Whole Genome Expression Profiling of Semitendinosus Tendons from Children with Diplegic and Tetraplegic Cerebral Palsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. RNA Extraction
2.3. RNA-Seq Analysis
2.4. Gene Ontology and Pathway Analysis
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
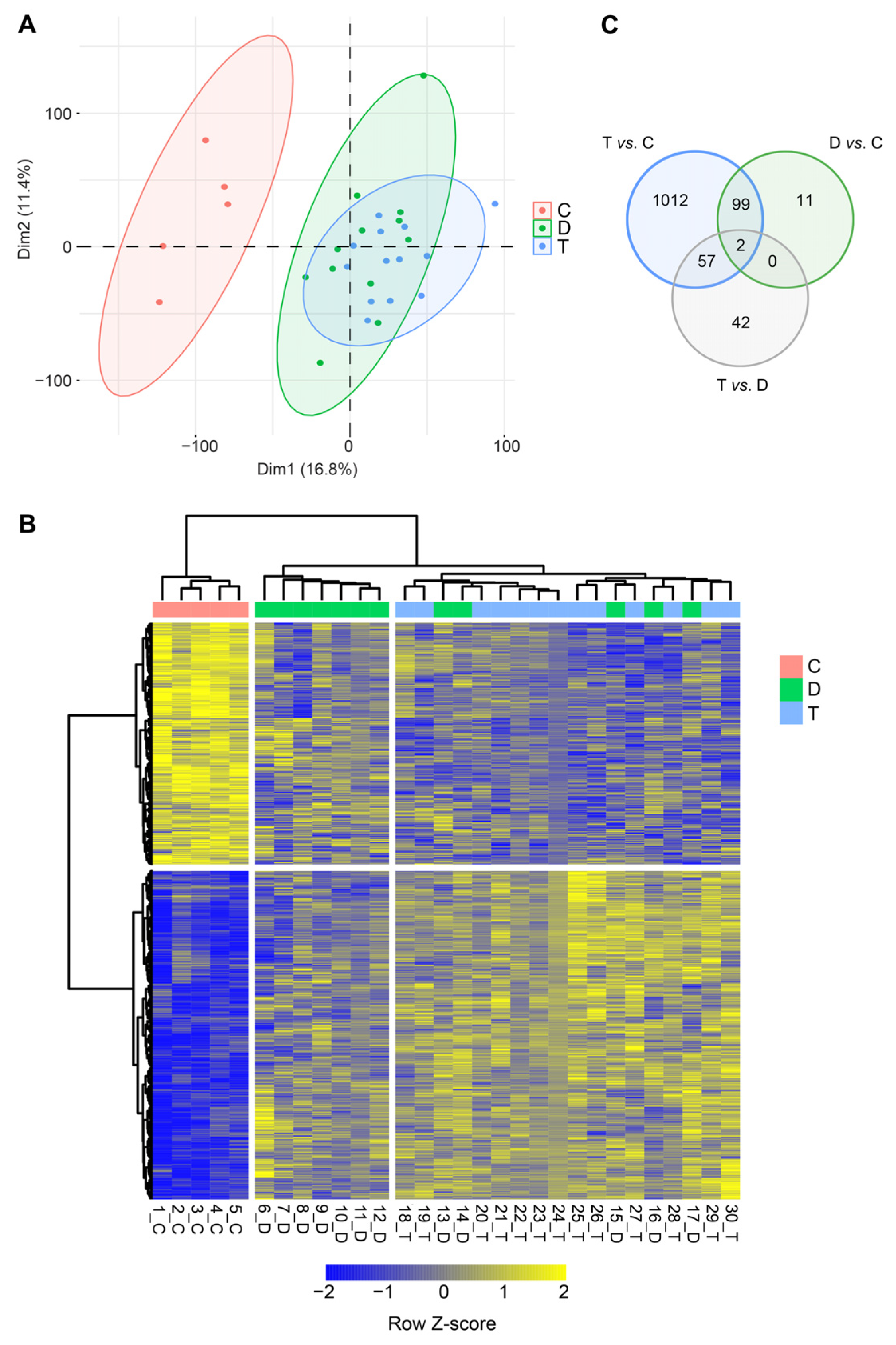
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannus, P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 2000, 10, 312–320. [Google Scholar] [CrossRef]
- Vogel, K.G. What happens when tendons bend and twist? Proteoglycans. J. Musculoskelet. Neuronal Interact. 2004, 4, 202–203. [Google Scholar] [PubMed]
- Ezura, Y.; Chakravarti, S.; Oldberg, A.; Chervoneva, I.; Birk, D.E. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000, 151, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Dunkman, A.A.; Buckley, M.R.; Mienaltowski, M.J.; Adams, S.M.; Thomas, S.J.; Satchell, L.; Kumar, A.; Pathmanathan, L.; Beason, D.P.; Iozzo, R.V.; et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013, 32, 3–13. [Google Scholar] [CrossRef]
- Boote, C.; Ma, Q.; Goh, K.L. Age-dependent mechanical properties of tail tendons in wild-type and mimecan gene-knockout mice—A preliminary study. J. Mech. Behav. Biomed. Mater. 2023, 139, 105672. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Arokiasamy, S.; Pataki, C.; Whiteford, J.R.; Couchman, J.R. Syndecan receptors: Pericellular regulators in development and inflammatory disease. Open Biol. 2021, 11, 200377. [Google Scholar] [CrossRef]
- Wang, J.H. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef]
- Bax, M.; Goldstein, M.; Rosenbaum, P.; Leviton, A.; Paneth, N.; Dan, B.; Jacobsson, B.; Damiano, D. Proposed definition and classification of cerebral palsy, April 2005. Dev. Med. Child Neurol. 2005, 47, 571. [Google Scholar] [CrossRef]
- Paneth, N.; Hong, T.; Korzeniewski, S. The Descriptive Epidemiology of Cerebral Palsy. Clin. Perinatol. 2006, 33, 251–267. [Google Scholar] [CrossRef]
- Yeargin-Allsopp, M.; Van Naarden Braun, K.; Doernberg, N.S.; Benedict, R.E.; Kirby, R.S.; Durkin, M.S. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: A multisite collaboration. Pediatrics 2008, 121, 547–554. [Google Scholar] [CrossRef]
- Surveillance of Cerebral Palsy in Europe. Prevalence and characteristics of children with cerebral palsy in Europe. Dev. Med. Child Neurol. 2002, 44, 633–640. [Google Scholar]
- Van Naarden Braun, K.; Doernberg, N.; Schieve, L.; Christensen, D.; Goodman, A.; Yeargin-Allsopp, M. Birth Prevalence of Cerebral Palsy: A Population-Based Study. Pediatrics 2016, 137, e20152872. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, H.N.; Farghaly, W.M.; Shehata, G.A.; Rageh, T.A.; Metwally, N.A.; Badry, R.; Sayed, M.A.; Abd El Hamed, M.; Abd-Elwarth, A.; Kandil, M.R. Cerebral palsy in Al-Quseir City, Egypt: Prevalence, subtypes, and risk factors. Neuropsychiatr. Dis. Treat. 2014, 10, 1267–1272. [Google Scholar] [PubMed]
