A Prospective Study on the Roles of the Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet-to-Lymphocyte Ratio (PLR) in Patients with Locally Advanced Rectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Histopathological Analysis
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
3.1. Reproducibility
3.2. Correlation with Clinicopathological Characteristics
3.3. Prognostic Value
3.4. Lymphocyte-to-Monocyte Ratio
3.5. Neutrophil-to-Lymphocyte Ratio
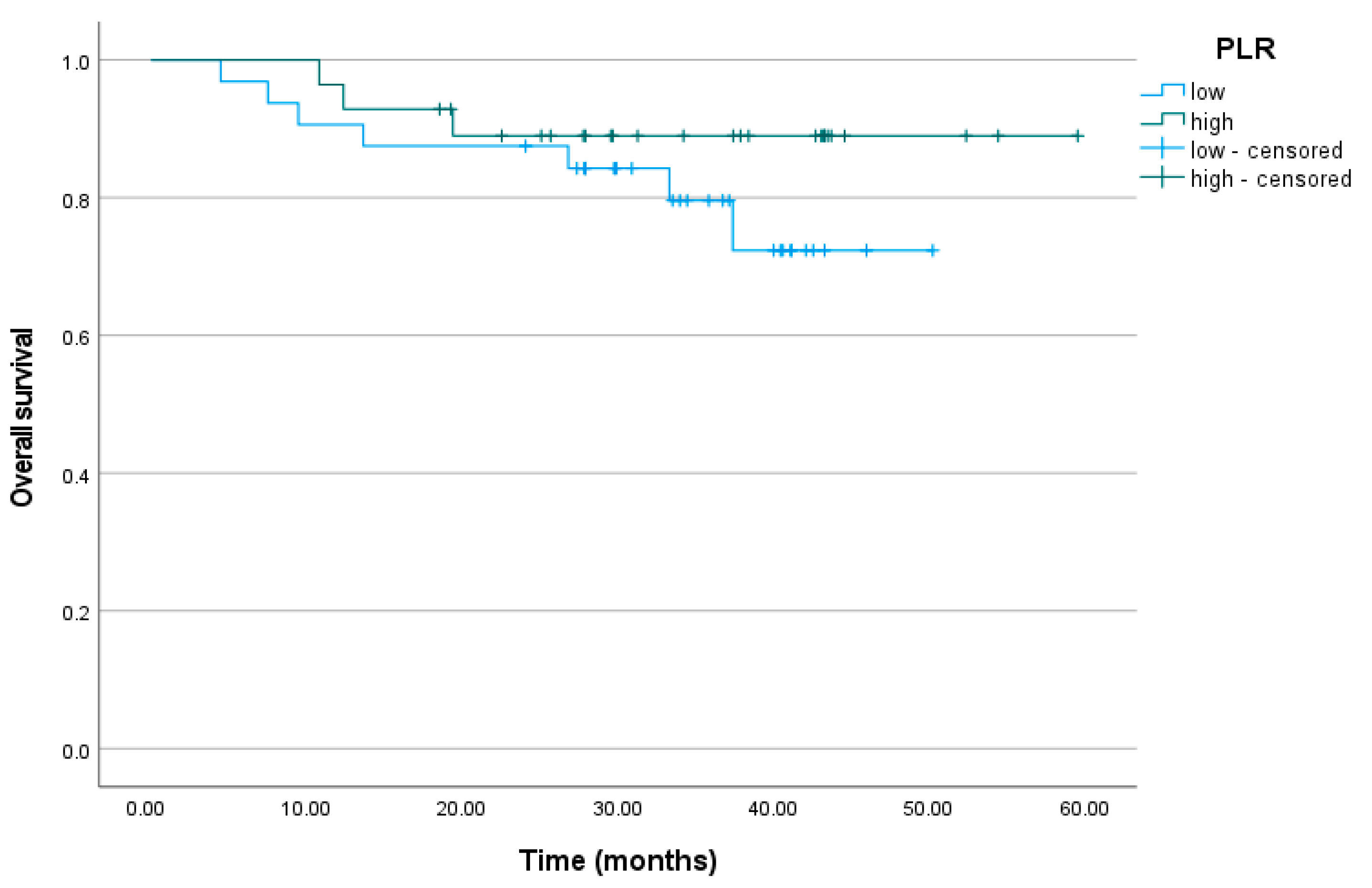
3.6. Platelet-to-Lymphocyte Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| X | First Measurement | Second Measurement | Third Measurement | All Measurements (Mean) | p-Value 1 |
|---|---|---|---|---|---|
| ALC (109/L), median (range) | 1.72 (0.70–3.79) | 1.65 (0.69–4.02) | 1.67 (0.52–3.92) | 1.67 (0.52–4.02) | 0.541 |
| ALC (109/L), mean (SD) | 1.84 (0.69) | 1.87 (0.76) | 1.86 (0.73) | 1.86 (0.69) | |
| AMC (109/L), median (range) | 0.61 (0.30–1.30) | 0.64 (0.27–5.26) | 0.64 (0.33–1.21) | 0.63 (0.27–5.26) | 0.800 |
| AMC (109/L), mean (SD) | 0.68 (0.25) | 0.77 (0.65) | 0.68 (0.24) | 0.71 (0.30) | |
| ANC (109/L), median (range) | 4.89 (2.42–12.36) | 5.10 (2.11–12.27) | 4.43 (2.35–13.69) | 4.84 (2.11–13.69) | 0.770 |
| ANC (109/L), mean (SD) | 5.43 (2.05) | 5.26 (2.00) | 5.02 (2.03) | 5.24 (1.91) | |
| Platelets (109/L), median (range) | 273.00 (116.00–666.00) | 254.00 (149.00–607.00) | 269.00 (152.00–601.00) | 264.00 (116.00–666.00) | 0.198 |
| Platelets (109/L), mean (SD) | 293.00 (109.60) | 284.00 (94.12) | 291.00 (101.82) | 289.00 (99.16) | |
| LMR, median (range) | 2.71 (1.11–6.42) | 2.95 (0.24–7.32) | 2.8 (0.87–6.98) | 2.89 (0.24–7.32) | 0.766 |
| LMR, mean (SD) | 2.94 (1.22) | 2.93 (1.25) | 2.91 (1.18) | 2.93 (1.11) | |
| NLR, median (range) | 2.71 (1.17–7.58) | 2.84 (0.81–8.96) | 2.47 (1.04–10.22) | 2.65 (0.81–10.22) | 0.344 |
| NLR, mean (SD) | 3.25 (1.47) | 3.13 (1.56) | 3.10 (1.80) | 3.16 (1.47) | |
| PLR, median (range) | 150.00 (67.00–551.00) | 141.00 (54.00–479.00) | 141.00 (67.00–430.00) | 142.00 (54.00–551.00) | 0.627 |
| PLR, mean (SD) | 179.00 (95.69) | 173.00 (91.15) | 180.00 (95.01) | 177.00 (87.90) |

| Measurement | p 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | Second vs. First | Third vs. First | Third vs. Second | ||||
| n (%) | CI95 for % 2 | n (%) | CI95 for % 2 | n (%) | CI95 for % 2 | ||||
| LMR | |||||||||
| LMR high (>2.6) | 34 (56.7) | 43.2–69.4 | 35 (58.3) | 44.5–70.9 | 33 (55.0) | 41.6–67.9 | >0.999 | 0.791 | 0.607 |
| LMR low (≤2.6) | 26 (43.3) | 30.6–56.8 | 25 (41.7) | 29.1–55.1 | 27 (45.0) | 32.1–58.4 | |||
| “True LMR-high/LMR-low”, the patients who remained in the same group (LMR high or LMR low) after the second/third measurements | x | X | 49 (81.7) | 69.6–90.5 | 41 (68.3) | 55.0–79.7 | x | x | 0.804 |
