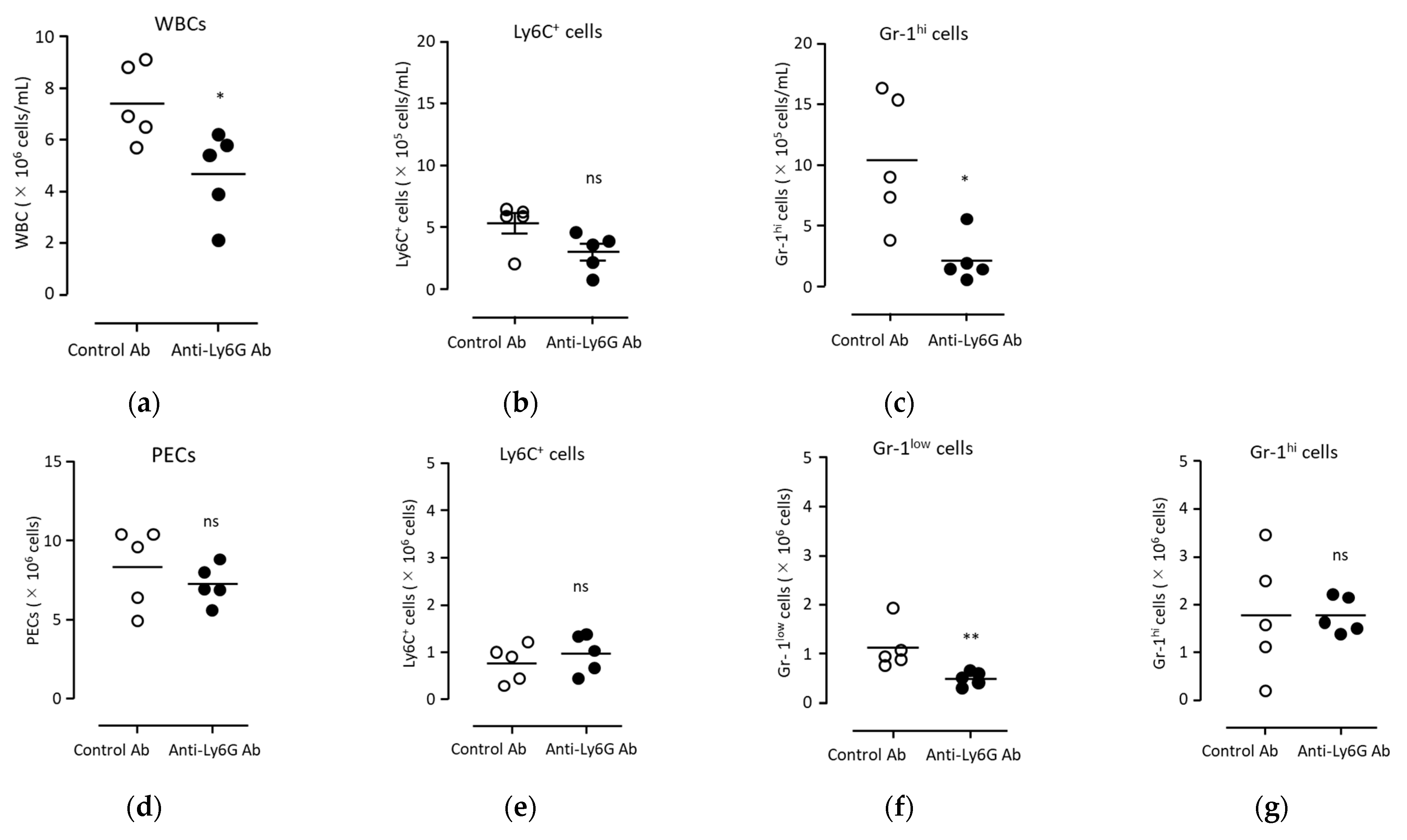
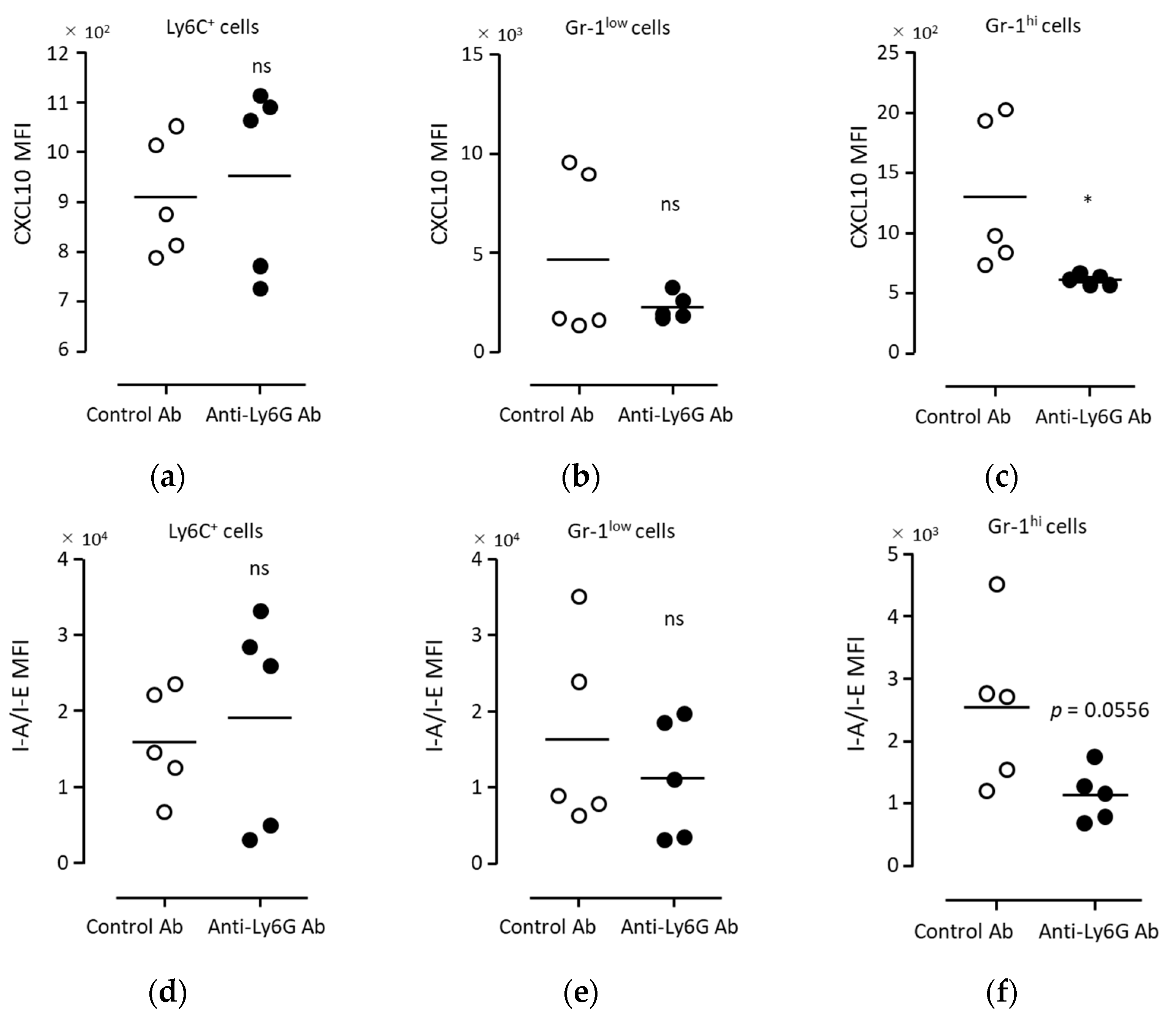
3.5. Anti-Tumor Immunity Was Elicited with Neutrophils Induced via BCG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2021, 3, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Kalafati, L.; Hatzioannou, A.; Hajishengallis, G.; Chavakis, T. The role of neutrophils in trained immunity. Immunol. Rev. 2022, 314, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Kostlin-Gille, N.; Gille, C. Myeloid-Derived Suppressor Cells in Pregnancy and the Neonatal Period. Front. Immunol. 2020, 11, 584712. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef] [PubMed]
- Ribechini, E.; Eckert, I.; Beilhack, A.; Du Plessis, N.; Walzl, G.; Schleicher, U.; Ritter, U.; Lutz, M.B. Heat-killed Mycobacterium tuberculosis prime-boost vaccination induces myeloid-derived suppressor cells with spleen dendritic cell-killing capability. JCI Insight 2019, 5, e128664. [Google Scholar] [CrossRef]
- Dorhoi, A.; Du Plessis, N. Monocytic Myeloid-Derived Suppressor Cells in Chronic Infections. Front. Immunol. 2017, 8, 1895. [Google Scholar] [CrossRef]
- Simons, M.P.; O’Donnell, M.A.; Griffith, T.S. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol. Oncol. 2008, 26, 341–345. [Google Scholar] [CrossRef]
- Liu, K.; Sun, E.; Lei, M.; Li, L.; Gao, J.; Nian, X.; Wang, L. BCG-induced formation of neutrophil extracellular traps play an important role in bladder cancer treatment. Clin. Immunol. 2019, 201, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Suttmann, H.; Riemensberger, J.; Bentien, G.; Schmaltz, D.; Stockle, M.; Jocham, D.; Bohle, A.; Brandau, S. Neutrophil granulocytes are required for effective Bacillus Calmette-Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res. 2006, 66, 8250–8257. [Google Scholar] [CrossRef] [PubMed]
- Khoyratty, T.E.; Ai, Z.; Ballesteros, I.; Eames, H.L.; Mathie, S.; Martin-Salamanca, S.; Wang, L.; Hemmings, A.; Willemsen, N.; von Werz, V.; et al. Distinct transcription factor networks control neutrophil-driven inflammation. Nat. Immunol. 2021, 22, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Sumagin, R. Emerging neutrophil plasticity: Terminally differentiated cells no more. J. Leukoc. Biol. 2021, 109, 473–475. [Google Scholar] [CrossRef]