- Chang, M.J.; Ma, H.I.; Lu, T.H. Estimating the prevalence of cerebral palsy in Taiwan: A comparison of different case definitions. Res. Dev. Disabil. 2015, 36, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Van Naarden Braun, K.; Doernberg, N.S.; Maenner, M.J.; Arneson, C.L.; Durkin, M.S.; Benedict, R.E.; Kirby, R.S.; Wingate, M.S.; Fitzgerald, R.; et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning—Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev. Med. Child Neurol. 2014, 56, 59–65. [Google Scholar] [CrossRef]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Primers 2016, 2, 15082. [Google Scholar]
- Handsfield, G.G.; Williams, S.; Khuu, S.; Lichtwark, G.; Stott, N.S. Muscle architecture, growth, and biological Remodelling in cerebral palsy: A narrative review. BMC Musculoskelet. Disord. 2022, 23, 233. [Google Scholar]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Gagliano, N.; Menon, A.; Martinelli, C.; Pettinari, L.; Panou, A.; Milzani, A.; Dalle-Donne, I.; Portinaro, N.M. Tendon structure and extracellular matrix components are affected by spasticity in cerebral palsy patients. Muscles Ligaments Tendons J. 2013, 3, 42–50. [Google Scholar] [CrossRef]
- Gagliano, N.; Pelillo, F.; Chiriva-Internati, M.; Picciolini, O.; Costa, F.; Schutt, R.C., Jr.; Gioia, M.; Portinaro, N. Expression profiling of genes involved in collagen turnover in tendons from cerebral palsy patients. Connect. Tissue Res. 2009, 50, 203–208. [Google Scholar] [CrossRef]
- Gagliano, N.; Pelillo, F.; Grizzi, F.; Picciolini, O.; Gioia, M.; Portinaro, N. Gene expression profile of extracellular matrix of tendons in cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 557–558. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Heino, J. The collagen family members as cell adhesion proteins. Bioessays 2007, 29, 1001–1010. [Google Scholar] [CrossRef]
- Gjaltema, R.A.; Bank, R.A. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, A.J.; Pancheri, N.M.; Schiele, N.R. Regulators of collagen crosslinking in developing and adult tendons. Eur. Cells Mater. 2022, 43, 130–152. [Google Scholar] [CrossRef] [PubMed]
- Beach, Z.M.; Bonilla, K.A.; Dekhne, M.S.; Sun, M.; Adams, T.H.; Adams, S.M.; Weiss, S.N.; Rodriguez, A.B.; Shetye, S.S.; Birk, D.E.; et al. Biglycan has a major role in maintenance of mature tendon mechanics. J. Orthop. Res. 2022, 40, 2546–2556. [Google Scholar] [CrossRef]
- Tasheva, E.S.; Koester, A.; Paulsen, A.Q.; Garrett, A.S.; Boyle, D.L.; Davidson, H.J.; Song, M.; Fox, N.; Conrad, G.W. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol. Vis. 2002, 8, 407–415. [Google Scholar]
