Multifaceted Tissue-Protective Functions of Polyvalent Immunoglobulin Preparations in Severe Infections—Interactions with Neutrophils, Complement, and Coagulation Pathways
Abstract
:1. Introduction
2. Endogenous Immunoglobulin Levels in Severe Infections
Polyvalent Immunoglobulins and Pathogen-Induced Tissue Damage
3. Polyvalent Immunoglobulins and Inflammation-Induced Tissue Damage
3.1. The Role of Immunoglobulins in the Modulation of Cytokine-Induced Inflammation
3.2. The Role of Immunoglobulins in the Modulation of Complement-Induced Inflammation
3.3. The Role of Natural IgM in Modulation of DAMP-Induced Inflammation
4. Indirect Tissue-Protective Functions of Polyvalent Immunoglobulins
4.1. Alveolar Damage in Severe Lung Infection
4.1.1. The Role of Neutrophils and NETs
4.1.2. The Role of Immunoglobulins
4.1.3. Nonclinical Studies Investigating the Supporting Role of Polyvalent Immunoglobulin in Alveolar Protection
4.1.4. Clinical Studies Investigating the Supporting Role of Polyvalent Immunoglobulins in Alveolar Protection
Studies with IVIg in sCAP
Studies with IgM/IgA-Enriched Ig Preparations in sCAP
Studies with IVIg Preparations in Sepsis
Studies with IgM/IgA-Enriched Ig Preparations in Sepsis
| REF | Indication | Ig Preparation (Dose) | N | Effect | Significant Difference | Marked Change | No Difference | Study Results |
|---|---|---|---|---|---|---|---|---|
| [140] | SARS-CoV | IgM/IgA-enriched Ig preparation (5 mL/kg/day for 3 consecutive days) | 12 | Lung scores | X | Treatment with Ig improved recovery from SARS-CoV infection, despite deterioration after initial steroid and ribavirin therapy. Scores derived from chest radiographs were based on the percentage of area involved, manifested as ground glass opacification, consolidation, or nodular shadow in each lung. Compared with Day 1, lung scores significantly improved on Days 5, 6, and 7 (p < 0.05) and oxygen requirement significantly improved on Days 6 and 7 (p < 0.05) after Ig treatment. | ||
| [142] | Severe and critical COVID-19 | IgM/IgA-enriched Ig preparation (5 mL/kg/day for 3 consecutive days) | 15 | Lung CT scores | X | Treatment with Ig improved recovery in SARS-CoV-2-infected patients based on lung CT data. CT scoring was based on lung involvement of each lobe, including ground glass opacity, crazy paving, and consolidation. Improvement in CT scores was observed in nine of ten patients who received Ig and were not on IMV. Treatment was not beneficial in patients already receiving IMV at the start of Ig treatment, 4 of 5 died. Patients with a lung CT score of ≥18 had an almost 4-fold increased risk of death versus those with lower scores. | ||
| [144] | Severe and critical COVID-19 | IgM/IgA-enriched Ig preparation (variable dosages) | 316 | VFD | X | Overall, no difference in the number of VFD (n = 182 received IMV at baseline) on Day 30 was observed with Igs and mortality rates were similar compared with control (28.8% vs. 31.8%). This was most likely caused by the heterogeneity of the treatment regimen and study population, including severe and critical COVID-19 patients. An improved, nearly statistically significant, benefit in 30-day survival (p = 0.063) was observed in a subgroup of patients receiving a high-dose Igs (>15 g/day for >3 days) and who were not on IMV at baseline (p = 0.068). | ||
| SURsg | X | |||||||
| [23] | sCAP | IgM/IgA-enriched Ig preparation (186.2 mg/kg/day for 5 days) | 160 | VFD | X | Overall, there was no statistically significant difference in VFDs between Ig treatment and control (mean: 11.0 vs. 9.6 days). Post hoc subset analyses in patients with CRP ≥ 70 mg/L (n = 124), IgM ≤ 0.8 g/L (n = 111), and high CRP/low IgM (n = 92) at baseline, showed a marked increase in the number of VFD (e.g., mean 12.6 vs. 8.7 days in the combined high CRP/low IgM subgroup, p = 0.031) and significant reductions in all-cause mortality compared with control (e.g., 11.8% vs. 36.6% in the combined subgroup, p = 0.006). | ||
| VFDsg | X | |||||||
| MORsg | X | |||||||
| [131] | sCAP receiving IMV and with septic shock | IVIg (5 g/day for 3 days) (dose regimen in Japan) | 1324 | VFD | X | Overall, there was a non-significant difference in the number of VFD in the Ig group compared with the control (8.3 vs. 9.4 days). No improvement was observed in the propensity sm (n = 1045) analysis (8.7 vs. 9.1 days). Similarly, no differences were found in 28-day mortality rates (37.8% vs. 35.3% overall and 36.7% vs. 36.0% in the sm groups). For the dose used in Japan clinically, no benefit was observed in pneumonia patients on IMV with septic shock. | ||
| VFDsm | X | |||||||
| MORsm | X | |||||||
| [136] | Severe COVID-19 | IVIg (20 g/day, total dose if ≤48 h: 64.4 ± 54.7 g, and if >48 h: 88.6 ± 71.1 g) | 58 | IMVsg | X | All patients were treated with Igs which were provided ≤48 h or >48 h after admission. In the subgroup receiving Igs early, the proportion of patients requiring IMV was significantly lower than in the >48 h group (6.7% vs. 32.1%). This positive effect was related to a statistically significant difference in 28-day mortality between the two groups (23.3% vs. 57.1%), a shorter length of hospital stay (11.5 ± 1.0 vs. 17.0 ± 1.6 days), and an ICU stay (9.5 ± 1.1 vs. 13.5 ± 1.6 days). | ||
| MORsg | X | |||||||
| HOSsg | X | |||||||
| [38] | Septic shock with severe respiratory failure | IgM/IgA-enriched Ig preparation (5 mL/kg/day over 3 days) | 33 | INF | X | Although CRP levels were significantly reduced in the Ig group on Days 4, 5, and 6, no significant difference in the median length of IMV and length of ICU stay was detected. Nevertheless, a trend was observed for the number of VD (median: 13 days vs. control: 17 days) and the length of stay in the ICU (median: 15 days vs. 26 days). See also Table 3. | ||
| VD | X | |||||||
| ICUd | X | |||||||
| [148] | Sepsis after surgery | IgM/IgA-enriched Ig preparation (5 mL/kg daily over 3 days) | 35 | IMV | X | Although a higher proportion of patients received IMV in the Ig group at baseline, the proportion of patients requiring IMV during the study increased slightly (83.3% to 88.8%) in the Ig group but markedly in the control group (64.7% to 100%). Additionally, the number of VD and mortality rate were lower in the Ig group compared with the control (9.9 vs. 12.8 days and 44.4% vs. 76.5%, respectively). Total time of hospitalization was decreased (13.3 vs. 15.9 days). The differences in this small study were not statistically significant. The observations were supported by a steady decrease in endotoxin levels after Ig treatment compared with control. | ||
| VD | X | |||||||
| MOR | X | |||||||
| HOS | X | |||||||
| EA | X | |||||||
| [149] | Severe infections with >90% IMV patients) | IgM/IgA-enriched Ig preparation (total dose 400 mL in 36 h) | 104 | VD | X | Overall, the number of VD was reduced significantly in the Ig group compared with the control (6.7 vs. 9.2 days) as well as in survivors (4.2 vs. 7.2 days) and in the high-risk postoperative “R3” group (5.5 vs. 12.7 days). Whereas the control group remained (mean) 21.5 days in ICU, those receiving Ig stayed only 14.8 days (p < 0.01). | ||
| VDsg | X | |||||||
| HOSsg | X | |||||||
| [150] | Septic shock within 24 h after onset of symptoms | IgM/IgA-enriched Ig preparation (250 mg/kg/day (20 mg/kg/h) for 3 consecutive days) | 168 | VFD | X | Marked improvement in lung function in the Ig group compared with control was observed in the overall population (median VFD: 22 vs. 12 days) and in the propensity sm population (n = 118 pairs; 20 vs. 10 days), although both were non-significant. Median number of ICU-free days increased markedly for Ig treatment (13 vs. 5 days) and in the sm population (13 vs. 4 days). Mortality rate was significantly reduced in the overall (25.0% vs. 46.1%, p = 0.004) and in the sm population and 25.4% vs. 45.8%, p = 0.021). See also Tables 2 and 3. | ||
| VFDsm | X | |||||||
| MOR | X | |||||||
| MORsm | X | |||||||
| [35] | Post-surgical intra-abdominal infections | IgM/IgA-enriched Ig preparation (total dose: 1300 mL over 3 days) | 64 | IMV | X | Although the difference was not significant, the duration of IMV was shorter in the Ig group. This effect was related to signs indicating a non-significant reduction in inflammation in the Ig group (reduction in the percentage of patients with fever, a larger decline in body temperature, a slightly larger decrease in TNF-α, endotoxin, and leukocyte levels, a stronger increase in platelets, and a faster and permanent decrease in PCT levels) and a shorter hospital stay (mean: 21 vs. 36 days). | ||
| INF | X | |||||||
| EA | X | |||||||
| HOS | X | |||||||
| [146] | Medical and surgical severe sepsis and septic shock (sepsis score: 12–27 and APACHE-II score 20–35) | IVIg (900 mg/kg total dose (600 mg/kg on day 0, 300 mg/kg on Day 1)) (low dose) | 624 | Lung | X | No beneficial effect of Ig treatment on pulmonary function was observed in the first 4 days, although the total duration of IMV overall and in the survivor subgroup was significantly shorter in the Ig group compared with the control group (mean: 11.3 vs. 13.4 days and 12.0 vs. 15.0 days, respectively). This study did not reveal a reduction in 28-day mortality by Ig treatment compared with the control (39.3% vs. 37.3%), but revealed a significantly higher ICU survival rate in the Ig group (60.7% vs. 54.5%). No effect of Ig treatment on plasma levels of IL-6 and TNF-receptors I and II was found. See also Table 3. | ||
| VD | X | |||||||
| VDsg | X | |||||||
| MOR | X | |||||||
| SUR(ICU) | X | |||||||
| INF | X |
4.2. Vascular Events in Systemic Infection
4.2.1. The Role of Neutrophils and NETs
4.2.2. Nonclinical Studies Investigating the Supportive Role of Polyvalent Immunoglobulins on Vascular Protection
4.2.3. Clinical Studies Investigating the Supporting Role of Polyvalent Immunoglobulins in Vascular Protection
| REF | Indication | Ig Preparation (Dose) | N | Effect | Significant Difference | Marked Change | No Difference | Study Results |
|---|---|---|---|---|---|---|---|---|
| [174] | Severe sepsis after surgery (sepsis score: 17–30) | IVIg (1000 mg/kg total dose (400 mg/kg on Days 0 and 1, and 200 mg/kg on Day 5)) (high dose) | 113 | Shock | X | Ig treatment resulted in a significant reduction in the number of patients developing and subsequently dying from septic shock (7% vs. 29%). Additionally, the ICU stay of survivors was markedly but not significantly reduced in patients receiving Ig compared with controls (mean: 19 vs. 26 days), and overall mortality was reduced significantly (33% vs. 64%). This study identified a subpopulation of septic patients that respond to high-dose Ig treatment. | ||
| ICUd | X | |||||||
| MOR | X | |||||||