| NLR | |||||||||
| NLR high (≥3.0) | 23 (38.3) | 26.1–51.8 | 27 (45.0) | 32.1–58.4 | 21 (35.0) | 23.1–48.4 | 0.455 | 0.754 | 0.146 |
| NLR low (<3.0) | 37 (61.7) | 48.2–73.9 | 33 (55.0) | 41.6–67.9 | 39 (65.0) | 51.6–76.9 | |||
| “True NLR-high/NLR-low”, the patients who remained in the same group (NLR high or NLR low) after the second/third measurements | x | X | 44 (73.3) | 60.3–83.9 | 41 (68.3) | 55.0–79.7 | x | x | 0.146 |
| PLR | |||||||||
| PLR high (≥150) | 29 (48.3) | 35.2–61.6 | 24 (40.0) | 27.6–53.5 | 26 (43.3) | 30.6–56.8 | 0.180 | 0.581 | 0.581 |
| PLR low (<150) | 31 (51.7) | 38.4–64.8 | 36 (60.0) | 46.5–72.4 | 34 (56.7) | 43.2–69.4 | |||
| “True PLR-high/PLR-low”, the patients who remained in the same group (PLR high or PLR low) after the second/third measurements | x | X | 47 (78.3) | 65.8–87.9 | 42 (70.0) | 56.8–81.2 | x | x | >0.999 |
| CEA | ||
|---|---|---|
| r | p-Value | |
| LMR avg * | −0.01 | 0.964 |
| NLR avg * | 0.19 | 0.183 |
| PLR avg * | 0.22 | 0.108 |
| CD8+ | Inflammatory Infiltrates | |||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| CPS | 0.56 | 0.002 | 0.51 | 0.005 |
References
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D.; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv22–iv40. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. JNCI J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Sargent, D.J.; E Tepper, J.; O’connell, M.J.; Allmer, C.; Smalley, S.R.; A Martenson, J.; Haller, D.G.; Mayer, R.J.; A Rich, T.; et al. Impact of T and N substage on survival and disease relapse in adjuvant rectal cancer: A pooled analysis. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 386–396. [Google Scholar] [CrossRef]
- Balch, G.C.; De Meo, A.; Guillem, J.G. Modern management of rectal cancer: A 2006 update. World J. Gastroenterol. 2006, 12, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.-R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.-K.; Putter, H.; Wiggers, T.; Rutten, H.J.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Engelen, S.; Maas, M.; Lahaye, M.; Leijtens, J.; van Berlo, C.; Jansen, R.; Breukink, S.; Dejong, C.; van de Velde, C.; Beets-Tan, R.; et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur. J. Cancer 2013, 49, 2311–2320. [Google Scholar] [CrossRef]
- Kripp, M.; Wieneke, J.; Kienle, P.; Welzel, G.; Brade, J.; Horisberger, K.; Wenz, F.; Post, S.; Gencer, D.; Hofmann, W.K.; et al. Intensified neoadjuvant chemoradiotherapy in locally advanced rectal cancer—Impact on long-term quality of life. Eur. J. Surg. Oncol. 2012, 38, 472–477. [Google Scholar] [CrossRef]