- Rees, S.G.; Waggett, A.D.; Kerr, B.C.; Probert, J.; Gealy, E.C.; Dent, C.M.; Caterson, B.; Hughes, C.E. Immunolocalisation and expression of keratocan in tendon. Osteoarthr. Cartil. 2009, 17, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wagner, A.; Gehwolf, R.; Yan, W.; Passini, F.S.; Thien, C.; Weissenbacher, N.; Lin, Z.; Lehner, C.; Teng, H.; et al. Load-induced regulation of tendon homeostasis by SPARC, a genetic predisposition factor for tendon and ligament injuries. Sci. Transl. Med. 2021, 13, eabe5738. [Google Scholar] [CrossRef]
- Alexandrov, V.P.; Naimov, S.I. A Prospectus of Tenomodulin. Folia Med. 2016, 58, 19–27. [Google Scholar] [CrossRef]
- Wang, J.H.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology—A minireview of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133–140. [Google Scholar] [CrossRef]
- Jones, F.S.; Jones, P.L. The tenascin family of ECM glycoproteins: Structure, function, and regulation during embryonic development and tissue remodeling. Dev. Dyn. 2000, 218, 235–259. [Google Scholar] [PubMed]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar]
- Docheva, D.; Popov, C.; Alberton, P.; Aszodi, A. Integrin signaling in skeletal development and function. Birth Defects Res. C Embryo Today 2014, 102, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Mousavizadeh, R.; Hojabrpour, P.; Eltit, F.; McDonald, P.C.; Dedhar, S.; McCormack, R.G.; Duronio, V.; Jafarnejad, S.M.; Scott, A. β1 integrin, ILK and mTOR regulate collagen synthesis in mechanically loaded tendon cells. Sci. Rep. 2020, 10, 12644. [Google Scholar] [CrossRef]
- Maeda, E.; Ye, S.; Wang, W.; Bader, D.L.; Knight, M.M.; Lee, D.A. Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech. Model. Mechanobiol. 2012, 11, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Disser, N.P.; Sugg, K.B.; Talarek, J.R.; Sarver, D.C.; Rourke, B.J.; Mendias, C.L. Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth. FASEB J. 2019, 33, 12680–12695. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Mao, Z.; Wei, X.; Lin, L.; Chen, L.; Wang, H.; Fu, X.; Zhang, J.; Yu, C. The roles of TGF-beta1 gene transfer on collagen formation during Achilles tendon healing. Biochem. Biophys. Res. Commun. 2009, 383, 235–239. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Yu, T.; An, S.; Deng, R.; Tan, X.; Crane, J.; Zhang, W.; Pan, D.; Wan, M.; et al. Inhibition of Integrin alphavbeta6 Activation of TGF-beta Attenuates Tendinopathy. Adv. Sci. 2022, 9, e2104469. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, B.; Li, Y.; Liu, X.; Guo, S.; Wang, C.; Li, S.; Wang, D. The Role of Vascular Endothelial Growth Factor in Tendon Healing. Front. Physiol. 2021, 12, 766080. [Google Scholar] [CrossRef]
- Liu, W.; Watson, S.S.; Lan, Y.; Keene, D.R.; Ovitt, C.E.; Liu, H.; Schweitzer, R.; Jiang, R. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol. Cell. Biol. 2010, 30, 4797–4807. [Google Scholar] [CrossRef]
- Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542. [Google Scholar] [CrossRef]
- Kayama, T.; Mori, M.; Ito, Y.; Matsushima, T.; Nakamichi, R.; Suzuki, H.; Ichinose, S.; Saito, M.; Marumo, K.; Asahara, H. Gtf2ird1-Dependent Mohawk Expression Regulates Mechanosensing Properties of the Tendon. Mol. Cell. Biol. 2016, 36, 1297–1309. [Google Scholar] [CrossRef]
- Riley, G. Tendinopathy—From basic science to treatment. Nat. Clin. Pr. Rheumatol. 2008, 4, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, D.; Tundo, G.R.; Fasciglione, G.F.; Gioia, M.; Bisicchia, S.; Gasbarra, E.; Ippolito, E.; Tarantino, U.; Coletta, M.; Marini, S. Role of metalloproteinases in tendon pathophysiology. Mini Rev. Med. Chem. 2014, 14, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Galloway, W.A.; Murphy, G.; Sandy, J.D.; Gavrilovic, J.; Cawston, T.E.; Reynolds, J.J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem. J. 1983, 209, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.; Murphy, G.; Breathnach, R. Human and rat malignant-tumor-associated mRNAs encode stromelysin-like metalloproteinases. Biochemistry 1989, 28, 5195–5203. [Google Scholar] [CrossRef]
- Fernandes, R.J.; Hirohata, S.; Engle, J.M.; Colige, A.; Cohn, D.H.; Eyre, D.R.; Apte, S.S. Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. J. Biol. Chem. 2001, 276, 31502–31509. [Google Scholar] [CrossRef]
- Colige, A.; Ruggiero, F.; Vandenberghe, I.; Dubail, J.; Kesteloot, F.; Van Beeumen, J.; Beschin, A.; Brys, L.; Lapiere, C.M.; Nusgens, B. Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens types I-III and V. J. Biol. Chem. 2005, 280, 34397–34408. [Google Scholar] [CrossRef]
- Vadon-Le Goff, S.; Kronenberg, D.; Bourhis, J.M.; Bijakowski, C.; Raynal, N.; Ruggiero, F.; Farndale, R.W.; Stocker, W.; Hulmes, D.J.; Moali, C. Procollagen C-proteinase enhancer stimulates procollagen processing by binding to the C-propeptide region only. J. Biol. Chem. 2011, 286, 38932–38938. [Google Scholar] [CrossRef]
- Hubmacher, D.; Wang, L.W.; Mecham, R.P.; Reinhardt, D.P.; Apte, S.S. Adamtsl2 deletion results in bronchial fibrillin microfibril accumulation and bronchial epithelial dysplasia—A novel mouse model providing insights into geleophysic dysplasia. Dis. Model. Mech. 2015, 8, 487–499. [Google Scholar] [CrossRef]
- Pingel, J.; Kampmann, M.L.; Andersen, J.D.; Wong, C.; Dossing, S.; Borsting, C.; Nielsen, J.B. Gene expressions in cerebral palsy subjects reveal structural and functional changes in the gastrocnemius muscle that are closely associated with passive muscle stiffness. Cell Tissue Res. 2021, 384, 513–526. [Google Scholar] [CrossRef]