| [36] | Severe sepsis | IgM/IgA-enriched preparation (250 mg/kg/day on 3 consecutive days) | 42 | Shock | X | Ig treatment resulted in a trend in the reduction of patients developing septic shock (38% vs. 57%). This vascular measure was supported by the fact that inflammation (PCT levels) was statistically significantly decreased in the Ig group compared with the control. Furthermore, the mortality was markedly but not significantly reduced in the Ig group in this small study (23.8% vs. 33.3%). | ||
| INF | X | |||||||
| MOR | X | |||||||
| [147] | Severe sepsis and septic shock | IgM/IgA-enriched preparation (5 mg/kg/day over 3 days) | 26 | VPT | X | No significant differences between or within the groups were detected for the use of vasopressor therapy. Nevertheless, a trend was observed, particularly on Day 3, where 45.4% of patients in the Ig group and 71.4% in the control group received vasopressors. See also Table 3. | ||
| VPTt | X | |||||||
| [175] | COVID-19 | IgM/IgA-enriched preparation (500 mg/kg/day over 2 consecutive days) | 47 | Shock | X | Ig treatment resulted in a significant reduction in the number of patients developing septic shock (12.5% vs. 21.7%). Most patients developed acute kidney (80%) and liver failure (80%) at the time of ICU admission, before the infusion of Ig. Some renal complications occurred at a higher rate in the Ig group compared with controls. Markedly fewer patients in the Ig group developed pulmonary embolism (12.5% vs. 26.1%), sepsis (20.8% vs. 39.1%), and mortality was significantly lower (37.5% vs. 56.5%). | ||
| PE | X | |||||||
| Sepsis | X | |||||||
| MOR | X | |||||||
| [176] | Sepsis or septic shock within 24 h after onset of symptoms | IgM/IgA-enriched preparation (250 mg/kg/day over 3 consecutive days) | 19 | PVDt | X | Persistent microcirculatory alterations during septic shock are associated with organ failure and death. Ig was able to significantly increase (21.7 ± 4.7 to 25.5 ± 5.1 mm/mm2) the sublingual PVD after 72 h, whereas it was reduced in the placebo group (25 ± 5.8 to 20.7 ± 4.1 mm/mm2). The MFI was significantly increased at 24 h in the Ig group (median: 2.68 to 2.93 AU), while it significantly decreased at 72 h in the placebo group (median: 2.83 to 2.67 AU). Infusion of Ig may be associated with an increase in the sublingual microvascular density and blood flow quality. The administration of Ig did not induce any significant variation in MAP or HR and did not correlate with variations in hemodynamic parameters or cytokine levels. | ||
| MFIt | X | |||||||
| [150] | Septic shock within 24 h after onset of symptoms | IgM/IgA-enriched preparation (250 mg/kg/day (20 mg/kg/h) for 3 consecutive days) | 168 | VPFD | X | Nearly no improvement in the use of vasopressors was observed in the Ig group compared with the control group overall (median VPFD: 24 vs. 23 days) or in the propensity score-matched population (n = 118 pairs; 24 vs. 22 days), despite the reductions in the 30-day mortality rate in the Ig group, which were significant in the overall and score-matched populations (25.0% vs. 46.1% and 25.4% vs. 45.8%). See also Tables 1 and 3. | ||
| VPFDsm | X | |||||||
| MOR | X | |||||||
| MORsm | X |
5. Prevention of Coagulopathy and Organ Failure by Immunoglobulins
5.1. The Role of Endothelial Cells, Platelets, Neutrophils, and Complement
5.2. Nonclinical Studies Investigating the Supporting Role of Polyvalent Immunoglobulins
5.3. Clinical Studies Investigating the Supporting Role of Polyvalent Immunoglobulins
5.3.1. Prevention of Coagulopathy
5.3.2. Prevention or Improvement of Organ Dysfunction
| REF | Indication | Ig Preparation (Dose) | N | Effect | Significant Difference | Marked Change | No Difference | Study Results |
|---|---|---|---|---|---|---|---|---|
| [30] | Sepsis | IVIg (1500 mg total dose (5.0 g/day for 3 days)) (dose regimen in Japan) | 41 | COAG | X | After IVIg treatment, a significant decrease compared with baseline was observed for various coagulation/fibrinolysis molecular markers (PT-INR (mean: 1.6 to 1.2), APTT (44.5 to 29.3), SF (30.1 to 14.7), PAI-1 (182 to 16.5)), markers for inflammation (CRP (mean: 16.3 to 9.9), PCT (32.2 to 6.3)), as well as the JAAM DIC score. Decrease of these markers after treatment was not significant for the control group. In both groups, only IL-6 (22943 to 85.5 vs. 1214 to 55) and TAT (8.2 to 3.0 vs. 20.5 to 3.7) decreased significantly after treatment. The DIC (p < 0.05) and SOFA (p < 0.01) scores significantly decreased after IVIg treatment compared with baseline. The 28-day mortality rate was markedly lower in the Ig group compared with the control (5.3% vs. 18.2%). | ||
| INF | X | |||||||
| DIC | X | |||||||
| SOFA | X | |||||||
| MOR | X | |||||||
| [147] | Severe sepsis and septic shock | IgM/IgA-enriched preparation (5 mg/kg/day over 3 days) | 26 | EAt | X | Endotoxin levels influence coagulation parameters. On Day 1, endotoxin was significantly decreased 6 and 12 h after Ig treatment and differed significantly compared with the control group following 6 h of treatment (0.26 ± 0.07 vs. 0.43 ± 0.07). The platelet count in the Ig group was significantly higher on Day 4 (mean: 200/nL vs. 87/nL). The fibrinogen concentration was significantly higher on Day 2 (475 mg/dL vs. 311 mg/dL, p = 0.016) and on Day 4 (420 mg/dL vs. 307 mg/dL, p = 0.017) compared with the control group. The levels of inflammatory markers and viscoelastic or aggregometric measures did not differ between groups. Regarding organ dysfunctions/failure, a marked change in the decrease in the SOFA score was observed with Ig treatment on Day 4 (mean: 11.7 to 7.0 vs. 10.6 to 9.5), and the use of dialysis increased in the control group (35.7% to 64.3% on Day 4) as compared with the Ig group (33.3% to 40%). | ||
| COAG | X | |||||||
| INF | X | |||||||
| SOFA | X | |||||||
| MOD | X | |||||||
| [204] | Gram-negative (endotoxemia) septic shock, <24 h after onset of symptoms | IgM/IgA-enriched preparation (Day 1: 600 mL; Days 2 and3: 300 mL) | 55 | EAt | X | Endotoxemia in patients with septic shock has physiological and biological consequences, resulting in higher APACHE-II scores. Ig treatment was associated with a significant decrease in endotoxin levels within 24 h of treatment, which was associated with a statistically significant decrease (p < 0.05) in the APACHE-II score after Day 5. In the control group, APACHE-II scores further increased until Day 10. This was related to a significant decrease in the 6-week mortality rate compared with the control group (1/27 (3.7%) vs. 9/28 (32.1%), p = 0.0082). | ||
| ASt | X | |||||||
| MOR | X | |||||||
| [146] | Medical and surgical severe sepsis and septic shock (sepsis score: 12–27 and APACHE-II score 20–35) | IVIg (900 mg/kg total dose (600 mg/kg on Day 0, 300 mg/kg on Day 1)) (low dose) | 624 | Lung | X | Regarding organ dysfunction, 90.5% of patients were mechanically ventilated at baseline. No beneficial effect of Ig treatment on pulmonary function was observed (see Table 1). A significantly stronger decline of the disease severity sepsis score (−1.21) and a more pronounced decline in the APACHE-II score (-1.25) in the Ig group compared with placebo were found. The improvement in APACHE-II was mainly due to improved GCS and decrease in leukocytes. The 28-day mortality rate was not significantly different between groups (39.3% in the Ig group vs. 37.3% in the control group), although the ICU survival rate was significantly higher in the Ig group (60.7% vs. 54.5%). | ||
| VD | X | |||||||
| VDsg | X | |||||||
| SS | X | X | ||||||
| AS | X | |||||||
| MOR | X | |||||||
| SUR(ICU) | X | |||||||
| [202] | STSS | IVIg (2000 mg/kg total dose (1 g/kg on Day 1 and 0.5 g/kg on Days 2 and 3)) (high dose) | 21 | SOFAt | X | Ig treatment resulted in improvement of organ dysfunction during treatment Days 1–3 in the Ig group, as assessed by a significant decrease in SOFA score. The resolution of shock in survivors (88 h vs. 122 h, not significant) was faster in the Ig group. The 28-mortality was 3.6-times higher in the placebo group (10% vs. 36%, not significant) and higher after 180 days (20% vs. 36%). Ig treatment significantly increased the neutralizing activity against superantigens. | ||
| Shock | X | |||||||
| MOR | X | |||||||
| SA | X | |||||||
| [38] | Septic shock accompanied by severe respiratory failure | IgM/IgA-enriched preparation (5 mL/kg/day via 8 h infusion over 3 days) | 33 | CRP | X | No significant resolution of organ failures was observed. Only a trend for length of IMV (median VD: 13 vs. 17). Markers of organ dysfunction and inflammation (platelets, PT, total protein, albumin, WBC) were nearly identical, except for CRP levels, which were significantly lower in the Ig group on Days 4, 5, and 6. Median length of ICU stay was not significantly different, but a decrease was observed in the Ig group (median: 15 vs. 26 days). The 28-day mortality rates were nearly identical in the two groups (25.0% vs. 29.4%). In this small study, at late-stage sepsis, organ dysfunction was likely driven by the host response rather than the infective pathogen itself. | ||
| INFt | X | |||||||
| MOD | X | |||||||
| VD | X | |||||||
| MOR | X | |||||||
| ICUd | X | |||||||
| [206] | Sepsis-induced MODs | IgM/IgA-enriched preparation (5 mg/kg/day over 3 days) | 118 | AS | X | Many factors affect mortality from MODs, including the number of affected organs and the degree of organ dysfunction. In this study, all patients had 2 or 3 organ dysfunctions, mostly respiratory and cardiovascular. Although APACHE-II scores decreased significantly on Day 4 in both the Ig (n = 56; from 27 to 16) and the control groups (n = 62; from 27 to 23), the effects were more pronounced in the Ig group. Ig treatment significantly increased the length of ICU stay (mean: 36 vs. 22 days), but decreased the overall (85.5% vs. 42.9%) and 28-day case fatality rate (69.3% vs. 25.0%). | ||
| MOR | X | |||||||
| [150] | Septic shock within 24 h after onset of symptoms | IgM/IgA-enriched preparation (250 mg/kg/day (20 mg/kg/h) for 3 consecutive days) | 168 | VFD | X | Improvement in organ dysfunction with Ig, although non-significant, was observed for the lung compared with control in the overall population (median VFD: 22 vs. 12 days) and in the propensity sm population (n = 118 pairs; 20 vs. 10 days), although both were non-significant. This was despite the fact that more patients required renal replacement therapy in the Ig group compared with the control group (overall: 30.4% vs. 22.4% and in sm: 28.8% vs. 20.3%). Sepsis and septic shock are the most important triggers of acute renal failure with a high mortality rate. However, the 30-day mortality was reduced by 21.1% (overall: 25.0% vs. 46.1%) in the Ig group despite the higher number of patients requiring renal replacement. See also Tables 1 and 2. | ||