- Song, W.; Wang, K.; Zhang, R.-j.; Zou, S.-b. Prognostic value of the lymphocyte monocyte ratio in patients with colorectal cancer: A meta-analysis. Medicine 2016, 95, e5540. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, Y.; Zheng, F. Prognostic significance of elevated preoperative neutrophil-to-lymphocyte ratio for patients with colorectal cancer undergoing curative surgery: A meta-analysis. Medicine 2019, 98, e14126. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, W.; Huang, K.; Yang, W.; Huang, L.; Cong, T.; Li, Q.; Qiu, M. Increased platelet-lymphocyte ratio closely relates to inferior clinical features and worse long-term survival in both resected and metastatic colorectal cancer: An updated systematic review and meta-analysis of 24 studies. Oncotarget 2017, 8, 32356–32369. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, A.; Dymicka-Piekarska, V. Fashionable, but What is Their Real Clinical Usefulness? NLR, LMR, and PLR as a Promising Indicator in Colorectal Cancer Prognosis: A Systematic Review. J. Inflamm. Res. 2023, 16, 69–81. [Google Scholar] [PubMed]
- Wu, Q.-B.; Wang, M.; Hu, T.; He, W.-B.; Wang, Z.-Q. Prognostic role of the lymphocyte-to-monocyte ratio in patients undergoing resection for nonmetastatic rectal cancer. Medicine 2016, 95, e4945. [Google Scholar] [CrossRef]
- Dudani, S.; Marginean, H.; Tang, P.A.; Monzon, J.G.; Raissouni, S.; Asmis, T.R.; Goodwin, R.A.; Gotfrit, J.; Cheung, W.Y.; Vickers, M.M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer 2019, 19, 664. [Google Scholar] [CrossRef]
- Zhou, X.; Du, Y.; Huang, Z.; Xu, J.; Qiu, T.; Wang, J.; Wang, T.; Zhu, W.; Liu, P. Prognostic value of PLR in various cancers: A meta-analysis. PLoS ONE 2014, 9, e101119. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef]
- Winiarek, M.; Rybski, S.; Spalek, M.; Krynski, J.; Zajac, L.; Kosakowska, E.; Zwolinski, J.; Rutkowski, A.; Michalski, W.; Bujko, K.; et al. Lymphocyte-to-monocyte ratio (LMR) is prognostic factor for selection of neoadjuvant treatment in locally advanced rectal cancer patients: Sub-set analysis of Polish-2 study. Ann. Oncol. 2017, 28, iii124–iii125. [Google Scholar] [CrossRef]
- Gawiński, C.; Winiarek, M.; Rybski, S.; Temnyk, M.; Kokoszyńska, K.; Wyrwicz, L. Reproducibility of pretreatment lymphocyte-to-monocyte ratio (LMR) in rectal cancer. J. Clin. Oncol. 2018, 36 (Suppl. S4), 715. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Shen, S.; Hou, Y. Inflammation and cancer: Paradoxical roles in tumorigenesis and implications in immunotherapies. Genes Dis. 2023, 10, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Q.; Ma, C.; Cao, W.Z.; Ning, Z.; Tan, G. Prognostic Significance of NLR, PLR, LMR and Tumor Infiltrating T Lymphocytes in Patients Undergoing Surgical Resection for Hilar Cholangiocarcinoma. Front. Oncol. 2022, 12, 908907. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Wang, Y.; Zhou, Y.; Quan, Q.; Zhang, Y.; Wang, H.; Zhang, B.; Xia, L. Immune cell concentrations among the primary tumor microenvironment in colorectal cancer patients predicted by clinicopathologic characteristics and blood indexes. J. Immunother. Cancer 2019, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, L.; Elsaka, A.; Zamzam, Y. Local and Systemic Inflammatory Markers as Prognostic and Predictive Markers In Locally Advanced Triple Negative Breast Cancer. Tumori J. 2020, 106 (Suppl. S1), 30. [Google Scholar] [CrossRef]
- Xiao, B.; Peng, J.; Zhang, R.; Xu, J.; Wang, Y.; Fang, Y.; Lin, J.; Pan, Z.; Wu, X. Density of CD8+ lymphocytes in biopsy samples combined with the circulating lymphocyte ratio predicts pathologic complete response to chemoradiotherapy for rectal cancer. Cancer Manag. Res. 2017, 9, 701–708. [Google Scholar] [CrossRef]
- Gawiński, C.; Michalski, W.; Mróz, A.; Wyrwicz, L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Tumor-Infiltrating Lymphocytes (TILs) in Left-Sided Colorectal Cancer Patients. Biology 2022, 11, 385. [Google Scholar] [CrossRef]
- Tang, Y.; Li, G.; Wu, S.; Tang, L.; Zhang, N.; Liu, J.; Zhang, S.; Yao, L. Programmed death ligand 1 expression in esophageal cancer following definitive chemoradiotherapy: Prognostic significance and association with inflammatory biomarkers. Oncol. Lett. 2018, 15, 4988–4996. [Google Scholar] [CrossRef]
- Akamine, T.; Takada, K.; Toyokawa, G.; Kinoshita, F.; Matsubara, T.; Kozuma, Y.; Haratake, N.; Takamori, S.; Hirai, F.; Tagawa, T.; et al. Association of preoperative serum CRP with PD-L1 expression in 508 patients with non-small cell lung cancer: A comprehensive analysis of systemic inflammatory markers. Surg. Oncol. 2018, 27, 88–94. [Google Scholar] [CrossRef]
- Gawiński, C.; Hołdakowska, A.; Wyrwicz, L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Extramural Vascular Invasion (EMVI) in Locally Advanced Rectal Cancer. Curr. Oncol. 2022, 30, 545–558. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wang, Y.; Luo, G.-Y.; Han, F.; Li, Y.-Q.; Zhou, Z.-G.; Xu, G.-L. Relationship Between PD-L1 Expression and CD8+ T-cell Immune Responses in Hepatocellular Carcinoma. J. Immunother. 2017, 40, 323–333. [Google Scholar] [CrossRef]
- Deng, M.; Li, S.-H.; Fu, X.; Yan, X.-P.; Chen, J.; Qiu, Y.-D.; Guo, R.-P. Relationship between PD-L1 expression, CD8+ T-cell infiltration and prognosis in intrahepatic cholangiocarcinoma patients. Cancer Cell Int. 2021, 21, 371. [Google Scholar] [CrossRef] [PubMed]