- Smith, L.R.; Chambers, H.G.; Subramaniam, S.; Lieber, R.L. Transcriptional abnormalities of hamstring muscle contractures in children with cerebral palsy. PLoS ONE 2012, 7, e40686. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Pontén, E.; Hedström, Y.; Ward, S.R.; Chambers, H.G.; Subramaniam, S.; Lieber, R.L. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC Med. Genom. 2009, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.C.; Corps, A.N.; Pennington, C.J.; Clark, I.M.; Edwards, D.R.; Bradley, M.M.; Hazleman, B.L.; Riley, G.P. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006, 54, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Jelinsky, S.A.; Rodeo, S.A.; Li, J.; Gulotta, L.V.; Archambault, J.M.; Seeherman, H.J. Regulation of gene expression in human tendinopathy. BMC Musculoskelet. Disord. 2011, 12, 86. [Google Scholar] [CrossRef]
- Ireland, D.; Harrall, R.; Curry, V.; Holloway, G.; Hackney, R.; Hazleman, B.; Riley, G. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001, 20, 159–169. [Google Scholar] [CrossRef]
- Dean, B.J.; Franklin, S.L.; Carr, A.J. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Jt. Res. 2012, 1, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef]
- Ellis, I.; Schnabel, L.V.; Berglund, A.K. Defining the Profile: Characterizing Cytokines in Tendon Injury to Improve Clinical Therapy. J. Immunol. Regen. Med. 2022, 16, 100059. [Google Scholar] [CrossRef]
- Jomaa, G.; Kwan, C.K.; Fu, S.C.; Ling, S.K.; Chan, K.M.; Yung, P.S.; Rolf, C. A systematic review of inflammatory cells and markers in human tendinopathy. BMC Musculoskelet. Disord. 2020, 21, 78. [Google Scholar] [CrossRef]
- Russo, V.; El Khatib, M.; Prencipe, G.; Citeroni, M.R.; Faydaver, M.; Mauro, A.; Berardinelli, P.; Cervero-Varona, A.; Haidar-Montes, A.A.; Turriani, M.; et al. Tendon Immune Regeneration: Insights on the Synergetic Role of Stem and Immune Cells during Tendon Regeneration. Cells 2022, 11, 434. [Google Scholar] [CrossRef]
- Langberg, H.; Rosendal, L.; Kjaer, M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J. Physiol. 2001, 534, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.; Karamanidis, K.; Albracht, K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J. Exp. Biol. 2007, 210, 2743–2753. [Google Scholar] [CrossRef] [PubMed]
- Couppe, C.; Kongsgaard, M.; Aagaard, P.; Hansen, P.; Bojsen-Moller, J.; Kjaer, M.; Magnusson, S.P. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J. Appl. Physiol. 2008, 105, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Arampatzis, A.; Peper, A.; Bierbaum, S.; Albracht, K. Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. J. Biomech. 2010, 43, 7. [Google Scholar] [CrossRef] [PubMed]
- Seynnes, O.R.; Erskine, R.M.; Maganaris, C.N.; Longo, S.; Simoneau, E.M.; Grosset, J.F.; Narici, M.V. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J. Appl. Physiol. 2009, 107, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bohm, S.; Mersmann, F.; Tettke, M.; Kraft, M.; Arampatzis, A. Human Achilles tendon plasticity in response to cyclic strain: Effect of rate and duration. J. Exp. Biol. 2014, 217, 4010–4017. [Google Scholar] [CrossRef]