| VFDsm | X | |||||||
| MOR | X | |||||||
| MORsm | X |
6. Conclusions and Future Use of Immunoglobulins
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; MacHado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, E1063–E1143. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Torres, A.; Nagavci, B.; Aliberti, S.; Antonelli, M.; Bassetti, M.; Bos, L.D.; Chalmers, J.D.; Derde, L.; de Waele, J.; et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 2023, 49, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Venet, F.; Gebeile, R.; Bancel, J.; Guignant, C.; Poitevin-Later, F.; Malcus, C.; Lepape, A.; Monneret, G. Assessment of plasmatic immunoglobulin G, A and M levels in septic shock patients. Int. Immunopharmacol. 2011, 11, 2086–2090. [Google Scholar] [CrossRef] [PubMed]
- Geier, C.; Schroder, J.; Tamm, A.; Dietz, S.; Nuding, S.; Holder, K.; Khandanpour, O.; Werdan, K.; Ebelt, H. Influence of the serum levels of immunoglobulins on clinical outcomes in medical intensive-care patients. Med. Klin. Intensivmed. Notfmed. 2017, 112, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Culshaw, N.; Post, B.; Tamayo, E.; Andaluz-Ojeda, D.; Bermejo-Martin, J.F.; Dietz, S.; Werdan, K.; Beale, R.; Spencer, J.; et al. Endogenous IgG hypogammaglobulinaemia in critically ill adults with sepsis: Systematic review and meta-analysis. Intensive Care Med. 2015, 41, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Dietz, S.; Lautenschlager, C.; Muller-Werdan, U.; Pilz, G.; Fraunberger, P.; Pasler, M.; Ebelt, H.; Walli, A.K.; Werdan, K.; Nuding, S. Serum IgG levels and mortality in patients with severe sepsis and septic shock: The SBITS data. Med. Klin. Intensivmed. Notfmed. 2017, 112, 462–470. [Google Scholar] [CrossRef]
- Taccone, F.S.; Stordeur, P.; De Backer, D.; Creteur, J.; Vincent, J.L. Gamma-globulin levels in patients with community-acquired septic shock. Shock 2009, 32, 379–385. [Google Scholar] [CrossRef]
- Tamayo, E.; Fernandez, A.; Almansa, R.; Carrasco, E.; Goncalves, L.; Heredia, M.; Andaluz-Ojeda, D.; March, G.; Rico, L.; Gomez-Herreras, J.I.; et al. Beneficial role of endogenous immunoglobulin subclasses and isotypes in septic shock. J. Crit. Care 2012, 27, 616–622. [Google Scholar] [CrossRef]
- Myrianthefs, P.M.; Boutzouka, E.; Baltopoulos, G.J. γ-globulin levels in patients with community-acquired septic shock. Shock 2010, 33, 556–557. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Rodriguez-Fernandez, A.; Herran-Monge, R.; Andaluz-Ojeda, D.; Muriel-Bombin, A.; Merino, P.; Garcia-Garcia, M.M.; Citores, R.; Gandia, F.; Almansa, R.; et al. Immunoglobulins IgG1, IgM and IgA: A synergistic team influencing survival in sepsis. J. Intern. Med. 2014, 276, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Fear, D.; Lavender, P.; Mare, T.; Beale, R.; Swanson, C.; Singer, M.; Spencer, J. Activation-associated accelerated apoptosis of memory B cells in critically ill patients with sepsis. Crit. Care Med. 2017, 45, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, N.J.; Schlievert, P.M. Suppression of immunoglobulin-secreting cells from human peripheral blood by toxic-shock-syndrome toxin-1. J. Infect. Dis. 1986, 153, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Krautz, C.; Maier, S.L.; Brunner, M.; Langheinrich, M.; Giamarellos-Bourboulis, E.J.; Gogos, C.; Armaganidis, A.; Kunath, F.; Grutzmann, R.; Weber, G.F. Reduced circulating B cells and plasma IgM levels are associated with decreased survival in sepsis—A meta-analysis. J. Crit. Care 2018, 45, 71–75. [Google Scholar] [CrossRef]
- de la Torre, M.C.; Bolibar, I.; Vendrell, M.; de Gracia, J.; Vendrell, E.; Rodrigo, M.J.; Boquet, X.; Torrebadella, P.; Yebenes, J.C.; Serra-Prat, M.; et al. Serum immunoglobulins in the infected and convalescent phases in community-acquired pneumonia. Respir. Med. 2013, 107, 2038–2045. [Google Scholar] [CrossRef]
- Andaluz-Ojeda, D.; Iglesias, V.; Bobillo, F.; Almansa, R.; Rico, L.; Gandia, F.; Loma, A.M.; Nieto, C.; Diego, R.; Ramos, E.; et al. Early natural killer cell counts in blood predict mortality in severe sepsis. Crit. Care 2011, 15, R243. [Google Scholar] [CrossRef]
- de la Torre, M.C.; Toran, P.; Serra-Prat, M.; Palomera, E.; Guell, E.; Vendrell, E.; Yebenes, J.C.; Torres, A.; Almirall, J. Serum levels of immunoglobulins and severity of community-acquired pneumonia. BMJ Open Respir. Res. 2016, 3, e000152. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Apostolidou, E.; Lada, M.; Perdios, I.; Gatselis, N.K.; Tsangaris, I.; Georgitsi, M.; Bristianou, M.; Kanni, T.; Sereti, K.; et al. Kinetics of circulating immunoglobulin M in sepsis: Relationship with final outcome. Crit. Care 2013, 17, R247. [Google Scholar] [CrossRef]
- Prucha, M.; Zazula, R.; Herold, I.; Dostal, M.; Hyanek, T.; Bellingan, G. Presence of hypogammaglobulinemia in patients with severe sepsis, septic shock, and SIRS is associated with increased mortality. J. Infect. 2014, 68, 297–299. [Google Scholar] [CrossRef]
- Prucha, M.; Zazula, R.; Herold, I.; Dostal, M.; Hyanek, T.; Bellingan, G. Presence of hypogammaglobulinemia—A risk factor of mortality in patients with severe sepsis, septic shock, and SIRS. Prague Med. Rep. 2013, 114, 246–257. [Google Scholar] [CrossRef]
- Justel, M.; Socias, L.; Almansa, R.; Ramirez, P.; Gallegos, M.C.; Fernandez, V.; Gordon, M.; Andaluz-Ojeda, D.; Nogales, L.; Rojo, S.; et al. IgM levels in plasma predict outcome in severe pandemic influenza. J. Clin. Virol. 2013, 58, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhu, J.; Jin, J.; Tong, C.; Zeng, W.; Deng, S.; Zou, S. Prognostic value of circulating lymphocyte B and plasma immunoglobulin M on septic shock and sepsis: A systematic review and meta-analysis. Am. J. Transl. Res. 2019, 11, 7223–7232. [Google Scholar] [PubMed]
- Welte, T.; Dellinger, R.P.; Ebelt, H.; Ferrer, M.; Opal, S.M.; Singer, M.; Vincent, J.L.; Werdan, K.; Martin-Loeches, I.; Almirall, J.; et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: A randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensive Care Med. 2018, 44, 438–448. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Lu, Z.; Zhang, L.; Fan, T.; Xiong, R.; Shen, X.; Feng, H.; Meng, H.; Lin, W.; Jiang, W.; et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 2020, 127, 104361. [Google Scholar] [CrossRef]
- de la Torre, M.C.; Palomera, E.; Serra-Prat, M.; Guell, E.; Yebenes, J.C.; Bermejo-Martin, J.F.; Almirall, J. IgG2 as an independent risk factor for mortality in patients with community-acquired pneumonia. J. Crit. Care 2016, 35, 115–119. [Google Scholar] [CrossRef]
- Akatsuka, M.; Tatsumi, H.; Sonoda, T.; Masuda, Y. Low immunoglobulin G level is associated with poor outcomes in patients with sepsis and septic shock. J. Microbiol. Immunol. Infect. 2020, 54, 728–732. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Vadász, I.; Wilhelm, J.; Walmrath, H.-D.; Seeger, W.; Birk, H.-W.; Jennert, B.; Dietrich, H.; Herold, S.; Trauth, J.; et al. Immunoglobulin deficiency as an indicator of disease severity in patients with COVID-19. Am. J. Physiol. Cell. Mol. Physiol. 2021, 320, L590–L599. [Google Scholar] [CrossRef]
- Weißmüller, S.; Schmidt, C.; Heinz, C.C. Multifaceted interactions of polyvalent immunoglobulin preparations with neutrophil and complement pathways in severe bacterial infections. Biomedicines, 2023; submitted. [Google Scholar]
- Kaul, R.; McGeer, A.; Norrby-Teglund, A.; Kotb, M.; Schwartz, B.; O’Rourke, K.; Talbot, J.; Low, D.E. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome—A comparative observational study. The Canadian Streptococcal Study Group. Clin. Infect. Dis. 1999, 28, 800–807. [Google Scholar] [CrossRef]
- Ishikura, H.; Nakamura, Y.; Kawano, Y.; Tanaka, J.; Mizunuma, M.; Ohta, D.; Nishida, T.; Murai, A. Intravenous immunoglobulin improves sepsis-induced coagulopathy: A retrospective, single-center observational study. J. Crit. Care 2015, 30, 579–583. [Google Scholar] [CrossRef]
- Ikeda, T.; Ikeda, K.; Taniuchi, H.; Suda, S.; Takahashi, Y. Background factors in patients receiving immunoglobulin administration and changes in sepsis markers. Crit. Care 2010, 14, P27. [Google Scholar] [CrossRef]
- Ikeda, T.; Ono, S.; Ueno, T.; Tanaka, H.; Suda, S. Changes in procalcitonin and presepsin before and after immunoglobulin administration in septic patients. Crit. Care 2015, 19, P62. [Google Scholar] [CrossRef]
- Kim, A.; Yokota, M.; Saito, N.; Takeda, M.; Harada, T.; Namiki, M.; Yaguchi, A. Intravenous immunoglobulin therapy could have efficacy in severe sepsis. Crit. Care 2013, 17, P39. [Google Scholar] [CrossRef]
- Ross, C.; Svenson, M.; Nielsen, H.; Lundsgaard, C.; Hansen, M.B.; Bendtzen, K. Increased in vivo antibody activity against interferon alpha, interleukin-1alpha, and interleukin-6 after high-dose Ig therapy. Blood 1997, 90, 2376–2380. [Google Scholar] [CrossRef] [PubMed]
- Reith, H.B.; Rauchschwalbe, S.K.; Mittelkotter, U.; Engemann, R.; Thiede, A.; Arnold, A.; Lissner, R. IgM-enriched immunoglobulin (pentaglobin) positively influences the course of post-surgical intra-abdominal infections. Eur. J. Med. Res. 2004, 9, 479–484. [Google Scholar] [PubMed]
- Tugrul, S.; Ozcan, P.E.; Akinci, O.; Seyhun, Y.; Cagatay, A.; Cakar, N.; Esen, F. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis [ISRCTN28863830]. Crit. Care 2002, 6, 357–362. [Google Scholar] [CrossRef] [PubMed]
- El-Nawawy, A.; El-Kinany, H.; Hamdy El-Sayed, M.; Boshra, N. Intravenous polyclonal immunoglobulin administration to sepsis syndrome patients: A prospective study in a pediatric intensive care unit. J. Trop. Pediatr. 2005, 51, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.; Mikor, A.; Leiner, T.; Molnar, Z.; Bogar, L.; Szakmany, T. Effects of IgM-enriched immunoglobulin therapy in septic-shock-induced multiple organ failure: Pilot study. J. Anesth. 2013, 27, 618–622. [Google Scholar] [CrossRef]
- Meisner, M.; Tschaikowsky, K.; Palmaers, T.; Schmidt, J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit. Care 1999, 3, 45–50. [Google Scholar] [CrossRef]
- Willuweit, K.; Bezinover, D.; Herzer, K.; Nowak, K.M.; Paul, A.; Saner, F.H. Efficacy of IgM-enriched immunoglobulin for vasopressor-resistant vasoplegic shock after liver transplantation. Transplantation 2019, 103, 381–386. [Google Scholar] [CrossRef]
- Duerr, C.; Bacher, A.; de Martin, A.; Sachet, M.; Sadeghi, K.; Baumann, S.; Heinz, C.; Spittler, A. The novel polyclonal Ab preparation trimodulin attenuates ex vivo endotoxin-induced immune reactions in early hyperinflammation. Innate Immun. 2019, 25, 374–388. [Google Scholar] [CrossRef]
