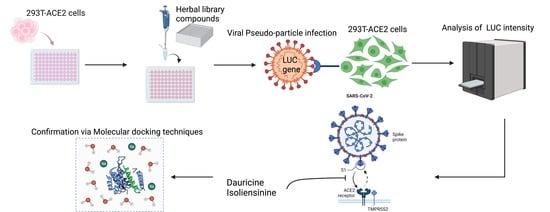
Herbal Compounds Dauricine and Isoliensinine Impede SARS-CoV-2 Viral Entry
Abstract
:1. Introduction
2. Methods and Materials
2.1. Herbal Compounds
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Viral Pseudo Particle (VPP) Assay
2.5. TMPRSS2 Protease Activity Assay
2.6. Spike-ACE2 Interaction Assay
2.7. Western Blot Analysis
2.8. Statistical Analysis
2.9. Molecular Docking
3. Results
3.1. Reduced Infection Rates Post-Drug Treatment with Dauricine and Isoliensinine
3.2. Cytotoxicity of Dauricine and Isoliensinine
3.3. Antiviral Activities of Dauricine and Isoliensinine
3.4. Dauricine and Isoliensinine Maintain Inhibitory Activities against Different Variants of VPPs
3.5. Dauricine and Isoliensinine Obstruct Spike Protein- ACE2 Interaction but Not TMPRSS2 Protease Activity
3.6. Molecular Modeling of Dauricine and Isoliensinine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.R.; Rennick, L.J.; Nambulli, S.; Robinson-McCarthy, L.R.; Bain, W.G.; Haidar, G.; Duprex, W.P. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science 2021, 371, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Belouzard, S.; Chu, V.C.; Whittaker, G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA 2009, 106, 5871–5876. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Johnson, B.A.; Xie, X.; Bailey, A.L.; Kalveram, B.; Lokugamage, K.G.; Muruato, A.; Zou, J.; Zhang, X.; Juelich, T.; Smith, J.K.; et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature 2021, 591, 293–299. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Muus, C.; Luecken, M.D.; Eraslan, G.; Sikkema, L.; Waghray, A.; Heimberg, G.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smillie, C.; et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat. Med. 2021, 27, 546–559. [Google Scholar] [CrossRef]
- Sakai, K.; Ami, Y.; Tahara, M.; Kubota, T.; Anraku, M.; Abe, M.; Nakajima, N.; Sekizuka, T.; Shirato, K.; Suzaki, Y.; et al. The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses. J. Virol. 2014, 88, 5608–5616. [Google Scholar] [CrossRef]
- Sato, K.; Hayashi, H.; Shimotai, Y.; Yamaya, M.; Hongo, S.; Kawakami, K.; Matsuzaki, Y.; Nishimura, H. TMPRSS2 Activates Hemagglutinin-Esterase Glycoprotein of Influenza C Virus. J. Virol. 2021, 95, e0129621. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Muller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef]
- Meng, B.; Abdullahi, A.; Ferreira, I.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Alipoor, S.D.; Mirsaeidi, M. SARS-CoV-2 cell entry beyond the ACE2 receptor. Mol. Biol. Rep. 2022, 49, 10715–10727. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Xie, Y.; Zhao, H.; Li, B.; Yu, X.; Wang, M.; Li, S.; Li, J. The efficacy of traditional Chinese medicine in the treatment of the COVID-19 pandemic in Henan Province: A retrospective study. Eur. J. Med. Res. 2023, 28, 78. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.C.; Shyur, L.F.; Jan, J.T.; Liang, P.H.; Kuo, C.J.; Arulselvan, P.; Wu, J.B.; Kuo, S.C.; Yang, N.S. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Tradit. Complement. Med. 2011, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lupala, C.S.; Ye, Y.; Chen, H.; Su, X.D.; Liu, H. Mutations on RBD of SARS-CoV-2 Omicron variant result in stronger binding to human ACE2 receptor. Biochem. Biophys. Res. Commun. 2022, 590, 34–41. [Google Scholar] [CrossRef]
- Park, H.J.; Gholam Zadeh, M.; Suh, J.H.; Choi, H.S. Dauricine Protects from LPS-Induced Bone Loss via the ROS/PP2A/NF-κB Axis in Osteoclasts. Antioxidants 2020, 9, 588. [Google Scholar] [CrossRef]