- Mysore, V.; Cullere, X.; Mears, J.; Rosetti, F.; Okubo, K.; Liew, P.X.; Zhang, F.; Madera-Salcedo, I.; Rosenbauer, F.; Stone, R.M.; et al. FcgammaR engagement reprograms neutrophils into antigen cross-presenting cells that elicit acquired anti-tumor immunity. Nat. Commun. 2021, 12, 4791. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A.; Yao, Y. Neutrophil plasticity: Acquisition of phenotype and functionality of antigen-presenting cell. J. Leukoc. Biol. 2015, 98, 489–496. [Google Scholar] [CrossRef]
- Takeda, Y.; Kato, T.; Ito, H.; Kurota, Y.; Yamagishi, A.; Sakurai, T.; Araki, A.; Nara, H.; Tsuchiya, N.; Asao, H. The pattern of GPI-80 expression is a useful marker for unusual myeloid maturation in peripheral blood. Clin. Exp. Immunol. 2016, 186, 373–386. [Google Scholar] [CrossRef]
- Hegde, S.; Leader, A.M.; Merad, M. MDSC: Markers, development, states, and unaddressed complexity. Immunity 2021, 54, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Bruger, A.M.; Dorhoi, A.; Esendagli, G.; Barczyk-Kahlert, K.; van der Bruggen, P.; Lipoldova, M.; Perecko, T.; Santibanez, J.; Saraiva, M.; Van Ginderachter, J.A.; et al. How to measure the immunosuppressive activity of MDSC: Assays, problems and potential solutions. Cancer Immunol. Immunother. 2019, 68, 631–644. [Google Scholar] [CrossRef]
- Takeda, Y.; Kato, T.; Nemoto, N.; Araki, A.; Gazi, M.Y.; Nara, H.; Asao, H. Augmentation of the expression of the eotaxin receptor on duodenal neutrophils by IL-21. Cytokine 2018, 110, 194–203. [Google Scholar] [CrossRef]
- Takeda, Y.; Nara, H.; Araki, A.; Asao, H. Human peripheral neutrophils express functional IL-21 receptors. Inflammation 2014, 37, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Paster, E.V.; Villines, K.A.; Hickman, D.L. Endpoints for mouse abdominal tumor models: Refinement of current criteria. Comp. Med. 2009, 59, 234–241. [Google Scholar] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Castellano, E.; Samba, C.; Esteso, G.; Simpson, L.; Vendrame, E.; Garcia-Cuesta, E.M.; Lopez-Cobo, S.; Alvarez-Maestro, M.; Linares, A.; Leibar, A.; et al. CyTOF analysis identifies unusual immune cells in urine of BCG-treated bladder cancer patients. Front. Immunol. 