- Lok, K.Z.; Basta, M.; Manzanero, S.; Arumugam, T.V. Intravenous immunoglobulin (IVIg) dampens neuronal toll-like receptor-mediated responses in ischemia. J. Neuroinflamm. 2015, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Suzuki, C.; Kobayashi, F.; Nakano, A.; Fujii, A.; Sakai, K.; Imada, T. Intravenous immunoglobulin preparation attenuates LPS-induced production of pro-inflammatory cytokines in human monocytic cells by modulating TLR4-mediated signaling pathways. Naunyn. Schmiedebergs. Arch. Pharmacol. 2012, 385, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Bohländer, F.; Riehl, D.; Weißmüller, S.; Gutscher, M.; Schüttrumpf, J.; Faust, S. Immunomodulation: Immunoglobulin preparations suppress hyperinflammation in a COVID-19 model via FcγRIIA and FcαRI. Front. Immunol. 2021, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- Bohländer, F.; Weißmüller, S.; Riehl, D.; Gutscher, M.; Schüttrumpf, J.; Faust, S. The functional role of IgA in the IgM/IgA-enriched immunoglobulin preparation trimodulin. Biomedicines 2021, 9, 1828. [Google Scholar] [CrossRef] [PubMed]
- Svenson, M.; Hansen, M.B.; Bendtzen, K. Binding of cytokines to pharmaceutically prepared human immunoglobulin. J. Clin. Investig. 1993, 92, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Shibata, Y.; Nishizawa, K. Intravenous immunoglobulin administration for patients with systemic inflammatory response syndrome. J. Jpn. Soc. Emerg. Med. 1997, 7, 307–308. [Google Scholar]
- Watanabe, M.; Uchida, K.; Nakagaki, K.; Kanazawa, H.; Trapnell, B.C.; Hoshino, Y.; Kagamu, H.; Yoshizawa, H.; Keicho, N.; Goto, H.; et al. Anti-cytokine autoantibodies are ubiquitous in healthy individuals. FEBS Lett. 2007, 581, 2017–2021. [Google Scholar] [CrossRef]
- Svenson, M.; Hansen, M.B.; Ross, C.; Diamant, M.; Rieneck, K.; Nielsen, H.; Bendtzen, K. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood 1998, 91, 2054–2061. [Google Scholar] [CrossRef]
- Roos, A.; Rieben, R.; Faber-Krol, M.C.; Daha, M.R. IgM-enriched human intravenous immunoglobulin strongly inhibits complement-dependent porcine cell cytotoxicity mediated by human xenoreactive antibodies. Xenotransplantation 2003, 10, 596–605. [Google Scholar] [CrossRef]
- Rieben, R.; Roos, A.; Muizert, Y.; Tinguely, C.; Gerritsen, A.F.; Daha, M.R. Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in vitro and in vivo in a rat model of acute inflammation. Blood 1999, 93, 942–951. [Google Scholar] [CrossRef]
- Lutz, H.U.; Stammler, P.; Jelezarova, E.; Nater, M.; Spath, P.J. High doses of immunoglobulin G attenuate immune aggregate-mediated complement activation by enhancing physiologic cleavage of C3b in C3bn-IgG complexes. Blood 1996, 88, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Spycher, M.; Matozan, K.; Minnig, K.; Zehnder, R.; Miescher, S.; Hoefferer, L.; Rieben, R. In vitro comparison of the complement-scavenging capacity of different intravenous immunoglobulin preparations. Vox Sang. 2009, 97, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Mollnes, T.E.; Hogasen, K.; Hoaas, B.F.; Michaelsen, T.E.; Garred, P.; Harboe, M. Inhibition of complement-mediated red cell lysis by immunoglobulins is dependent on the IG isotype and its C1 binding properties. Scand. J. Immunol. 1995, 41, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Weißmüller, S.; Bohländer, F.; Germer, M.; König, M.; Staus, A.; Wartenberg-Demand, A.; Heinz, C.C.; Schüttrumpf, J. The dual role of a polyvalent IgM/IgA-enriched immunoglobulin preparation in activating and inhibiting the complement system. Biomedicines 2021, 9, 817. [Google Scholar] [CrossRef]
- Walpen, A.J.; Laumonier, T.; Aebi, C.; Mohacsi, P.J.; Rieben, R. Immunoglobulin M-enriched intravenous immunoglobulin inhibits classical pathway complement activation, but not bactericidal activity of human serum. Xenotransplantation 2004, 11, 141–148. [Google Scholar] [CrossRef]
- Basta, M.; Van Goor, F.; Luccioli, S.; Billings, E.M.; Vortmeyer, A.O.; Baranyi, L.; Szebeni, J.; Alving, C.R.; Carroll, M.C.; Berkower, I.; et al. F(ab)’2-mediated neutralization of C3a and C5a anaphylatoxins: A novel effector function of immunoglobulins. Nat. Med. 2003, 9, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Miletic, V.D.; Hester, C.G.; Frank, M.M. Regulation of complement activity by immunoglobulin. I. Effect of immunoglobulin isotype on C4 uptake on antibody-sensitized sheep erythrocytes and solid phase immune complexes. J. Immunol. 1996, 156, 749–757. [Google Scholar] [CrossRef]
- Basta, M.; Kirshbom, P.; Frank, M.M.; Fries, L.F. Mechanism of therapeutic effect of high-dose intravenous immunoglobulin. Attenuation of acute, complement-dependent immune damage in a guinea pig model. J. Clin. Investig. 1989, 84, 1974–1981. [Google Scholar] [CrossRef]
- Basta, M. Ambivalent effect of immunoglobulins on the complement system: Activation versus inhibition. Mol. Immunol. 2008, 45, 4073–4079. [Google Scholar] [CrossRef]
- Ghosh, M.; Rana, S. The anaphylatoxin C5a: Structure, function, signaling, physiology, disease, and therapeutics. Int. Immunopharmacol. 2023, 118, 110081. [Google Scholar] [CrossRef]
- Denk, S.; Neher, M.D.; Messerer, D.A.C.; Wiegner, R.; Nilsson, B.; Rittirsch, D.; Nilsson-Ekdahl, K.; Weckbach, S.; Ignatius, A.; Kalbitz, M.; et al. Complement C5a functions as a master switch for the pH balance in neutrophils exerting fundamental immunometabolic effects. J. Immunol. 2017, 198, 4846–4854. [Google Scholar] [CrossRef]
- Denk, S.; Taylor, R.P.; Wiegner, R.; Cook, E.M.; Lindorfer, M.A.; Pfeiffer, K.; Paschke, S.; Eiseler, T.; Weiss, M.; Barth, E.; et al. Complement C5a-induced changes in neutrophil morphology during inflammation. Scand. J. Immunol. 2017, 86, 143–155. [Google Scholar] [CrossRef]
- Ibrahim, F.B.; Pang, S.J.; Melendez, A.J. Anaphylatoxin signaling in human neutrophils. A key role for sphingosine kinase. J. Biol. Chem. 2004, 279, 44802–44811. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hack, C.E.; Nuijens, J.H.; Felt-Bersma, R.J.; Schreuder, W.O.; Eerenberg-Belmer, A.J.; Paardekooper, J.; Bronsveld, W.; Thijs, L.G. Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am. J. Med. 1989, 86, 20–26. [Google Scholar] [CrossRef]
- Xu, R.; Lin, F.; Bao, C.; Huang, H.; Ji, C.; Wang, S.; Jin, L.; Sun, L.; Li, K.; Zhang, Z.; et al. Complement 5a receptor-mediated neutrophil dysfunction is associated with a poor outcome in sepsis. Cell Mol. Immunol. 2016, 13, 103–109. [Google Scholar] [CrossRef]
- Rittirsch, D.; Redl, H.; Huber-Lang, M. Role of complement in multiorgan failure. Clin. Dev. Immunol. 2012, 2012, 962927. [Google Scholar] [CrossRef]
- Bengtson, A.; Heideman, M. Altered anaphylatoxin activity during induced hypoperfusion in acute and elective abdominal aortic surgery. J. Trauma. 1986, 26, 631–637. [Google Scholar] [CrossRef]
- Nakae, H.; Endo, S.; Inada, K.; Yoshida, M. Chronological changes in the complement system in sepsis. Surg. Today 1996, 26, 225–229. [Google Scholar] [CrossRef]
- Ohta, R.; Torii, Y.; Imai, M.; Kimura, H.; Okada, N.; Ito, Y. Serum concentrations of complement anaphylatoxins and proinflammatory mediators in patients with 2009 H1N1 influenza. Microbiol. Immunol. 2011, 55, 191–198. [Google Scholar] [CrossRef]
- Noris, M.; Benigni, A.; Remuzzi, G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020, 98, 314–322. [Google Scholar] [CrossRef]
- Holter, J.C.; Pischke, S.E.; de Boer, E.; Lind, A.; Jenum, S.; Holten, A.R.; Tonby, K.; Barratt-Due, A.; Sokolova, M.; Schjalm, C.; et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. USA 2020, 117, 25018–25025. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiao, H.; Guo, R.; Li, Y.; Shen, B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg. Microbes Infect. 2015, 4, e28. [Google Scholar] [CrossRef]
- Carvelli, J.; Demaria, O.; Vely, F.; Batista, L.; Chouaki Benmansour, N.; Fares, J.; Carpentier, S.; Thibult, M.L.; Morel, A.; Remark, R.; et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020, 588, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.R.W.; Bruchfeld, A.N.; Harper, L.; Schaier, M.; Venning, M.C.; Hamilton, P.; Burst, V.; Grundmann, F.; Jadoul, M.; Szombati, I.; et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J. Am. Soc. Nephrol. 2017, 28, 2756–2767. [Google Scholar] [CrossRef]
- Horiuchi, T.; Tsukamoto, H. Complement-targeted therapy: Development of C5- and C5a-targeted inhibition. Inflamm. Regen. 2016, 36, 11. [Google Scholar] [CrossRef]
- Vlaar, A.P.J.; Witzenrath, M.; van Paassen, P.; Heunks, L.M.A.; Mourvillier, B.; de Bruin, S.; Lim, E.H.T.; Brouwer, M.C.; Tuinman, P.R.; Saraiva, J.F.K.; et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 1137–1146. [Google Scholar] [CrossRef]
- Kaveri, S.V.; Silverman, G.J.; Bayry, J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J. Immunol. 2012, 188, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Silverman, G.J.; Gronwall, C.; Vas, J.; Chen, Y. Natural autoantibodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discov. Med. 2009, 8, 151–156. [Google Scholar] [PubMed]
- Boes, M. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 2000, 37, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Gronwall, C.; Akhter, E.; Oh, C.; Burlingame, R.W.; Petri, M.; Silverman, G.J. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin. Immunol. 2012, 142, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.J. Natural IgM antibodies against oxidation-specific epitopes. J. Clin. Immunol. 2010, 30 (Suppl. S1), S56–S60. [Google Scholar] [CrossRef] [PubMed]
- Lobo, P.I. Role of natural autoantibodies and natural IgM anti-leucocyte autoantibodies in health and disease. Front. Immunol. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Boes, M.; Prodeus, A.P.; Schmidt, T.; Carroll, M.C.; Chen, J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1998, 188, 2381–2386. [Google Scholar] [CrossRef]
- Quartier, P.; Potter, P.K.; Ehrenstein, M.R.; Walport, M.J.; Botto, M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur. J. Immunol. 2005, 35, 252–260. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wild, T.; Xiong, Y.; Sylvers, P.; Zhang, Y.; Zhang, L.; Wahl, L.; Wahl, S.M.; Kozlowski, S.; Notkins, A.L. Polyreactive antibodies plus complement enhance the phagocytosis of cells made apoptotic by UV-light or HIV. Sci. Rep. 2013, 3, 2271. [Google Scholar] [CrossRef]
- Elkon, K.B. (Ed.) Apoptosis and its relevance to autoimmunity. In Current Directions in Autoimmunity; Karger AG: Basel, Switzerland, 2005; Volume 9, pp. 120–142. ISBN 978-3-8055-8036-6. [Google Scholar]