- Sudoyo, A.W.; Kurniawan, A.N.; Kusumo, G.D.; Putra, T.P.; A Rexana, F.; Yunus, M.; Budiyati, A.D.; Kurniawan, D.; Utama, A.; Utomo, A.R. Increased CD8 Tumor Infiltrating Lymphocytes in Colorectal Cancer Microenvironment Supports an Adaptive Immune Resistance Mechanism of PD-L1 Expression. Asian Pac. J. Cancer Prev. 2019, 20, 3421–3427. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chang, Q.; Liu, Y.; Chen, L.; Wei, G.; Yang, J.; Zheng, P.; He, F.; Wang, W.; Ming, L. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: A posteriori and big-data-based. J. Clin. Lab. Anal. 2018, 32, e22228. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.; Camacho-Rivera, M.; Taioli, E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS ONE 2014, 9, e112361. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, N.Y.; Na, S.H.; Youn, Y.H.; Shin, C.S. Reference values of neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018, 97, e11138. [Google Scholar] [CrossRef]
- Green, J.; Bin Mahmood, S.U.; Mori, M.; Yousef, S.; Mangi, A.A.; Geirsson, A. Stability across time of the neutrophil-lymphocyte and lymphocyte-neutrophil ratios and associations with outcomes in cardiac surgery patients. J. Cardiothorac. Surg. 2019, 14, 164. [Google Scholar] [CrossRef]
- Stotz, M.; Pichler, M.; Absenger, G.; Szkandera, J.; Arminger, F.; Schaberl-Moser, R.; Samonigg, H.; Stojakovic, T.; Gerger, A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br. J. Cancer 2014, 110, 435–440. [Google Scholar] [CrossRef]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sakurai, K.; Yamazoe, S.; Kimura, K.; Toyokawa, T.; Amano, R.; Tanaka, H.; et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 9966–9973. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, H.; Liang, L.; Li, G.; Fan, M.; Wu, Y.; Zhu, J.; Zhang, Z. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat. Oncol. 2014, 9, 295. [Google Scholar] [CrossRef]
- Kim, T.G.; Park, W.; Kim, H.; Choi, D.H.; Park, H.C.; Kim, S.-H.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y.; et al. Baseline neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in rectal cancer patients following neoadjuvant chemoradiotherapy. Tumori J. 2018, 105, 434–440. [Google Scholar] [CrossRef]
- Ishikawa, D.; Nishi, M.; Takasu, C.; Kashihara, H.; Tokunaga, T.; Higashijima, J.; Yoshikawa, K.; Shimada, M. The Role of Neutrophil-to-lymphocyte Ratio on the Effect of CRT for Patients With Rectal Cancer. Vivo 2020, 34, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Portale, G.; Cavallin, F.; Valdegamberi, A.; Frigo, F.; Fiscon, V. Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio Are Not Prognostic Biomarkers in Rectal Cancer Patients with Curative Resection. J. Gastrointest. Surg. 2018, 22, 1611–1618. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Seruga, B.; Ocana, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; Mcnamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Rashtak, S.; Ruan, X.; Druliner, B.R.; Liu, H.; Therneau, T.; Mouchli, M.; Boardman, L.A. Peripheral Neutrophil to Lymphocyte Ratio Improves Prognostication in Colon Cancer. Clin. Color. Cancer 2017, 16, 115–123.e3. [Google Scholar] [CrossRef]