- Yang, X.; Huang, M.; Yang, J.; Wang, J.; Zheng, S.; Ma, X.; Cai, J.; Deng, S.; Shu, G.; Yang, G. Activity of Isoliensinine in Improving the Symptoms of Type 2 Diabetic Mice via Activation of AMP-Activated Kinase and Regulation of PPARgamma. J. Agric. Food Chem. 2017, 65, 7168–7178. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Zhou, S.J. Dauricine combined with clindamycin inhibits severe pneumonia co-infected by influenza virus H5N1 and Streptococcus pneumoniae in vitro and in vivo through NF-κB signaling pathway. J. Pharmacol. Sci. 2018, 137, 12–19. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.L.; Zhou, Z.W.; Long, H.Z.; Luo, H.Y.; Wen, D.D.; Cheng, L.; Gao, L.C. Isoliensinine: A Natural Compound with “Drug-Like” Potential. Front. Pharmacol. 2021, 12, 630385. [Google Scholar] [CrossRef]
- Zhang, D.; Hamdoun, S.; Chen, R.; Yang, L.; Ip, C.K.; Qu, Y.; Li, R.; Jiang, H.; Yang, Z.; Chung, S.K.; et al. Identification of natural compounds as SARS-CoV-2 entry inhibitors by molecular docking-based virtual screening with bio-layer interferometry. Pharmacol. Res. 2021, 172, 105820. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Zhang, Y.-N.; Li, X.-D.; Zhang, H.-Q.; Xiao, S.-Q.; Deng, F.; Yuan, Z.-M.; Ye, H.-Q.; Zhang, B. A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2. Signal Transduct. Target. Ther. 2020, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Huang, B.; Ruhan, A.; Li, W.; Wang, W.; Deng, Y.; Tan, W. Development and effectiveness of pseudotyped SARS-CoV-2 system as determined by neutralizing efficiency and entry inhibition test in vitro. Biosaf. Health 2020, 2, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Kalkeri, R.; Cai, Z.; Lin, S.; Farmer, J.; Kuzmichev, Y.V.; Koide, F. SARS-CoV-2 Spike Pseudoviruses: A Useful Tool to Study Virus Entry and Address Emerging Neutralization Escape Phenotypes. Microorganisms 2021, 9, 1744. [Google Scholar] [CrossRef]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Barcena, M.; et al. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Neerukonda, S.N.; Vassell, R.; Herrup, R.; Liu, S.; Wang, T.; Takeda, K.; Yang, Y.; Lin, T.L.; Wang, W.; Weiss, C.D. Establishment of a well-characterized SARS-CoV-2 lentiviral pseudovirus neutralization assay using 293T cells with stable expression of ACE2 and TMPRSS2. PLoS ONE 2021, 16, e0248348. [Google Scholar] [CrossRef]
- Hsu, K.C.; Chen, Y.F.; Lin, S.R.; Yang, J.M. iGEMDOCK: A graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinform. 2011, 12 (Suppl. 1), S33. [Google Scholar] [CrossRef]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput.-Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef]
- Vangone, A.; Schaarschmidt, J.; Koukos, P.; Geng, C.; Citro, N.; Trellet, M.E.; Xue, L.C.; Bonvin, A. Large-scale prediction of binding affinity in protein-small ligand complexes: The PRODIGY-LIG web server. Bioinformatics 2019, 35, 1585–1587. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Chen, H.F.; Wang, W.J.; Chen, C.Y.; Chang, W.C.; Hsueh, P.R.; Peng, S.L.; Wu, C.S.; Chen, Y.; Huang, H.Y.; Shen, W.J.; et al. The natural tannins oligomeric proanthocyanidins and punicalagin are potent inhibitors of infection by SARS-CoV-2. eLife 2023, 12, e84899. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, W.H.; Chen, H.F.; Huang, L.M.; Gao, J.Y.; Lin, C.W.; Wang, Y.C.; Yang, C.S.; Liu, Y.L.; Hou, M.H.; et al. Tafenoquine and its derivatives as inhibitors for the severe acute respiratory syndrome coronavirus 2. J. Biol. Chem. 2022, 298, 101658. [Google Scholar] [CrossRef] [PubMed]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Alabed, M.; Temsah, M.H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther. Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gheware, A.; Ray, A.; Rana, D.; Bajpai, P.; Nambirajan, A.; Arulselvi, S.; Mathur, P.; Trikha, A.; Arava, S.; Das, P.; et al. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci. Rep. 2022, 12, 4058. [Google Scholar] [CrossRef]
- Gillim-Ross, L.; Taylor, J.; Scholl, D.R.; Ridenour, J.; Masters, P.S.; Wentworth, D.E. Discovery of novel human and animal cells infected by the severe acute respiratory syndrome coronavirus by replication-specific multiplex reverse transcription-PCR. J. Clin. Microbiol. 2004, 42, 3196–3206. [Google Scholar] [CrossRef] [PubMed]