- Tsiantoulas, D.; Perkmann, T.; Afonyushkin, T.; Mangold, A.; Prohaska, T.A.; Papac-Milicevic, N.; Millischer, V.; Bartel, C.; Horkko, S.; Boulanger, C.M.; et al. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 2015, 56, 440–448. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood 2017, 130, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, G.; Afonyushkin, T.; Goderle, L.; Puhm, F.; Schrottmaier, W.; Taqi, S.; Schwameis, M.; Ay, C.; Pabinger, I.; Jilma, B.; et al. Natural IgM antibodies inhibit microvesicle-driven coagulation and thrombosis. Blood 2021, 137, 1406–1415. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Nederkoorn, P.J.; Vermeij, J.-D.; Dijkgraaf, M.G.; van de Beek, D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, L.; Smith, C.J.; McColl, B.W. Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nat. Commun. 2017, 8, 15051. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, L.; Harris, A.J.; Malbon, A.; Daniels, M.J.; Younas, M.; Grainger, J.R.; Allan, S.M.; Smith, C.J.; McColl, B.W. Treatment with IgM-enriched intravenous immunoglobulins (IgM-IVIg) enhances clearance of stroke-associated bacterial lung infection. Immunology. 2022, 167, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.M.; Midwood, K.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef]
- Goulopoulou, S.; McCarthy, C.G.; Webb, R.C. Toll-like receptors in the vascular system: Sensing the dangers within. Pharmacol. Rev. 2016, 68, 142–167. [Google Scholar] [CrossRef]
- Ferrer, M.; Travierso, C.; Cilloniz, C.; Gabarrus, A.; Ranzani, O.T.; Polverino, E.; Liapikou, A.; Blasi, F.; Torres, A. Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS ONE 2018, 13, e0191721. [Google Scholar] [CrossRef]
- Zambon, M.; Vincent, J.L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008, 133, 1120–1127. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- de Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell. Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Pinegin, B.V. Neutrophil extracellular traps: Mechanisms of formation and role in health and disease. Biochemistry 2014, 79, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, D.; Jabłońska, E.; Garley, M.; Ratajczak-Wrona, W.; Iwaniuk, A. New aspects of the biology of neutrophil extracellular traps. Scand. J. Immunol. 2016, 84, 317–322. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Urrutia, R.; Yipp, B.G.; Jenne, C.N.; Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012, 12, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.R.; Kulkarni, O.P.; Mulay, S.R.; Darisipudi, M.N.; Romoli, S.; Thomasova, D.; Scherbaum, C.R.; Hohenstein, B.; Hugo, C.; Müller, S.; et al. Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J. Am. Soc. Nephrol. 2015, 26, 2399–2413. [Google Scholar] [CrossRef]
- Allam, R.; Kumar, S.V.R.; Darisipudi, M.N.; Anders, H.-J. Extracellular histones in tissue injury and inflammation. J. Mol. Med. 2014, 92, 465–472. [Google Scholar] [CrossRef]
- Giacalone, V.D.; Margaroli, C.; Mall, M.A.; Tirouvanziam, R. Neutrophil adaptations upon recruitment to the lung: New concepts and implications for homeostasis and disease. Int. J. Mol. Sci. 2020, 21, 851. [Google Scholar] [CrossRef]
- Camicia, G.; Pozner, R.; de Larranaga, G. Neutrophil extracellular traps in sepsis. Shock 2014, 42, 286–294. [Google Scholar] [CrossRef]
- Lipinska-Gediga, M. Neutrophils, NETs, NETosis—Old or new factors in sepsis and septic shock? Anaesthesiol. Intensive Ther. 2017, 49, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Colon, D.F.; Wanderley, C.W.; Franchin, M.; Silva, C.M.; Hiroki, C.H.; Castanheira, F.V.S.; Donate, P.B.; Lopes, A.H.; Volpon, L.C.; Kavaguti, S.K.; et al. Neutrophil extracellular traps (NETs) exacerbate severity of infant sepsis. Crit. Care 2019, 23, 113. [Google Scholar] [CrossRef]
- Fujie, K.; Shinguh, Y.; Inamura, N.; Yasumitsu, R.; Okamoto, M.; Okuhara, M. Release of neutrophil elastase and its role in tissue injury in acute inflammation: Effect of the elastase inhibitor, FR134043. Eur. J. Pharmacol. 1999, 374, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, O.Z.; Palaniyar, N. NET balancing: A problem in inflammatory lung diseases. Front. Immunol. 2013, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Chambers, R.C. The mercurial nature of neutrophils: Still an enigma in ARDS? Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L217–L230. [Google Scholar] [CrossRef]
- Lefrancais, E.; Mallavia, B.; Zhuo, H.; Calfee, C.S.; Looney, M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 2018, 3, e98178. [Google Scholar] [CrossRef]
- Bachofen, M.; Weibel, E.R. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin. Chest Med. 1982, 3, 35–56. [Google Scholar] [CrossRef]
- Gao, X.; Yan, X.; Yin, Y.; Lin, X.; Zhang, Q.; Xia, Y.; Cao, J. Therapeutic targeting of apoptosis inhibitor of macrophage/CD5L in sepsis. Am. J. Respir. Cell Mol. Biol. 2019, 60, 323–334. [Google Scholar] [CrossRef]
- Hiramoto, E.; Tsutsumi, A.; Suzuki, R.; Matsuoka, S.; Arai, S.; Kikkawa, M.; Miyazaki, T. The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci. Adv. 2018, 4, eaau1199. [Google Scholar] [CrossRef]
- Blandino, R.; Baumgarth, N. Secreted IgM: New tricks for an old molecule. J. Leukoc. Biol. 2019, 106, 1021–1034. [Google Scholar] [CrossRef]
- Stehr, S.N.; Knels, L.; Weissflog, C.; Schober, J.; Haufe, D.; Lupp, A.; Koch, T.; Heller, A.R. Effects of IGM-enriched solution on polymorphonuclear neutrophil function, bacterial clearance, and lung histology in endotoxemia. Shock 2008, 29, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ates, I.; Dogan, N.; Aksoy, M.; Halici, Z.; Gundogdu, C.; Keles, M.S. The protective effects of IgM-enriched immunoglobulin and erythropoietin on the lung and small intestine tissues of rats with induced sepsis: Biochemical and histopathological evaluation. Pharm. Biol. 2015, 53, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Lin, N.T.; Subeq, Y.M.; Lee, R.P.; Chen, I.H.; Hsu, B.G. Erythropoietin protects severe haemorrhagic shock-induced organ damage in conscious rats. Injury 2010, 41, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, R.A.; van Kaam, A.H.; Haitsma, J.J.; Verbrugge, S.J.; Delreu, F.; Lachmann, B. Immunoglobulin M-enriched intravenous polyclonal immunoglobulins reduce bacteremia following Klebsiella pneumoniae infection in an acute respiratory distress syndrome rat model. Exp. Lung Res. 2004, 30, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Yasumoto, H.; Shimizu, M.; Hamaoka, S.; Kinoshita, M.; Akiyama, K.; Sawa, T. IV immunoglobulin for acute lung injury and bacteremia in Pseudomonas aeruginosa pneumonia*. Crit. Care Med. 2016, 44, e12–e24. [Google Scholar] [CrossRef]
- Hagiwara, J.; Yamada, M.; Motoda, N.; Yokota, H. Intravenous immunoglobulin attenuates cecum ligation and puncture-induced acute lung injury by inhibiting apoptosis of alveolar epithelial cells. J. Nippon. Med. Sch. 2020, 87, 129–137. [Google Scholar] [CrossRef]
- Rockman, S.; Lowther, S.; Camuglia, S.; Vandenberg, K.; Taylor, S.; Fabri, L.; Miescher, S.; Pearse, M.; Middleton, D.; Kent, S.J.; et al. Intravenous immunoglobulin protects against severe pandemic influenza infection. EBioMedicine 2017, 19, 119–127. [Google Scholar] [CrossRef]
- Hung, I.F.N.; To, K.K.W.; Lee, C.K.; Lee, K.L.; Yan, W.W.; Chan, K.; Chan, W.M.; Ngai, C.W.; Law, K.I.; Chow, F.L.; et al. Hyperimmune IV immunoglobulin treatment: A multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 2013, 144, 464–473. [Google Scholar] [CrossRef]
- Tagami, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Intravenous immunoglobulin and mortality in pneumonia patients with septic shock: An observational nationwide study. Clin. Infect. Dis. 2015, 61, 385–392. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Zhang, F.; Xia, Y. Evaluation of the effect of intravenous immunoglobulin dosing on mortality in patients with sepsis: A network meta-analysis. Clin. Ther. 2019, 41, 1823–1838.e4. [Google Scholar] [CrossRef]
- Soares, M.O.; Welton, N.J.; Harrison, D.A.; Peura, P.; Shankar-Hari, M.; Harvey, S.E.; Madan, J.; Ades, A.E.; Rowan, K.M.; Palmer, S.J. Intravenous immunoglobulin for severe sepsis and septic shock: Clinical effectiveness, cost-effectiveness and value of a further randomised controlled trial. Crit. Care 2014, 18, 649. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Kirkpatrick, A.W.; Delaney, A. Polyclonal intravenous immunoglobulin for the treatment of severe sepsis and septic shock in critically ill adults: A systematic review and meta-analysis. Crit. Care Med. 2007, 35, 2686–2692. [Google Scholar] [PubMed]
- Liu, X.; Zhang, Y.; Lu, L.; Li, X.; Wu, Y.; Yang, Y.; Li, T.; Cao, W. Benefits of high-dose intravenous immunoglobulin on mortality in patients with severe COVID-19: An updated systematic review and meta-analysis. Front. Immunol. 2023, 14, 1116738. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Cao, S.; Dong, H.; Li, Q.; Chen, E.; Zhang, W.; Yang, L.; Fu, S.; Wang, R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-G.; Xie, S.-M.; Zhang, J.; Zheng, F.; Jiang, D.-X.; Li, K.-Y.; Zuo, Q.; Yan, Y.-S.; Liu, J.-Y.; Xie, Y.-L.; et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints 2020, 2020030065. [Google Scholar] [CrossRef]
- Cao, W.; Liu, X.; Bai, T.; Fan, H.; Hong, K.; Song, H.; Han, Y.; Lin, L.; Ruan, L.; Li, T. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect. Dis. 2020, 7, ofaa102. [Google Scholar] [CrossRef]
- Marcec, R.; Dodig, V.M.; Radanovic, I.; Likic, R. Intravenous immunoglobulin (IVIg) therapy in hospitalised adult COVID-19 patients: A systematic review and meta-analysis. Rev. Med. Virol. 2022, 32, e2397. [Google Scholar] [CrossRef]
- Ho, J.C.; Wu, A.Y.; Lam, B.; Ooi, G.C.; Khong, P.L.; Ho, P.L.; Chan-Yeung, M.; Zhong, N.S.; Ko, C.; Lam, W.K.; et al. Pentaglobin in steroid-resistant severe acute respiratory syndrome. Int. J. Tuberc. Lung Dis. 2004, 8, 1173–1179. [Google Scholar]
- Carannante, N.; Fiorentino, G.; Corcione, A.; Di Sarno, R.; Spatarella, M.; Maturo, N.; Fragranza, F.; Di Micco, P. Administration of immunoglobulins in SARS-CoV-2-positive patient is associated with fast clinical and radiological healing: Case report. Front. Med. 2020, 7, 388. [Google Scholar] [CrossRef]
- Tabarsi, P.; Hashemian, S.M.R.; Bauhofer, A.; Savadkoohi, A.A.; Ghadimi, S.; Haseli, S.; Dastan, F. IgM-enriched immunoglobulin in COVID-19: Case series of 15 severely ill SARS-CoV-2-infected patients. Int. Immunopharmacol. 2021, 99, 107998. [Google Scholar] [CrossRef]
- Agafina, A.; Aguiar, V.C.; Rossovskaya, M.; Fartoukh, M.S.; Thiéry, G.; Timsit, J.-F.; Gordeev, I.; Protsenko, D.; Carbone, J.; Pellegrini, R.; et al. Efficacy and Safety of Trimodulin in Patients with Severe COVID-19: A Randomised, Placebo-Controlled, Double-Blind, Multicentre, Phase II Trial (ESsCOVID) (in Preparation). Available online: https://clinicaltrials.gov/ (accessed on 7 November 2023).