- Virchow, R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr. Rev. 1989, 47, 23–25. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Wang, L.; Zhang, C. Interplay between inflammatory tumor microenvironment and cancer stem cells. Oncol. Lett. 2018, 16, 679–686. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Antonio, N.; Bønnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P.M. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef] [PubMed]
- Tazzyman, S.; Lewis, C.E.; Murdoch, C. Neutrophils: Key mediators of tumour angiogenesis. Int. J. Exp. Pathol. 2009, 90, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, W. Neutrophils diminish T-cell immunity to foster gastric cancer progression: The role of GM-CSF/PD-L1/PD-1 signalling pathway. Gut 2017, 66, 1878–1880. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef]
- Lin, R.J.; Afshar-Kharghan, V.; Schafer, A.I. Paraneoplastic thrombocytosis: The secrets of tumor self-promotion. Blood 2014, 124, 184–187. [Google Scholar] [CrossRef] [PubMed]






| All Patients (n = 60) | |
|---|---|
| Age (years), median (range) | 66.5 (29–89) |
| Sex, n (%) | |
| Male | 43 (71.7) |
| Female | 17 (28.3) |
| BMI, n (%) | - |
| <18.5 | 1 (2) |
| 18.5–25 | 19 (32) |
| 25–30 | 23 (38) |
| ≥30 | 17 (28) |
| Smokers, n (%) | 15 (25) |
| Non-smokers, n (%) | 45 (75) |
| CEA (ng/mL), median (range) | 21.89 (0.86–69.96) |
| Normal level (<5.0 ng/mL), n (%) | 28 (47) |
| Elevated level (≥5.0 ng/mL), n (%) | 32 (53) |
| Tumor, n (%) | |
| T3 | 55 (91.7) |
| T4 | 5 (8.3) |
| Lymph nodes, n (%) | |
| N0 | 8 (13.3) |
| N1 | 35 (58.3) |
| N2 | 16 (26.7) |
| Nx | 1 (1.7) |
| Grade, n (%) | |
| G1 | 2 (3.3) |
| G2 | 42 (70) |
| G3 | 2 (3.3) |
| Gx | 14 (23.3) |
| Stage, n (%) | |
| II–IIIA | 8 (13.3) |
| IIIB | 41 (68.3) |
| IIIC | 10 (16.7) |
| Tumor localization, n (%) | |
| Low rectum | 28 (47) |
| Middle rectum | 24 (40) |
| High rectum | 8 (13) |
| Time between measurements (days), median (range) | |
| 1st–2nd | 9 (1–42) |
| 2nd–3rd | 11 (1–34) |
| 1st–3rd | 21 (7–55) |
| % Change | n | Mean | Standard Deviation | Standard Error | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| L | 60 | 4.76 | 30.23 | 3.9 | 0.75 | −60.61 | 92.86 |
| M | 60 | 3.88 | 24.39 | 3.1 | 4.78 | −40.00 | 85.71 |
| N | 60 | −5.59 | 20.57 | 2.7 | −8.20 | −47.70 | 43.66 |
| WBC | 60 | −2.39 | 17.28 | 2.2 | −3.86 | −39.78 | 42.12 |
| PLT | 60 | 1.29 | 15.30 | 2.0 | −0.70 | −29.32 | 44.60 |
| LMR Avg * | NLR Avg * | PLR Avg * | ||||
|---|---|---|---|---|---|---|