2022, 13, 970931. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, H.; Takeda, Y.; Takei, H.; Shinzawa, H.; Takahashi, T.; Sendo, F. In vivo regulation of rat neutrophil apoptosis occurring spontaneously or induced with TNF-alpha or cycloheximide. J. Immunol. 1995, 154, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Segura, M.; Sicilia, J.; Ballesteros, I.; Hidalgo, A. Strategies of neutrophil diversification. Nat. Immunol. 2023, 24, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Suttmann, H.; Lehan, N.; Bohle, A.; Brandau, S. Stimulation of neutrophil granulocytes with Mycobacterium bovis bacillus Calmette-Guerin induces changes in phenotype and gene expression and inhibits spontaneous apoptosis. Infect. Immun. 2003, 71, 4647–4656. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Sun, E.L.; Wang, L.N.; Nian, X.W.; Ma, C.Q. Gene Expression in Human Polymorphonuclear Neutrophils (PMNs) Stimulated by Bacillus Calmette-Guerin (BCG). Inflammation 2020, 43, 2098–2108. [Google Scholar] [CrossRef]
- Markman, J.L.; Porritt, R.A.; Wakita, D.; Lane, M.E.; Martinon, D.; Noval Rivas, M.; Luu, M.; Posadas, E.M.; Crother, T.R.; Arditi, M. Loss of testosterone impairs anti-tumor neutrophil function. Nat. Commun. 2020, 11, 1613. [Google Scholar] [CrossRef]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016, 28, 119–128. [Google Scholar] [CrossRef]
- Ashiru, O.; Esteso, G.; Garcia-Cuesta, E.M.; Castellano, E.; Samba, C.; Escudero-Lopez, E.; Lopez-Cobo, S.; Alvarez-Maestro, M.; Linares, A.; Ho, M.M.; et al. BCG Therapy of Bladder Cancer Stimulates a Prolonged Release of the Chemoattractant CXCL10 (IP10) in Patient Urine. Cancers 2019, 11, 940. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Alvear-Arias, J.J.; Carrillo, C.; Villar, J.P.; Garcia-Betancourt, R.; Pena-Pichicoi, A.; Fernandez, A.; Fernandez, M.; Carmona, E.M.; Pupo, A.; Neely, A.; et al. Expression of H(v)1 proton channels in myeloid-derived suppressor cells (MDSC) and its potential role in T cell regulation. Proc. Natl. Acad. Sci. USA 2022, 119, e2104453119. [Google Scholar] [CrossRef] [PubMed]