- Rahmel, T.; Kraft, F.; Haberl, H.; Achtzehn, U.; Brandenburger, T.; Neb, H.; Jarczak, D.; Dietrich, M.; Magunia, H.; Zimmer, F.; et al. Intravenous IgM-enriched immunoglobulins in critical COVID-19: A multicentre propensity-weighted cohort study. Crit. Care 2022, 26, 204. [Google Scholar] [CrossRef] [PubMed]
- Tascini, C.; Cotrufo, M.; Sozio, E.; Fanin, M.; Dellai, F.; Zanus Forte, A.; Cesselli, D.; DE Stefanis, P.; Ripoli, A.; Sbrana, F.; et al. Potential role of IgM-enriched immunoglobulin as adjuvant treatment in severe SARS-CoV-2 infection. Minerva Anestesiol. 2023, 89, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Werdan, K.; Pilz, G.; Bujdoso, O.; Fraunberger, P.; Neeser, G.; Schmieder, R.E.; Viell, B.; Marget, W.; Seewald, M.; Walger, P.; et al. Score-based immunoglobulin G therapy of patients with sepsis: The SBITS study. Crit. Care Med. 2007, 35, 2693–2701. [Google Scholar]
- Wand, S.; Klages, M.; Kirbach, C.; Warszawska, J.; Meybohm, P.; Zacharowski, K.; Koch, A. IgM-enriched immunoglobulin attenuates systemic endotoxin activity in early severe sepsis: A before-after cohort study. PLoS ONE 2016, 11, e0160907. [Google Scholar] [CrossRef] [PubMed]
- Wesoly, C.; Kipping, N.; Grundmann, R. Immunoglobulin therapy of postoperative sepsis. Z. Exp. Chir. Transpl. Kunstl. Organe 1990, 23, 213–216. [Google Scholar]
- Just, H.M.; Metzger, M.; Vogel, W.; Pelka, R.B. Effect of adjuvant immunoglobulin therapy on infections in patients in an surgical intensive care unit. Results of a randomized controlled study. Klin. Wochenschr. 1986, 64, 245–256. [Google Scholar] [CrossRef]
- Cavazzuti, I.; Serafini, G.; Busani, S.; Rinaldi, L.; Biagioni, E.; Buoncristiano, M.; Girardis, M. Early therapy with IgM-enriched polyclonal immunoglobulin in patients with septic shock. Intensive Care Med. 2014, 40, 1888–1896. [Google Scholar] [CrossRef]
- Cui, J.; Wei, X.; Lv, H.; Li, Y.; Li, P.; Chen, Z.; Liu, G. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: A meta-analysis with trial sequential analysis. Ann. Intensive Care 2019, 9, 27. [Google Scholar] [CrossRef]
- Tan, P.; Luscinskas, F.W.; Homer-Vanniasinkam, S. Cellular and molecular mechanisms of inflammation and thrombosis. Eur. J. Vasc. Endovasc. Surg. 1999, 17, 373–389. [Google Scholar] [CrossRef]
- Lerman, Y.V.; Kim, M. Neutrophil migration under normal and sepsis conditions. Cardiovasc. Hematol. Disord. Drug Targets 2015, 15, 19–28. [Google Scholar] [CrossRef]
- Jersmann, H.P.; Hii, C.S.; Ferrante, J.V.; Ferrante, A. Bacterial lipopolysaccharide and tumor necrosis factor alpha synergistically increase expression of human endothelial adhesion molecules through activation of NF-kappaB and p38 mitogen-activated protein kinase signaling pathways. Infect. Immun. 2001, 69, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Drost, E.M.; Kassabian, G.; Meiselman, H.J.; Gelmont, D.; Fisher, T.C. Increased rigidity and priming of polymorphonuclear leukocytes in sepsis. Am. J. Respir. Crit. Care Med. 1999, 159, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, L.B. Sepsis/septic shock: Participation of the microcirculation: An abbreviated review. Crit. Care Med. 1996, 24, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Koike, Y.; Shimura, T.; Okigami, M.; Ide, S.; Toiyama, Y.; Okugawa, Y.; Inoue, Y.; Araki, T.; Uchida, K.; et al. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS ONE 2014, 9, e111888. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, S.; Wang, Y.; Rahman, M.; Syk, I.; Zhang, E.; Thorlacius, H. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L586–L596. [Google Scholar] [CrossRef]
- Czaikoski, P.G.; Mota, J.M.S.C.; Nascimento, D.C.; Sônego, F.; Castanheira, F.V.e.S.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef]
- Xu, C.; Poirier, B.; Duong Van Huyen, J.P.; Lucchiari, N.; Michel, O.; Chevalier, J.; Kaveri, S. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins: A possible mechanism of action in vascular diseases. Am. J. Pathol. 1998, 153, 1257–1266. [Google Scholar] [CrossRef]
- Ichiyama, T.; Ueno, Y.; Isumi, H.; Niimi, A.; Matsubara, T.; Furukawa, S. An immunoglobulin agent (IVIG) inhibits NF-kappaB activation in cultured endothelial cells of coronary arteries in vitro. Inflamm. Res. 2004, 53, 253–256. [Google Scholar] [CrossRef]
- Radder, C.M.; Beekhuizen, H.; Kanhai, H.H.H.; Brand, A. Effect of maternal anti-HPA-1a antibodies and polyclonal IVIG on the activation status of vascular endothelial cells. Clin. Exp. Immunol. 2004, 137, 216–222. [Google Scholar] [CrossRef]
- Matsuda, A.; Morita, H.; Unno, H.; Saito, H.; Matsumoto, K.; Hirao, Y.; Munechika, K.; Abe, J. Anti-inflammatory effects of high-dose IgG on TNF-alpha-activated human coronary artery endothelial cells. Eur. J. Immunol. 2012, 42, 2121–2131. [Google Scholar] [CrossRef]
- Ito, Y.; Lukita-Atmadja, W.; Machen, N.W.; Baker, G.L.; McCuskey, R.S. Effect of intravenous immunoglobulin G on the TNFalpha-mediated hepatic microvascular inflammatory response. Shock 1999, 11, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Lukita-Atmadja, W.; Machen, N.W.; Baker, G.L.; McCuskey, R.S. High doses of intravenous immunoglobulin G enhance Kupffer cell phagocytic function during the late phase of sepsis and endotoxemia in rats. Shock 2000, 13, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, B.M.; Herx, L.M.; Gill, V.; Metz, L.M.; Kubes, P. IVIg therapy in brain inflammation: Etiology-dependent differential effects on leucocyte recruitment. Brain 2004, 127, 2649–2656. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Clark, W.M. Strokes, thromboembolic events, and IVIg: Rare incidents blemish an excellent safety record. Neurology 2003, 60, 1736–1737. [Google Scholar] [CrossRef]
- Calverley, D.C.; Brass, E.; Hacker, M.R.; Tsao-Wei, D.D.; Espina, B.M.; Pullarkat, V.A.; Hodis, H.N.; Groshen, S. Potential role of platelet FcgammaRIIA in collagen-mediated platelet activation associated with atherothrombosis. Atherosclerosis 2002, 164, 261–267. [Google Scholar] [CrossRef]
- Jang, J.E.; Hidalgo, A.; Frenette, P.S. Intravenous immunoglobulins modulate neutrophil activation and vascular injury through FcgammaRIII and SHP-1. Circ. Res. 2012, 110, 1057–1066. [Google Scholar] [CrossRef]
- Gill, V.; Doig, C.; Knight, D.; Love, E.; Kubes, P. Targeting adhesion molecules as a potential mechanism of action for intravenous immunoglobulin. Circulation 2005, 112, 2031–2039. [Google Scholar] [CrossRef]
- Hoffman, J.N.; Fertmann, J.M.; Vollmar, B.; Laschke, M.W.; Jauch, K.W.; Menger, M.D. Immunoglobulin M-enriched human intravenous immunoglobulins reduce leukocyte-endothelial cell interactions and attenuate microvascular perfusion failure in normotensive endotoxemia. Shock 2008, 29, 133–139. [Google Scholar] [CrossRef]
- Dominioni, L.; Dionigi, R.; Zanello, M.; Chiaranda, M.; Dionigi, R.; Acquarolo, A.; Ballabio, A.; Sguotti, C. Effects of high-dose IgG on survival of surgical patients with sepsis scores of 20 or greater. Arch. Surg. 1991, 126, 236–240. [Google Scholar] [CrossRef]