| Median (Range) | p-Value | Median (Range) | p-Value | Median (Range) | p-Value | |
| T | ||||||
| T3 | 2.94 (1.12–6.91) | 0.470 | 2.71 (1.01–6.90) | 0.336 | 142.46 (62.70–452.56) | 0.377 |
| T4 | 2.49 (1.19–3.84) | 3.10 (2.07–8.92) | 185.88 (97.58–390.89) | |||
| N | ||||||
| 0 | 3.52 (1.50–5.34) | 0.714 | 2.27 (2.06–4.07) | 0.457 | 116.35 (89.14–145.30) | 0.033 (0 vs. 1 and 2) |
| 1 | 2.91 (1.14–6.91) | 2.91 (1.01–8.92) | 147.27 (62.70–452.56) | |||
| 2 | 147.27 (62.70–452.56) | 2.68 (1.94–5.44) | 164.41 (93.47–321.83) | |||
| CR | ||||||
| No pCR | 3.02 (1.12–6.91) | 0.867 | 2.71 (1.01–6.90) | 0.796 | 136.65 (62.70–392.60) | 0.309 |
| pCR | 2.85 (1.80–5.23) | 2.56 (1.91–6.39) | 153.55 (93.47–452.56) | |||
| Progression/inoperability | ||||||
| No | 2.86 (1.12–6.91) | 0.990 | 2.71 (1.01–8.92) | 0.805 | 144.43 (62.70–452.56) | 0.931 |
| Yes | 2.98 (1.14–3.86) | 2.72 (1.76–6.15) | 149.28 (95.91–349.34) | |||
| CPS | CD8+ | Inflammatory Infiltrate | ||||
|---|---|---|---|---|---|---|
| R | p Value | R | p Value | r | p Value | |
| LMR avg * | 0.45 | 0.016 | 0.21 | 0.266 | 0.38 | 0.044 |
| NLR avg * | −0.19 | 0.316 | −0.08 | 0.691 | −0.15 | 0.447 |
| PLR avg * | 0.16 | 0.401 | 0.06 | 0.744 | 0.09 | 0.626 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawiński, C.; Mróz, A.; Roszkowska-Purska, K.; Sosnowska, I.; Derezińska-Wołek, E.; Michalski, W.; Wyrwicz, L. A Prospective Study on the Roles of the Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet-to-Lymphocyte Ratio (PLR) in Patients with Locally Advanced Rectal Cancer. Biomedicines 2023, 11, 3048. https://doi.org/10.3390/biomedicines11113048
Gawiński C, Mróz A, Roszkowska-Purska K, Sosnowska I, Derezińska-Wołek E, Michalski W, Wyrwicz L. A Prospective Study on the Roles of the Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet-to-Lymphocyte Ratio (PLR) in Patients with Locally Advanced Rectal Cancer. Biomedicines. 2023; 11(11):3048. https://doi.org/10.3390/biomedicines11113048
Chicago/Turabian StyleGawiński, Cieszymierz, Andrzej Mróz, Katarzyna Roszkowska-Purska, Iwona Sosnowska, Edyta Derezińska-Wołek, Wojciech Michalski, and Lucjan Wyrwicz. 2023. "A Prospective Study on the Roles of the Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet-to-Lymphocyte Ratio (PLR) in Patients with Locally Advanced Rectal Cancer" Biomedicines 11, no. 11: 3048. https://doi.org/10.3390/biomedicines11113048
APA StyleGawiński, C., Mróz, A., Roszkowska-Purska, K., Sosnowska, I., Derezińska-Wołek, E., Michalski, W., & Wyrwicz, L. (2023). A Prospective Study on the Roles of the Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet-to-Lymphocyte Ratio (PLR) in Patients with Locally Advanced Rectal Cancer. Biomedicines, 11(11), 3048. https://doi.org/10.3390/biomedicines11113048