- Pires De Souza, G.A.; Le Bideau, M.; Boschi, C.; Wurtz, N.; Colson, P.; Aherfi, S.; Devaux, C.; La Scola, B. Choosing a cellular model to study SARS-CoV-2. Front. Cell. Infect. Microbiol. 2022, 12, 1003608. [Google Scholar] [CrossRef]
- Pagani, I.; Ghezzi, S.; Alberti, S.; Poli, G.; Vicenzi, E. Origin and evolution of SARS-CoV-2. Eur. Phys. J. Plus 2023, 138, 157. [Google Scholar] [CrossRef]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, Y.; Wang, Y.C.; Wang, W.J.; Yang, C.S.; Tsai, C.L.; Hou, M.H.; Chen, H.F.; Shen, Y.C.; Hung, M.C. Tannic acid suppresses SARS-CoV-2 as a dual inhibitor of the viral main protease and the cellular TMPRSS2 protease. Am. J. Cancer Res. 2020, 10, 4538–4546. [Google Scholar] [PubMed]
- Xiang, Y.; Zhai, G.; Li, Y.; Wang, M.; Chen, X.; Wang, R.; Xie, H.; Zhang, W.; Ge, G.; Zhang, Q.; et al. Ginkgolic acids inhibit SARS-CoV-2 and its variants by blocking the spike protein/ACE2 interplay. Int. J. Biol. Macromol. 2023, 226, 780–792. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.; Thakur, N.; Peacock, T.P.; Bialy, D.; Elrefaey, A.M.E.; Bogaardt, C.; Horton, D.L.; Ho, S.; Kankeyan, T.; Carr, C.; et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat. Microbiol. 2022, 7, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Ord, M.; Faustova, I.; Loog, M. The sequence at Spike S1/S2 site enables cleavage by furin and phospho-regulation in SARS-CoV2 but not in SARS-CoV1 or MERS-CoV. Sci. Rep. 2020, 10, 16944. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Saied, A.A.; Mitra, S.; Alhumaydhi, F.A.; Emran, T.B.; Wilairatana, P. Omicron variant (B.1.1.529) and its sublineages: What do we know so far amid the emergence of recombinant variants of SARS-CoV-2? Biomed. Pharmacother. 2022, 154, 113522. [Google Scholar] [CrossRef]
- Jiao, Y.; Xing, Y.; Sun, Y. Impact of E484Q and L452R Mutations on Structure and Binding Behavior of SARS-CoV-2 B.1.617.1 Using Deep Learning AlphaFold2, Molecular Docking and Dynamics Simulation. Int. J. Mol. Sci. 2023, 24, 11564. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Meng, B.; Kemp, S.A.; Papa, G.; Datir, R.; Ferreira, I.; Marelli, S.; Harvey, W.T.; Lytras, S.; Mohamed, A.; Gallo, G.; et al. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7. Cell Rep. 2021, 35, 109292. [Google Scholar] [CrossRef]
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef]
- Zech, F.; Schniertshauer, D.; Jung, C.; Herrmann, A.; Cordsmeier, A.; Xie, Q.; Nchioua, R.; Prelli Bozzo, C.; Volcic, M.; Koepke, L.; et al. Spike residue 403 affects binding of coronavirus spikes to human ACE2. Nat. Commun. 2021, 12, 6855. [Google Scholar] [CrossRef]








| Cell Line | Compound | a IC50 (μM) | b CC50 (μM) | c SI |
|---|---|---|---|---|
| 293T-ACE2 | Dauricine | 1.26 ± 0.5 | 39.79 ± 7.59 | 31.83 |
| Isoliensinine | 0.29 ± 0.11 | 43.58 ± 0.94 | 150.28 | |
| VeroE6 | Dauricine | 1.47 ± 0.18 | 25.36 ± 0.72 | 17.25 |
| Isoliensinine | 0.45 ± 0.04 | 54.13 ± 5.9 | 120.29 |
| ΔG Prediction (Kcal/mol) | |
|---|---|
| B.1.1.529 and Dauricine | −4.96 |
| B.1.1.529 and Isoliensinine | −5.04 |
| B.1.617 and Isoliensinine | −4.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabrell, S.N.; Li, Y.-C.; Yamaguchi, H.; Chen, H.-F.; Hung, M.-C. Herbal Compounds Dauricine and Isoliensinine Impede SARS-CoV-2 Viral Entry. Biomedicines 2023, 11, 2914. https://doi.org/10.3390/biomedicines11112914
Dabrell SN, Li Y-C, Yamaguchi H, Chen H-F, Hung M-C. Herbal Compounds Dauricine and Isoliensinine Impede SARS-CoV-2 Viral Entry. Biomedicines. 2023; 11(11):2914. https://doi.org/10.3390/biomedicines11112914
Chicago/Turabian StyleDabrell, Shaneek Natoya, Yi-Chuan Li, Hirohito Yamaguchi, Hsiao-Fan Chen, and Mien-Chie Hung. 2023. "Herbal Compounds Dauricine and Isoliensinine Impede SARS-CoV-2 Viral Entry" Biomedicines 11, no. 11: 2914. https://doi.org/10.3390/biomedicines11112914
APA StyleDabrell, S. N., Li, Y.-C., Yamaguchi, H., Chen, H.-F., & Hung, M.-C. (2023). Herbal Compounds Dauricine and Isoliensinine Impede SARS-CoV-2 Viral Entry. Biomedicines, 11(11), 2914. https://doi.org/10.3390/biomedicines11112914