- Dominioni, L.; Bianchi, V.; Imperatori, A.; Minoia, G.; Dionigi, R. High-dose intravenous IgG for treatment of severe surgical infections. Dig. Surg. 1996, 13, 430–434. [Google Scholar] [CrossRef]
- Corona, A.; Richini, G.; Simoncini, S.; Zangrandi, M.; Biasini, M.; Russo, G.; Pasqua, M.; Santorsola, C.; Gregorini, C.; Giordano, C. Treating critically ill patients experiencing SARS-CoV-2 severe infection with Ig-M and Ig-A enriched Ig-G infusion. Antibiotics 2021, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Domizi, R.; Adrario, E.; Damiani, E.; Scorcella, C.; Carsetti, A.; Giaccaglia, P.; Casarotta, E.; Gabbanelli, V.; Pantanetti, S.; Lamura, E.; et al. IgM-enriched immunoglobulins (Pentaglobin) may improve the microcirculation in sepsis: A pilot randomized trial. Ann. Intensive Care 2019, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Berlot, G.; Vassallo, M.C.; Busetto, N.; Bianchi, M.; Zornada, F.; Rosato, I.; Tartamella, F.; Prisco, L.; Bigotto, F.; Bigolin, T.; et al. Relationship between the timing of administration of IgM and IgA enriched immunoglobulins in patients with severe sepsis and septic shock and the outcome: A retrospective analysis. J. Crit. Care 2012, 27, 167–171. [Google Scholar] [CrossRef]
- Berlot, G.; Zanchi, S.; Moro, E.; Tomasini, A.; Bixio, M. The role of the intravenous IgA and IgM-enriched immunoglobulin preparation in the treatment of sepsis and septic shock. J. Clin. Med. 2023, 12, 4645. [Google Scholar] [CrossRef]
- Berlot, G.; Vassallo, M.C.; Busetto, N.; Nieto Yabar, M.; Istrati, T.; Baronio, S.; Quarantotto, G.; Bixio, M.; Barbati, G.; Dattola, R.; et al. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann. Intensive Care 2018, 8, 122. [Google Scholar] [CrossRef]
- Chauhan, A.J.; Wiffen, L.J.; Brown, T.P. COVID-19: A collision of complement, coagulation and inflammatory pathways. J. Thromb. Haemost. 2020, 18, 2110–2117. [Google Scholar] [CrossRef]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M.; Filler, S.G. Endothelial cells, tissue factor and infectious diseases. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2003, 36, 987–991. [Google Scholar] [CrossRef]
- Pate, M.; Damarla, V.; Chi, D.S.; Negi, S.; Krishnaswamy, G. Endothelial cell biology: Role in the inflammatory response. Adv. Clin. Chem. 2010, 52, 109–130. [Google Scholar] [CrossRef]
- Jenne, C.N.; Urrutia, R.; Kubes, P. Platelets: Bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013, 35, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Zucoloto, A.Z.; Jenne, C.N. Platelet-neutrophil interplay: Insights into neutrophil extracellular trap (NET)-driven coagulation in infection. Front. Cardiovasc. Med. 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.P.; Miller-Dorey, S.; Jenne, C.N. Platelets and coagulation in infection. Clin. Transl. Immunol. 2016, 5, e89. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Sarma, J.V.; Zetoune, F.S.; Rittirsch, D.; Neff, T.A.; McGuire, S.R.; Lambris, J.D.; Warner, R.L.; Flierl, M.A.; Hoesel, L.M.; et al. Generation of C5a in the absence of C3: A new complement activation pathway. Nat. Med. 2006, 12, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Amara, U.; Flierl, M.A.; Rittirsch, D.; Klos, A.; Chen, H.; Acker, B.; Bruckner, U.B.; Nilsson, B.; Gebhard, F.; Lambris, J.D.; et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010, 185, 5628–5636. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Yin, W.; Ghebrehiwet, B. Platelet mediated complement activation. Adv. Exp. Med. Biol. 2008, 632, 81–91. [Google Scholar] [CrossRef]
- Kastl, S.P.; Speidl, W.S.; Kaun, C.; Rega, G.; Assadian, A.; Weiss, T.W.; Valent, P.; Hagmueller, G.W.; Maurer, G.; Huber, K.; et al. The complement component C5a induces the expression of plasminogen activator inhibitor-1 in human macrophages via NF-kappaB activation. J. Thromb. Haemost. 2006, 4, 1790–1797. [Google Scholar] [CrossRef]
- Voves, C.; Wuillemin, W.A.; Zeerleder, S. International Society on Thrombosis and Haemostasis score for overt disseminated intravascular coagulation predicts organ dysfunction and fatality in sepsis patients. Blood Coagul. Fibrinolysis 2006, 17, 445–451. [Google Scholar] [CrossRef]
- Asakura, H.; Suga, Y.; Yoshida, T.; Ontachi, Y.; Mizutani, T.; Kato, M.; Ito, T.; Morishita, E.; Yamazaki, M.; Miyamoto, K.; et al. Pathophysiology of disseminated intravascular coagulation (DIC) progresses at a different rate in tissue factor-induced and lipopolysaccharide-induced DIC models in rats. Blood Coagul. Fibrinolysis 2003, 14, 221–228. [Google Scholar] [CrossRef]
- Asakura, H.; Takahashi, Y.; Kubo, A.; Ontachi, Y.; Hayashi, T.; Omote, M.; Arahata, M.; Kadohira, Y.; Maekawa, M.; Yamazaki, M.; et al. Immunoglobulin preparations attenuate organ dysfunction and hemostatic abnormality by suppressing the production of cytokines in lipopolysaccharide-induced disseminated intravascular coagulation in rats. Crit. Care Med. 2006, 34, 2421–2425. [Google Scholar] [CrossRef]
- Wiebe, F.; Handtke, S.; Wesche, J.; Schnarre, A.; Palankar, R.; Wolff, M.; Jahn, K.; Voß, F.; Weißmüller, S.; Schüttrumpf, J.; et al. Polyvalent immunoglobulin preparations inhibit pneumolysin-induced platelet destruction. Thromb. Haemost. 2022, 122, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K.; Handtke, S.; Palankar, R.; Weissmuller, S.; Nouailles, G.; Kohler, T.P.; Wesche, J.; Rohde, M.; Heinz, C.; Aschenbrenner, A.F.; et al. Pneumolysin induces platelet destruction, not platelet activation, which can be prevented by immunoglobulin preparations in vitro. Blood Adv. 2020, 4, 6315–6326. [Google Scholar] [CrossRef] [PubMed]
- Katz, U.; Achiron, A.; Sherer, Y.; Shoenfeld, Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun. Rev. 2007, 6, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Romero-Garrido, J.A.; López-Granados, E.; Borobia, A.M.; Pérez, T.; Medrano, N.; Rueda, C.; Tong, H.Y.; Herrero, A.; Frías, J. Symptomatic thromboembolic events in patients treated with intravenous-immunoglobulins: Results from a retrospective cohort study. Thromb. Res. 2014, 133, 1045–1051. [Google Scholar] [CrossRef]
- Marie, I.; Maurey, G.; Hervé, F.; Hellot, M.F.; Levesque, H. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br. J. Dermatol. 2006, 155, 714–721. [Google Scholar] [CrossRef]
- Pilz, G.; Fateh-Moghadam, S.; Werdan, K. Scoring systems for the early detection of septic complications in intensive care patients. Krankenpfl. J. 1991, 29, 483–492. [Google Scholar]
- Werdan, K.; Pilz, G. Supplemental immune globulins in sepsis: A critical appraisal. Clin. Exp. Immunol. 1996, 104 (Suppl. S1), S83–S90. [Google Scholar] [CrossRef]
- Hamed, G.; Rizk, A.; Aziz, K.A.; Andraos, A.; Aziz, A.A.; ElNaggar, A.; Kamal, A.; Mokhtar, S. Comparative study between intravenous immunoglobulins and standard treatment in septic patients. Crit. Care 2008, 12 (Suppl. S2), P454. [Google Scholar] [CrossRef]
- Darenberg, J.; Ihendyane, N.; Sjölin, J.; Aufwerber, E.; Haidl, S.; Follin, P.; Andersson, J.; Norrby-Teglund, A. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: A European randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2003, 37, 333–340. [Google Scholar] [CrossRef]