- Bohm, S.; Mersmann, F.; Arampatzis, A. Human tendon adaptation in response to mechanical loading: A systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med. Open 2015, 1, 7. [Google Scholar] [CrossRef]
- Rosager, S.; Aagaard, P.; Dyhre-Poulsen, P.; Neergaard, K.; Kjaer, M.; Magnusson, S.P. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand. J. Med. Sci. Sports 2002, 12, 90–98. [Google Scholar] [CrossRef]
- Sponbeck, J.K.; Perkins, C.L.; Berg, M.J.; Rigby, J.H. Achilles Tendon Cross Sectional Area Changes Over a Division I NCAA Cross Country Season. Int. J. Exerc. Sci. 2017, 10, 1226–1234. [Google Scholar]
- Kongsgaard, M.; Reitelseder, S.; Pedersen, T.G.; Holm, L.; Aagaard, P.; Kjaer, M.; Magnusson, S.P. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol. 2007, 191, 111–121. [Google Scholar] [CrossRef]



| Patient | Gender | Age | Type |
|---|---|---|---|
| 1 | F | 15.8 | C |
| 2 | M | 26.4 | C |
| 3 | M | 14.6 | C |
| 4 | M | 16.1 | C |
| 5 | M | 16.8 | C |
| 6 | M | 21.1 | D |
| 7 | M | 15.5 | D |
| 8 | M | 17.1 | D |
| 9 | M | 15.0 | D |
| 10 | F | 5.6 | D |
| 11 | M | 16.5 | D |
| 12 | M | 10.5 | D |
| 13 | M | 11.0 | D |
| 14 | M | 15.7 | D |
| 15 | F | 11.4 | D |
| 16 | M | 17.1 | D |
| 17 | M | 10.1 | D |
| 18 | M | 11.5 | T |
| 19 | F | 11.0 | T |
| 20 | F | 14.5 | T |
| 21 | M | 16.2 | T |
| 22 | M | 4.9 | T |
| 23 | F | 14.0 | T |
| 24 | M | 14.9 | T |
| 25 | F | 12.8 | T |
| 26 | F | 11.6 | T |
| 27 | M | 13.3 | T |
| 28 | M | 9.2 | T |
| 29 | M | 16.2 | T |
| 30 | M | 13.0 | T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemska, S.; Serio, S.; Larcher, V.; Beltrame, G.; Portinaro, N.M.; Bang, M.-L. Whole Genome Expression Profiling of Semitendinosus Tendons from Children with Diplegic and Tetraplegic Cerebral Palsy. Biomedicines 2023, 11, 2918. https://doi.org/10.3390/biomedicines11112918
Nemska S, Serio S, Larcher V, Beltrame G, Portinaro NM, Bang M-L. Whole Genome Expression Profiling of Semitendinosus Tendons from Children with Diplegic and Tetraplegic Cerebral Palsy. Biomedicines. 2023; 11(11):2918. https://doi.org/10.3390/biomedicines11112918
Chicago/Turabian StyleNemska, Simona, Simone Serio, Veronica Larcher, Giulia Beltrame, Nicola Marcello Portinaro, and Marie-Louise Bang. 2023. "Whole Genome Expression Profiling of Semitendinosus Tendons from Children with Diplegic and Tetraplegic Cerebral Palsy" Biomedicines 11, no. 11: 2918. https://doi.org/10.3390/biomedicines11112918
APA StyleNemska, S., Serio, S., Larcher, V., Beltrame, G., Portinaro, N. M., & Bang, M.-L. (2023). Whole Genome Expression Profiling of Semitendinosus Tendons from Children with Diplegic and Tetraplegic Cerebral Palsy. Biomedicines, 11(11), 2918. https://doi.org/10.3390/biomedicines11112918