- Nitto, T.; Inoue, T.; Node, K. Alternative spliced variants in the pantetheinase family of genes expressed in human neutrophils. Gene 2008, 426, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Wang, J.X.; Parisini, E.; Dascher, C.C.; Nigrovic, P.A. Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 2013, 94, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Bucher, K.; Schmitt, F.; Autenrieth, S.E.; Dillmann, I.; Nurnberg, B.; Schenke-Layland, K.; Beer-Hammer, S. Fluorescent Ly6G antibodies determine macrophage phagocytosis of neutrophils and alter the retrieval of neutrophils in mice. J. Leukoc. Biol. 2015, 98, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Stackowicz, J.; Jonsson, F.; Reber, L.L. Mouse Models and Tools for the in vivo Study of Neutrophils. Front. Immunol. 2019, 10, 3130. [Google Scholar] [CrossRef]
- Reber, L.L.; Gillis, C.M.; Starkl, P.; Jonsson, F.; Sibilano, R.; Marichal, T.; Gaudenzio, N.; Berard, M.; Rogalla, S.; Contag, C.H.; et al. Neutrophil myeloperoxidase diminishes the toxic effects and mortality induced by lipopolysaccharide. J. Exp. Med. 2017, 214, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, J.Z.; Orosz, A.; Zajta, E.; Kasa, O.; Nemeth, T.; Simon, E.; Fodor, S.; Csonka, K.; Baratki, B.L.; Kovesdi, D.; et al. Myeloid-Specific Deletion of Mcl-1 Yields Severely Neutropenic Mice That Survive and Breed in Homozygous Form. J. Immunol. 2018, 201, 3793–3803. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. Antibodies against neutrophil LY6G do not inhibit leukocyte recruitment in mice in vivo. Blood 2013, 121, 241–242. [Google Scholar] [CrossRef]

| Classification | Number |
|---|---|
| Age in years, median (range) | 67 (50–89) |
| Male | 10 |
| Female | 4 |
| pTis | 6 |
| pTa | 4 |
| pT1 | 4 |
| Intermediate 1 | 2 |
| High 1 | 11 |
| Very high 1 | 1 |
| Gene Symbol | Description | Log2 Ratio 1 | p-Value 2 |
|---|---|---|---|
| CXCL10 | C-X-C motif chemokine ligand 10 | 8.59 | 1.68 × 10−7 |
| HLA-DQα1 | Major histocompatibility complex, class II, DQ alpha 1 | 8.44 | 4.53 × 10−4 |
| CXCL9 | C-X-C motif chemokine ligand 9 | 8.04 | 4.48 × 10−4 |
| CXCL11 | C-X-C motif chemokine ligand 11 | 5.06 | 4.71 × 10−4 |