- Iizuka, Y.; Sanui, M.; Sasabuchi, Y.; Lefor, A.K.; Hayakawa, M.; Saito, S.; Uchino, S.; Yamakawa, K.; Kudo, D.; Takimoto, K.; et al. Low-dose immunoglobulin G is not associated with mortality in patients with sepsis and septic shock. Crit. Care 2017, 21, 181. [Google Scholar] [CrossRef]
- Schedel, I.; Dreikhausen, U.; Nentwig, B.; Hockenschnieder, M.; Rauthmann, D.; Balikcioglu, S.; Coldewey, R.; Deicher, H. Treatment of gram-negative septic shock with an immunoglobulin preparation: A prospective, randomized clinical trial. Crit. Care Med. 1991, 19, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Foster, D.; Vincent, J.L.; Cook, D.J.; Cohen, J.; Dellinger, R.P.; Opal, S.; Abraham, E.; Brett, S.J.; Smith, T.; et al. Diagnostic and prognostic implications of endotoxemia in critical illness: Results of the MEDIC study. J. Infect. Dis. 2004, 190, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, L.; Aynali, G.; Aynali, A.; Alaca, A.; Kutuk, S.; Ceylan, B.G. The effects of adjuvant immunoglobulin M-enriched immunoglobulin therapy on mortality rate and renal function in sepsis-induced multiple organ dysfunction syndrome: Retrospective analysis of intensive care unit patients. J. Int. Med. Res. 2012, 40, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Karatzas, S.; Boutzouka, E.; Venetsanou, K.; Myrianthefs, P.; Fildisis, G.; Baltopoulos, G. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis: Another point of view. Crit. Care 2002, 6, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Kreymann, K.G.; de Heer, G.; Nierhaus, A.; Kluge, S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit. Care Med. 2007, 35, 2677–2685. [Google Scholar]
- De Rosa, F.G.; Corcione, S.; Tascini, C.; Pasero, D.; Rocchetti, A.; Massaia, M.; Berlot, G.; Solidoro, P.; Girardis, M. A Position Paper on IgM-Enriched Intravenous Immunoglobulin Adjunctive Therapy in Severe Acute Bacterial Infections: The TO-PIRO SCORE Proposal. New Microbiol. 2019, 42, 176–180. [Google Scholar]
- Kakoullis, L.; Pantzaris, N.D.; Platanaki, C.; Lagadinou, M.; Papachristodoulou, E.; Velissaris, D. The use of IgM-enriched immunoglobulin in adult patients with sepsis. J. Crit. Care 2018, 47, 30–35. [Google Scholar] [CrossRef]
- Neilson, A.R.; Burchardi, H.; Schneider, H. Cost-effectiveness of immunoglobulin M-enriched immunoglobulin (Pentaglobin) in the treatment of severe sepsis and septic shock. J. Crit. Care 2005, 20, 239–249. [Google Scholar] [CrossRef]
- Busani, S.; Damiani, E.; Cavazzuti, I.; Donati, A.; Girardis, M. Intravenous immunoglobulin in septic shock: Review of the mechanisms of action and meta-analysis of the clinical effectiveness. Minerva Anestesiol. 2016, 82, 559–572. [Google Scholar]
- Norrby-Teglund, A.; Haque, K.N.; Hammarstrom, L. Intravenous polyclonal IgM-enriched immunoglobulin therapy in sepsis: A review of clinical efficacy in relation to microbiological aetiology and severity of sepsis. J. Intern. Med. 2006, 260, 509–516. [Google Scholar] [CrossRef]
- Rodriguez, A.; Rello, J.; Neira, J.; Maskin, B.; Ceraso, D.; Vasta, L.; Palizas, F. Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery. Shock 2005, 23, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Tascini, C.; Fraganza, F.; Salani, F.; Sozio, E.; Rossi, M.; Sbrana, F.; Carannante, N.; Chiesa, M.D.; Ripoli, A.; Bertolino, G.; et al. Potential role of IgM-enriched immunoglobulin as adjuvant treatment for invasive meningococcal disease. Intensive Care Med. 2018, 44, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.; Wilson, C.; Curtis, N.; Norrby-Teglund, A.; Sriskandan, S. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: A systematic review and meta-analysis. Clin. Infect. Dis. 2018, 67, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Tziolos, N.; Routsi, C.; Katsenos, C.; Tsangaris, I.; Pneumatikos, I.; Vlachogiannis, G.; Theodorou, V.; Prekates, A.; Antypa, E.; et al. Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins. Clin. Microbiol. Infect. 2016, 22, 499–506. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Use of intravenous immunoglobulins in sepsis therapy—A clinical view. Int. J. Mol. Sci. 2020, 21, 5543. [Google Scholar] [CrossRef]
- Popov, D.; Yaroustovsky, M.; Lobacheva, G. Prevention of infectious complications after heart surgery in children: Procalcitonin-guided strategy. Kardiochir. Torakochirurgia Pol. 2014, 11, 140–144. [Google Scholar] [CrossRef]
- Busani, S.; Roat, E.; Tosi, M.; Biagioni, E.; Coloretti, I.; Meschiari, M.; Gelmini, R.; Brugioni, L.; De Biasi, S.; Girardis, M. Adjunctive Immunotherapy with Polyclonal Ig-M Enriched Immunoglobulins for Septic Shock: From Bench to Bedside. The Rationale for a Personalized Treatment Protocol. Front. Med. 2021, 8, 616511. [Google Scholar] [CrossRef]
- Biagioni, E.; Tosi, M.; Berlot, G.; Castiglione, G.; Corona, A.; De Cristofaro, M.G.; Donati, A.; Feltracco, P.; Forfori, F.; Fragranza, F.; et al. Adjunctive IgM-enriched immunoglobulin therapy with a personalised dose based on serum IgM-titres versus standard dose in the treatment of septic shock: A randomised controlled trial (IgM-fat trial). BMJ Open 2021, 11, e036616. [Google Scholar] [CrossRef]
- Amreen, S.; Brar, S.K.; Perveen, S.; Chaudhry, M.R.; AlBabtain, S.; Khan, S. Clinical Efficacy of Intravenous Immunoglobulins in Management of Toxic Shock Syndrome: An Updated Literature Review. Cureus 2021, 13, 1–8. [Google Scholar] [CrossRef]
- Cagri Dinleyici, E.; Frey, G.; Kola, E.; Wippermann, U.; Bauhofer, A.; Staus, A.; Griffiths, P.; Azharry, M.; Rohsiswatmo, R. Clinical efficacy of IgM-enriched immunoglobulin as adjunctive therapy in neonatal and pediatric sepsis: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1239014. [Google Scholar] [CrossRef]
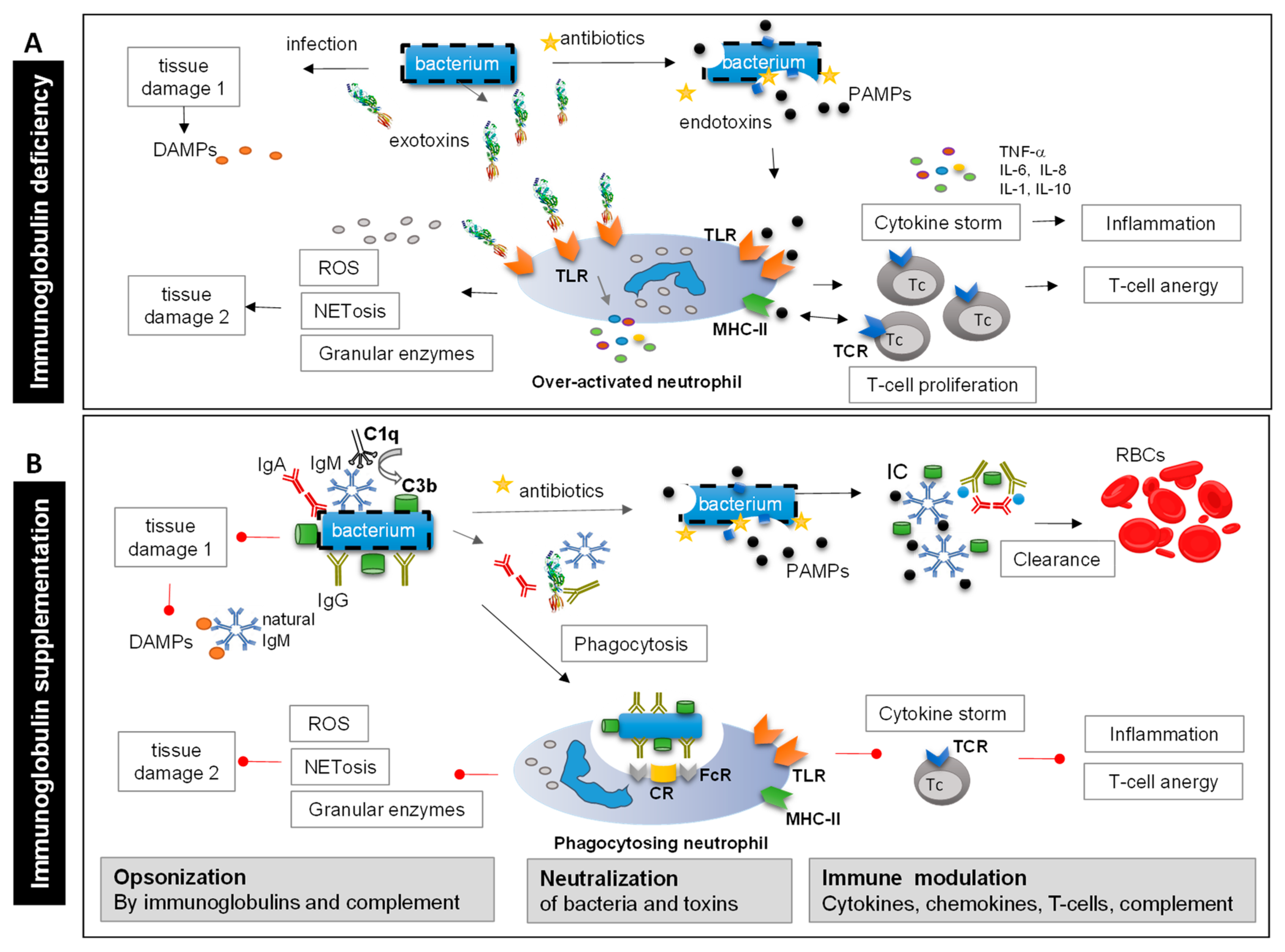
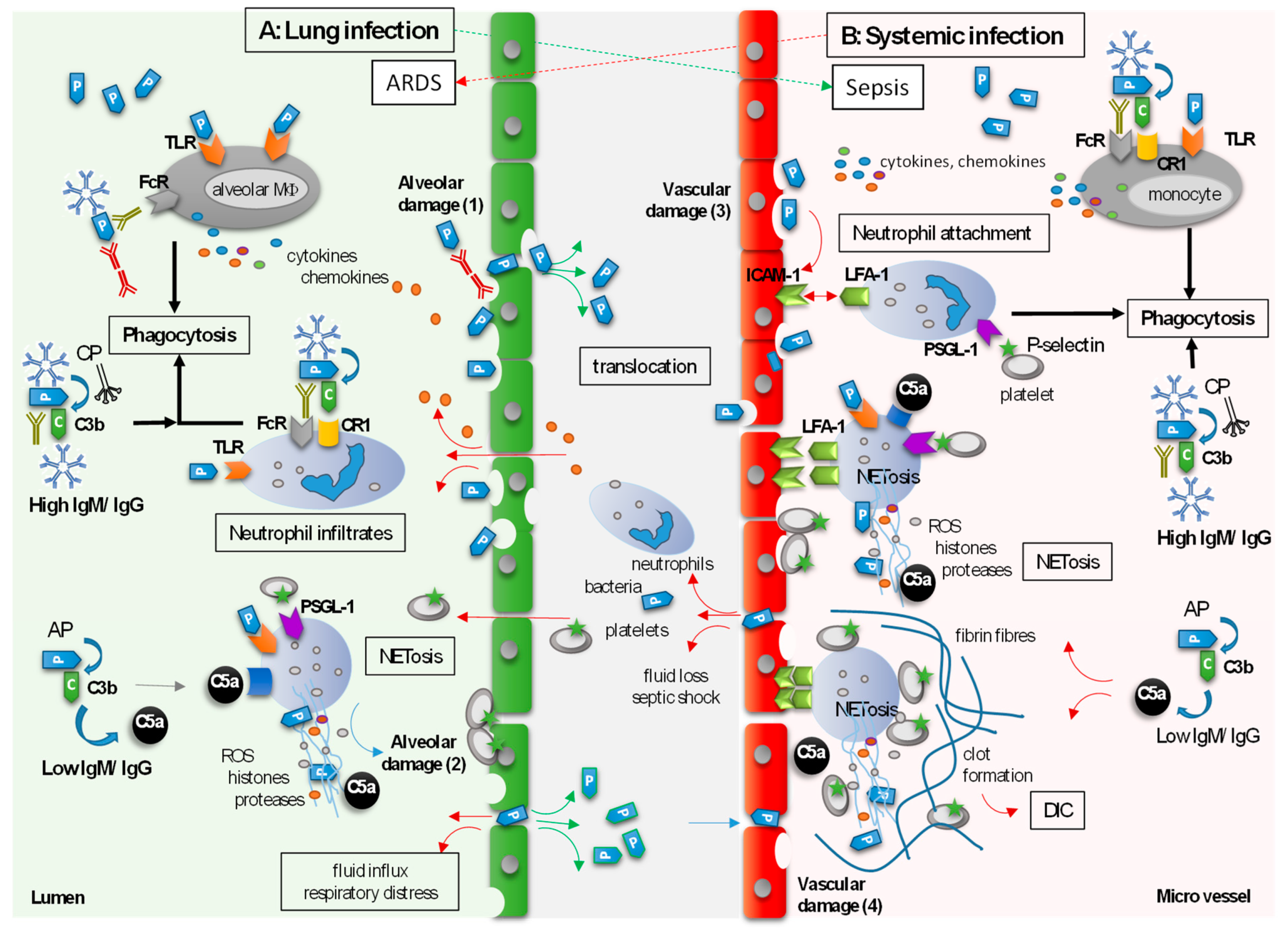
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, C.; Weißmüller, S.; Heinz, C.C. Multifaceted Tissue-Protective Functions of Polyvalent Immunoglobulin Preparations in Severe Infections—Interactions with Neutrophils, Complement, and Coagulation Pathways. Biomedicines 2023, 11, 3022. https://doi.org/10.3390/biomedicines11113022
Schmidt C, Weißmüller S, Heinz CC. Multifaceted Tissue-Protective Functions of Polyvalent Immunoglobulin Preparations in Severe Infections—Interactions with Neutrophils, Complement, and Coagulation Pathways. Biomedicines. 2023; 11(11):3022. https://doi.org/10.3390/biomedicines11113022
Chicago/Turabian StyleSchmidt, Carolin, Sabrina Weißmüller, and Corina C. Heinz. 2023. "Multifaceted Tissue-Protective Functions of Polyvalent Immunoglobulin Preparations in Severe Infections—Interactions with Neutrophils, Complement, and Coagulation Pathways" Biomedicines 11, no. 11: 3022. https://doi.org/10.3390/biomedicines11113022
APA StyleSchmidt, C., Weißmüller, S., & Heinz, C. C. (2023). Multifaceted Tissue-Protective Functions of Polyvalent Immunoglobulin Preparations in Severe Infections—Interactions with Neutrophils, Complement, and Coagulation Pathways. Biomedicines, 11(11), 3022. https://doi.org/10.3390/biomedicines11113022