| HLA-DPβ1 | Major histocompatibility complex, class II, DP beta 1 | 4.96 | 1.34 × 10−3 |
| HLA-DPα1 | Major histocompatibility complex, class II, DP alpha 1 | 4.81 | 5.77 × 10−4 |
| HLA-DRβ1 | Major histocompatibility complex, class II, DR beta 1 | 4.50 | 3.02 × 10−3 |
| HLA-DQA2 | Major histocompatibility complex, class II, DQ alpha 2 | 4.40 | 3.50 × 10−4 |
| TNFSF10 (TRAIL) | Tumor necrosis factor superfamily member 10 | 1.04 | 3.49 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, Y.; Kato, T.; Sabrina, S.; Naito, S.; Ito, H.; Emi, N.; Kuboki, Y.; Takai, Y.; Fukuhara, H.; Ushijima, M.; et al. Intracellular Major Histocompatibility Complex Class II and C-X-C Motif Chemokine Ligand 10-Expressing Neutrophils Indicate the State of Anti-Tumor Activity Induced by Bacillus Calmette–Guérin. Biomedicines 2023, 11, 3062. https://doi.org/10.3390/biomedicines11113062
Takeda Y, Kato T, Sabrina S, Naito S, Ito H, Emi N, Kuboki Y, Takai Y, Fukuhara H, Ushijima M, et al. Intracellular Major Histocompatibility Complex Class II and C-X-C Motif Chemokine Ligand 10-Expressing Neutrophils Indicate the State of Anti-Tumor Activity Induced by Bacillus Calmette–Guérin. Biomedicines. 2023; 11(11):3062. https://doi.org/10.3390/biomedicines11113062
Chicago/Turabian StyleTakeda, Yuji, Tomoyuki Kato, Saima Sabrina, Sei Naito, Hiromi Ito, Naoto Emi, Yuya Kuboki, Yuki Takai, Hiroki Fukuhara, Masaki Ushijima, and et al. 2023. "Intracellular Major Histocompatibility Complex Class II and C-X-C Motif Chemokine Ligand 10-Expressing Neutrophils Indicate the State of Anti-Tumor Activity Induced by Bacillus Calmette–Guérin" Biomedicines 11, no. 11: 3062. https://doi.org/10.3390/biomedicines11113062
APA StyleTakeda, Y., Kato, T., Sabrina, S., Naito, S., Ito, H., Emi, N., Kuboki, Y., Takai, Y., Fukuhara, H., Ushijima, M., Narisawa, T., Yagi, M., Kanno, H., Sakurai, T., Nishida, H., Araki, A., Shimotai, Y., Nagashima, M., Nouchi, Y., ... Asao, H. (2023). Intracellular Major Histocompatibility Complex Class II and C-X-C Motif Chemokine Ligand 10-Expressing Neutrophils Indicate the State of Anti-Tumor Activity Induced by Bacillus Calmette–Guérin. Biomedicines, 11(11), 3062. https://doi.org/10.3390/biomedicines11113062






